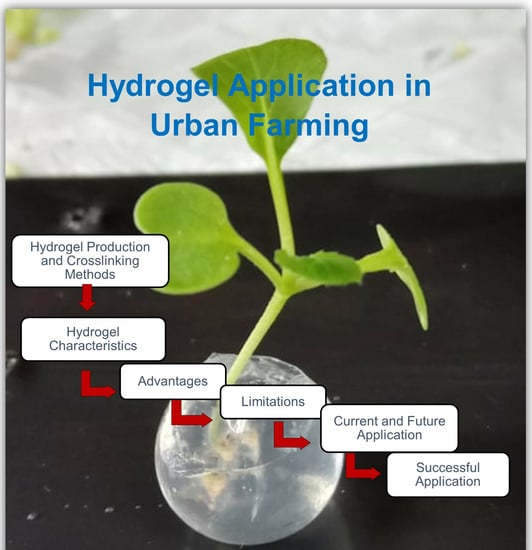
Hydrogel Application in Urban Farming: Potentials and Limitations—A Review
Abstract
1. Introduction
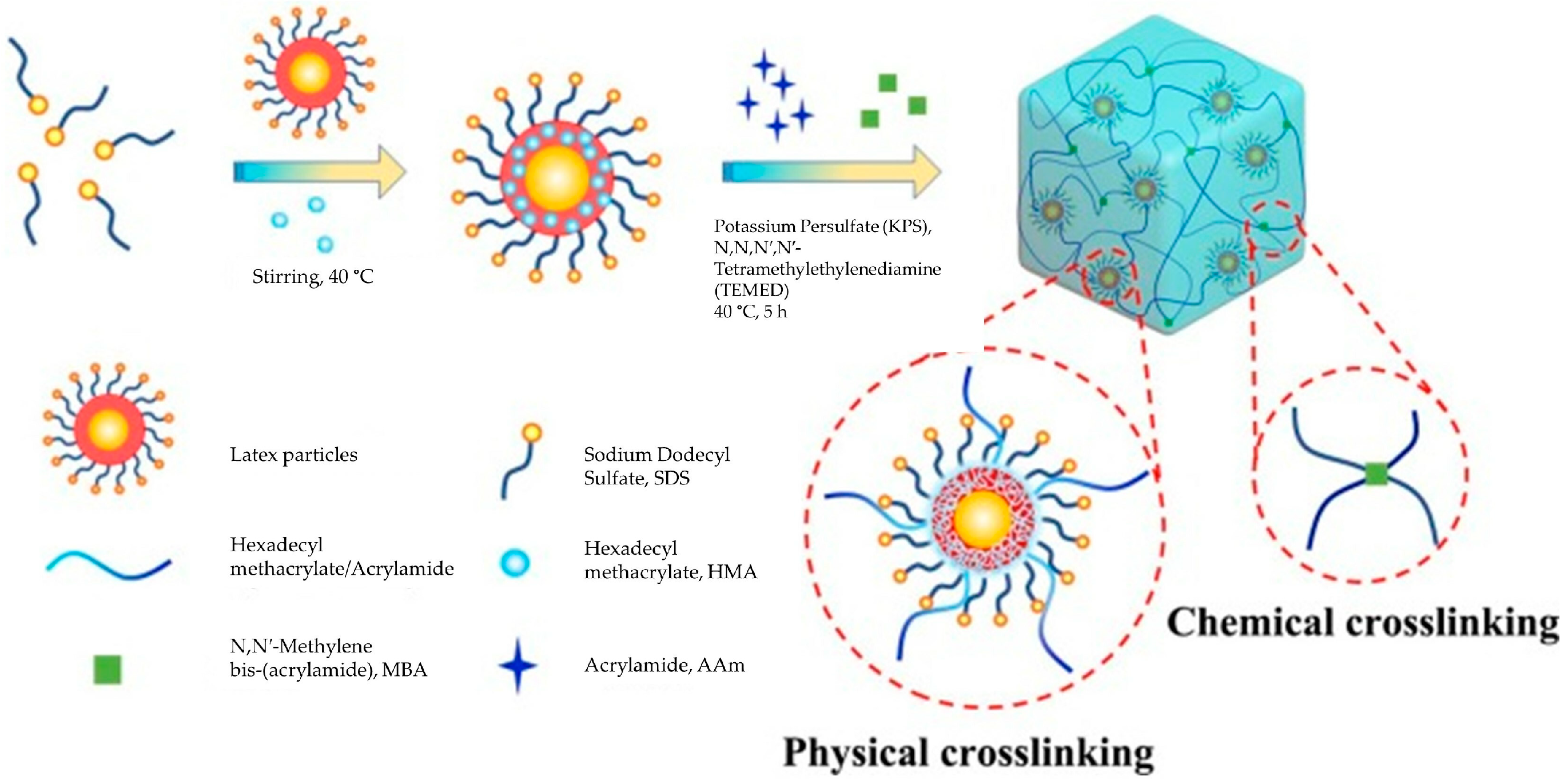
2. Hydrogel Production and Crosslinking Methods
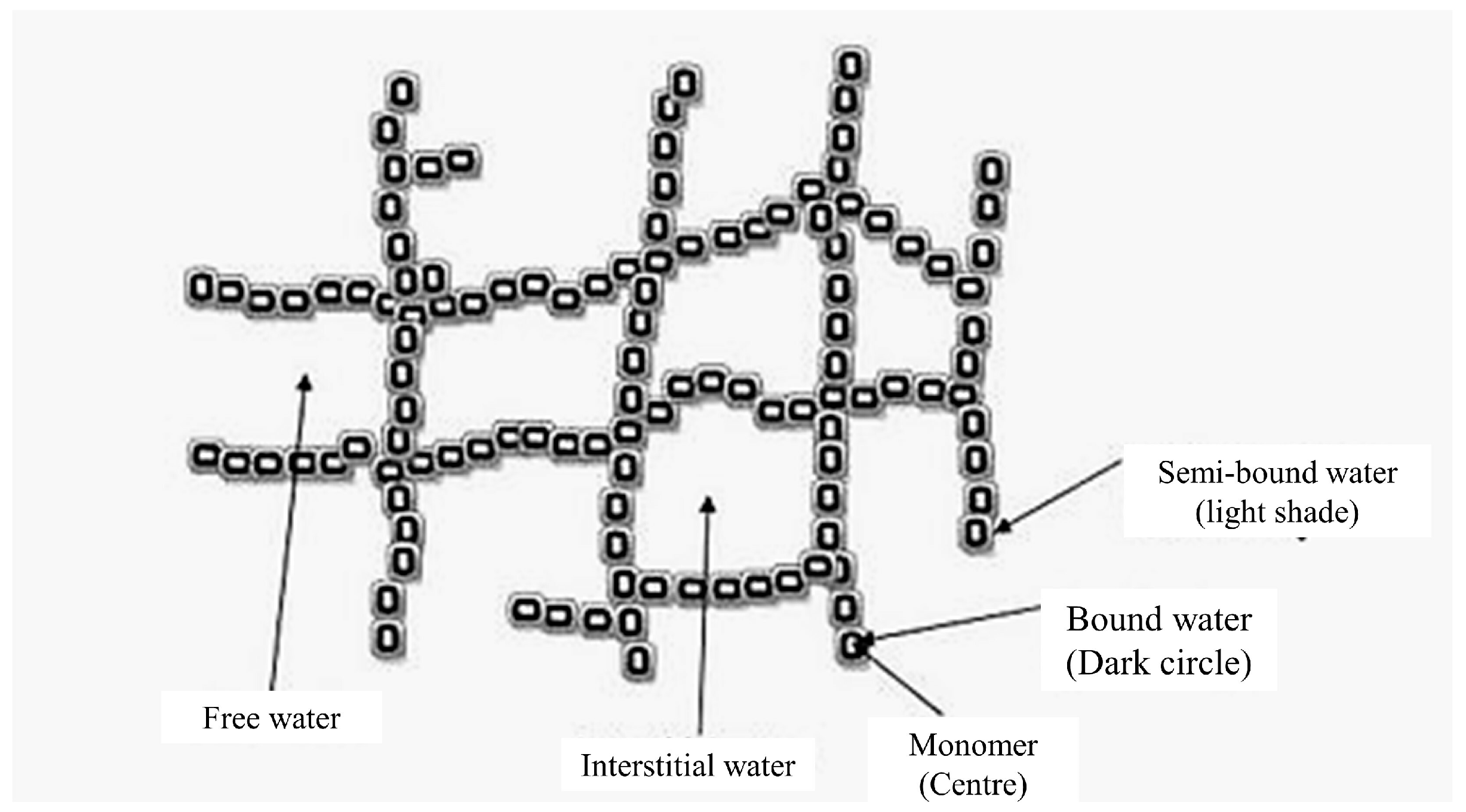
3. Hydrogel Characteristics
4. Advantages of Hydrogel in Urban Farming
4.1. Hydrogel as a Potting Medium
4.2. Alternative to Soilless Agriculture
4.3. Efficient Irrigation and Fertiliser Application
5. Limitations of Hydrogel in Urban Farming
Structural Stability and Physical Integrity of Hydrogel
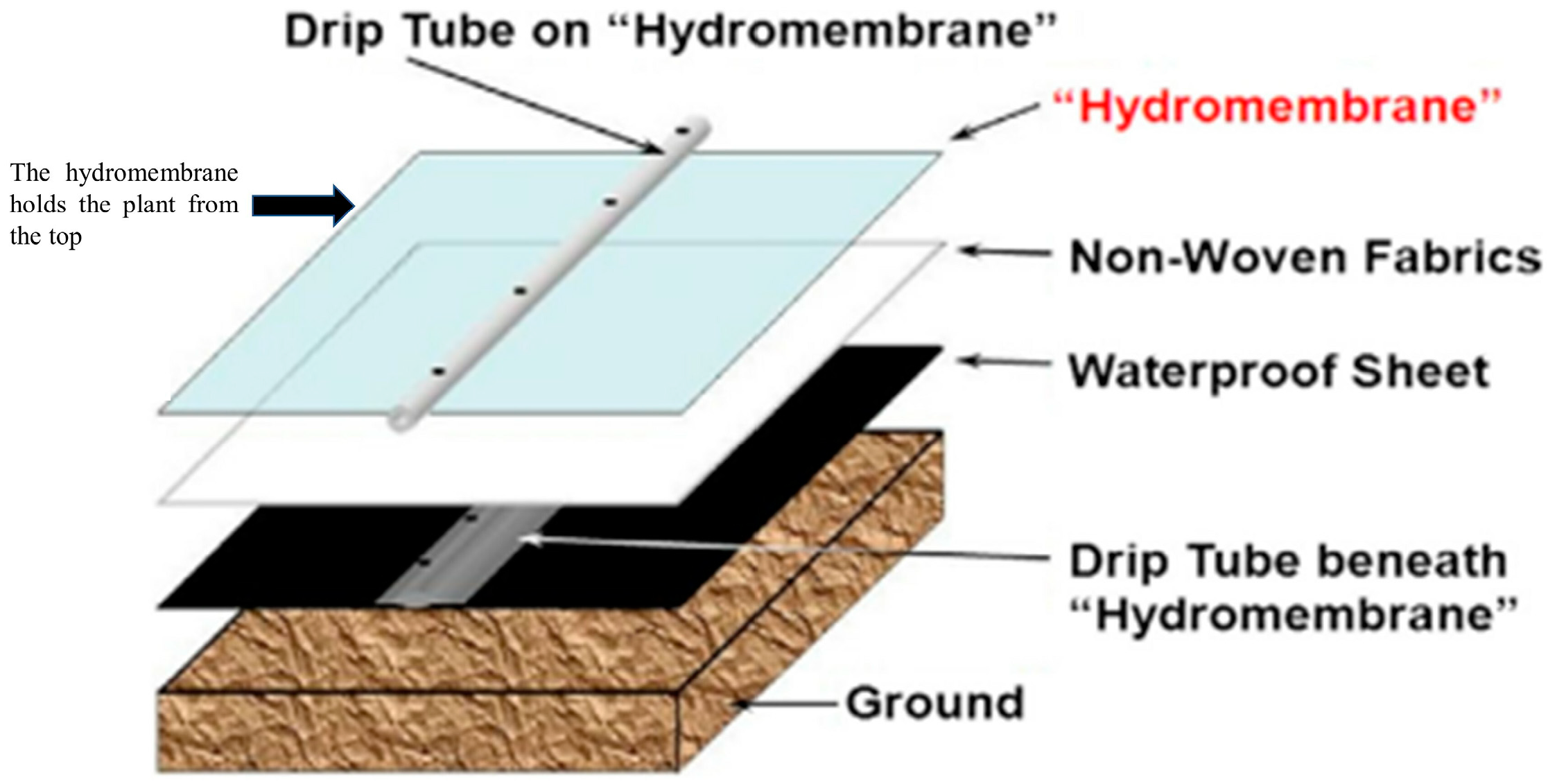
6. Current and Future Applications of Hydrogel in Urban Farming
7. Successful Applications of Hydrogel-Based Products
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertram, G.C. The State of Food and Agriculture; United Nations Food and Agriculture Organization (FAO): Rome, Italy, 2016; Volume 59, ISBN 9789251093740. [Google Scholar]
- Benke, K.; Tomkins, B. Future Food-Production Systems: Vertical Farming and Controlled-Environment Agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Kwon, C.; Heo, J.; Lemmon, Z.H.; Capua, Y.; Hutton, S.F.; Van Eck, J.; Park, S.J.; Lippman, Z.B. Rapid Customization of Solanaceae Fruit Crops for Urban Agriculture. Nat. Biotechnol. 2020, 38, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, D.; Karak, N. Biodegradable Superabsorbent Hydrogel for Water Holding in Soil and Controlled-Release Fertilizer. J. Appl. Polym. Sci. 2020, 137, 1–12. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-Based Hydrogel Materials: Chemistry, Properties and Their Prospective Applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Neethu, T.M.; Dubey, P.K.; Kaswala, A.R. Prospects and Applications of Hydrogel Technology in Agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3155–3162. [Google Scholar] [CrossRef]
- Xu, H.; Yeum, K.; Yoon, Y.; Ju, J. Effect of Hydrogels in Three Substrates on Growth and Ornamental Quality of Apple Mint (Mentha suaveolens) in Unirrigated Green Roofs. J. Hortic. 2019, 6, 1–5. [Google Scholar] [CrossRef]
- Luan, L.Q.; Xo, D.H. Preparation of Oligoalginate Immobilized Hydrogel by Radiation and Its Application for Hydroponic Culture. Radioisotopes 2017, 66, 171–179. [Google Scholar] [CrossRef]
- Durpekova, S.; Filatova, K.; Cisar, J.; Ronzova, A.; Kutalkova, E.; Sedlarik, V. A Novel Hydrogel Based on Renewable Materials for Agricultural Application. Int. J. Polym. Sci. 2020, 2020. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of Cellulose-Based Hydrogel: A Review. J. Mater. Res. Technol. 2020, 10, 935–952. [Google Scholar] [CrossRef]
- Tholibon, D.; Tharazi, I.; Sulong, A.B.; Muhamad, N.; Ismail, N.F.; Radzi, M.K.F.M.; Radzuan, N.A.M.; Hui, D. Kenaf Fiber Composites: A Review on Synthetic and Biodegradable Polymer Matrix. J. Kejuruter. 2019, 31, 65–76. [Google Scholar]
- Rico-García, D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Hernández-Olmos, S.L.; Guerrero-Ramírez, G.L.; Vilas-Vilela, J.L. Lignin-Based Hydrogels: Synthesis and Applications. Polymers 2020, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Zhang, S.; Huang, L.; Wang, H.; Wang, W.; Ye, Q. Starch-Based Hydrogel Loading with Carbendazim for Controlled-Release and Water Absorption. Carbohydr. Polym. 2015, 125, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y. Biomaterial Technology for Tissue Engineering Applications. J. R. Soc. Interface 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, C.K.; Upadhyay, N.; Jain, A.; Verma, A.; Narayana Charyulu, R.; Jain, S. Hydrogels-Promising Candidates for Tissue Engineering; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780323353038. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2013, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current Progress on Bio-Based Polymers and Their Future Trends. Prog. Biomater. 2013, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kaco, H.; Zakaria, S.; Razali, N.F.; Chia, C.H.; Zhang, L.; Jani, S.M. Properties of Cellulose Hydrogel from Kenaf Core Prepared via Pre-Cooled Dissolving Method. Sains Malaysiana 2014, 43, 1221–1229. [Google Scholar]
- Baharin, K.W.; Zakaria, S.; Ellis, A.V.; Talip, N.; Kaco, H.; Gan, S.; Zailan, F.D.; Ain Syed Hashim, S.N. Factors Affecting Cellulose Dissolution of Oil Palm Empty Fruit Bunch and Kenaf Pulp in NaOH/Urea Solvent. Sains Malaysiana 2018, 47, 377–386. [Google Scholar] [CrossRef]
- Mane, S.; Ponrathnam, S.; Chavan, N. Effect of Chemical Crosslinking on Properties of Polymer Microbeads: A Review. Can. Chem. Trans. 2016, 3, 473–485. [Google Scholar] [CrossRef]
- Yang, F.; Hlushko, R.; Wu, D.; Sukhishvili, S.A.; Du, H.; Tian, F. Ocean Salinity Sensing Using Long-Period Fiber Gratings Functionalized with Layer-by-Layer Hydrogels. ACS Omega 2019, 4, 2134–2141. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of Synthesis of Hydrogels … A Review. Saudi Pharm. J. 2015, 24, 554–559. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-Linking in Hydrogels - A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-Based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Das, D.; Prakash, P.; Rout, P.K.; Baladhare, S. Synthesis and Characterization of Superabsorbent Cellulose-Based Hydrogel for Agriculture Application. Starch 2021, 73, 1–10. [Google Scholar] [CrossRef]
- Leick, S.; Henning, S.; Degen, P.; Rehage, H. Deformation of Liquid-Filled Calcium Alginate Capsules in a Spinning Drop Apparatus Deformation of Liquid-Filled Calcium Alginate Capsules in a Spinning Drop Apparatus. Phys. Chem. Chem. Phys. 2010, 12, 2950–2958. [Google Scholar] [CrossRef]
- Wong, L.C.; Leh, C.P.; Goh, C.F. Designing Cellulose Hydrogels from Non-Woody Biomass. Carbohydr. Polym. 2021, 264, 1–17. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Sari, M.; Oppermanna, W.; Okay, O. Tough and Self-Healing Hydrogels Formed via Hydrophobic Interactions. Adv. Pharm. Bull. 2011, 44, 4997–5005. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Bao, Y. Advances in Cellulose-Based Superabsorbent Hydrogels. RSC Adv. 2015, 5, 59745–59757. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Salleh, K.M.; Zakaria, S.; Sajab, M.S.; Gan, S.; Chia, C.H.; Jaafar, S.N.S.; Amran, U.A. Chemically Crosslinked Hydrogel and Its Driving Force towards Superabsorbent Behaviour. Int. J. Biol. Macromol. 2018, 118, 1422–1430. [Google Scholar] [CrossRef]
- Milani, P.; França, D.; Balieiro, A.G.; Faez, R. Polymers and Its Applications in Agriculture. Polimeros 2017, 27, 256–266. [Google Scholar] [CrossRef]
- Cai, M.H.; Chen, X.Y.; Fu, L.Q.; Du, W.L.; Yang, X.; Mou, X.Z.; Hu, P.Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, X.; Ren, X.; Gao, G. The Role of Chemical and Physical Crosslinking in Different Deformation Stages of Hybrid Hydrogels. Eur. Polym. J. 2018, 100, 86–95. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Tanaka, T. Collapse of Gels and the Critical Endpoint. Phys. Rev. Lett. 1978, 40, 820–823. [Google Scholar] [CrossRef]
- Beckett, L.E.; Lewis, J.T.; Tonge, T.K.; Korley, L.T.J. Enhancement of the Mechanical Properties of Hydrogels with Continuous Fibrous Reinforcement. ACS Biomater. Sci. Eng. 2020, 6, 5453–5473. [Google Scholar] [CrossRef]
- Chen, G.; Tang, W.; Wang, X.; Zhao, X.; Chen, C.; Zhu, Z. Applications of Hydrogels with Special Physical Properties in Biomedicine. Polymers 2019, 11, 1420. [Google Scholar] [CrossRef]
- Li, W.; Wu, D.; Hu, D.; Zhu, S.; Pan, C.; Jiao, Y.; Li, L.; Luo, B.; Zhou, C.; Lu, L. Stress-Relaxing Double-Network Hydrogel for Chondrogenic Differentiation of Stem Cells. Mater. Sci. Eng. C 2019, 107, 110333. [Google Scholar] [CrossRef]
- Koivisto, J.T.; Joki, T.; Zhang, H.; Li, Z.; Li, D.; Cells, E.S. Viscoelastic Behaviour of Hydrogel-Based Composites for Tissue Engineering under Mechanical Load. Biomed. Mater. 2017, 12, 25004. [Google Scholar]
- Yan, C.; Pochan, D.J. Rheological Properties of Peptide-Based Hydrogels for Biomedical and Other Applications. Chem. Soc. Rev. 2011, 39, 3528–3540. [Google Scholar] [CrossRef]
- Zigon-Branc, S.; Markovic, M.; Van Hoorick, J.; Van Vlierberghe, S.; Dubruel, P.; Zerobin, E.; Baudis, S.; Ovsianikov, A. Impact of Hydrogel Stiffness on Differentiation of Human Adipose-Derived Stem Cell Microspheroids. Tissue Eng. Part A 2019, 25, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mayumi, K.; Creton, C.; Narita, T. Rheological Properties of Tough Hydrogels Based on an Associating Polymer with Permanent and Transient Crosslinks: Effects of Crosslinking Density. J. Rheol. 2017, 61, 1371–1383. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Xu, X.; Su, Y.; Yue, Q.; Gao, B. Characterization, Swelling and Slow-Release Properties of a New Controlled Release Fertilizer Based on Wheat Straw Cellulose Hydrogel. J. Taiwan Inst. Chem. Eng. 2016, 60, 564–572. [Google Scholar] [CrossRef]
- Singhal, R.; Gupta, K. A Review: Tailor-Made Hydrogel Structures (Classifications and Synthesis Parameters). Polym. Plast. Technol. Eng. 2015, 55, 54–70. [Google Scholar] [CrossRef]
- López-Velázquez, J.C.; Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Qui-Zapata, J.A.; García-Morales, S.; Navarro-López, D.E.; Luna-Bárcenas, G.; Vassallo-Brigneti, E.C.; García-Carvajal, Z.Y. Gelatin–Chitosan–PVA Hydrogels and Their Application in Agriculture. J. Chem. Technol. Biotechnol. 2019, 94, 3495–3504. [Google Scholar] [CrossRef]
- Dai, H.; Huang, H. Enhanced Swelling and Responsive Properties of Pineapple Peel Arboxymethyl Cellulose-g-Poly(Acrylic Acid-Co-Acrylamide) Superabsorbent Hydrogel by the Introduction of Carclazyte. J. Agric. Food Chem. 2017, 65, 565–574. [Google Scholar] [CrossRef]
- Cerqueira, S.; Ana, B.; Diana, S.; Costa, B.; Taman, M.N.; Lanceros-me, C.M.C.S.; Ribelles, J.L.G.; Sentanin, F.; Pawlicka, A.; Manuela, M. Thermal—Mechanical Behaviour of Chitosan—Cellulose Derivative Thermoreversible Hydrogel Films. Cellulose 2015, 22, 1911–1929. [Google Scholar] [CrossRef]
- Toniato, T.V.; Stocco, T.D.; Santos, D.; Santanna, L.B.; Tim, C.R.; Roberta, F.; Silva-filho, E.C.; Campana-filho, S.P.; Santanna, L.B.; Tim, C.R.; et al. Hybrid Chitosan/Amniotic Membrane-Based Hydrogels for Articular Cartilage Tissue Engineering Application. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 961–970. [Google Scholar] [CrossRef]
- Salleh, K.M.; Zakaria, S.; Sajab, M.S.; Gan, S.; Kaco, H. Superabsorbent Hydrogel from Oil Palm Empty Fruit Bunch Cellulose and Sodium Carboxymethylcellulose. Int. J. Biol. Macromol. 2019, 131, 50–59. [Google Scholar] [CrossRef]
- Rollon, R.J.C.; Batac, R.A.; Batac, R.A.; Maglines, S.M. Effects of Carbonized Rice Hull and Arbuscular Mycorrhizal Fungi Application on Potting Media Chemical Properties, Growth and Nutrient Uptake of Falcata (Paraserianthes falcataria L.). Int. J. Agron. Agric. Res. 2018, 13, 93–101. [Google Scholar]
- Tahat, M.M.; Alananbeh, K.M.; Othman, Y.A.; Leskovar, D.I. Soil Health and Sustainable Agriculture. Sustain. 2020, 12, 4859. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef]
- Alshrouf, A. Hydroponics, Aeroponic and Aquaponic as Compared with Conventional Farming. Am. Sci. Res. J. Eng. 2017, 27, 247–255. [Google Scholar]
- Tm, S.; Thakur, N.; Sharma, P. Use of Alternative Growing Media in Ornamental Plants. Int. J. Chem. Stud. 2020, 8, 188–194. [Google Scholar] [CrossRef]
- Dede, G.; Pekarchuk, O.; Ozer, H.; Dede, O.H. Alternative Growing Media Components for Green Wall Designs in Terms of Lightweight. In Proceedings of the 2nd International Congress on Engineering and Achitecture, Marmaris, Turkey, 22–24 April 2019; pp. 373–383. [Google Scholar]
- Thakur, T.; Grewal, H.S. Influence of Potting Media Compositions on Flower Production of Chrysanthemum (Chrysanthemum Morifolium Ramat) Cultivar Kikiobiory. J. Plant Nutr. 2019, 42, 1861–1867. [Google Scholar] [CrossRef]
- Nazim, S.G. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2019, 9, 1384. [Google Scholar] [CrossRef]
- Mohammad Padzil, F.N.; Lee, S.H.; Ainun, Z.M.A.A.; Lee, C.H.; Abdullah, L.C. Potential of Oil Palm Empty Fruit Bunch Resources in Nanocellulose Hydrogel Production for Versatile Applications: A Review. Materials 2020, 13, 1245. [Google Scholar] [CrossRef]
- Deenavarman, M.; Lourdusamy, D.K.; Thangaselvabai, T.; Venktesan, K. Effect of Different Media Incorporated with Pusa Hydrogel on Growth and Watering Frequency of Potted Foliage Plant, Arrowhead (Syngonium Podophyllum Schott.). Ann. Hortic. 2018, 11, 67. [Google Scholar] [CrossRef]
- Calcagnile, P.; Sibillano, T.; Giannini, C.; Sannino, A.; Demitri, C. Biodegradable Poly(Lactic Acid)/Cellulose-Based Superabsorbent Hydrogel Composite Material as Water and Fertilizer Reservoir in Agricultural Applications. J. Appl. Polym. Sci. 2019, 136, 1–9. [Google Scholar] [CrossRef]
- Abobatta, W. Impact of Hydrogel Polymer in Agricultural Sector. Adv. Agric. Environ. Sci. Open Access 2018, 1, 59–64. [Google Scholar] [CrossRef]
- Suprabawati, A.; Aisyah, L.S.; Firzatullah, M.R. Crosslinked CMC-Urea Hydrogel Made from Natural Carboxymethyl Cellulose (CMC) as Slow-Release Fertilizer Coating. AIP Conf. Proc. 2020, 2243, 1–7. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and Characterization of Slow-Release Fertilizer Encapsulated by Starch-Based Superabsorbent Polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.A.C.; Jorge, M.H.A.; Bortolin, A.; Boiteux, L.S.; Ribeiro, C.; Marconcini, J.M. Growth of Tomato Seedlings in Substrates Containing a Nanocomposite Hydrogel with Calcium Montmorillonite (NC-MMT). Hortic. Bras. 2019, 37, 199–203. [Google Scholar] [CrossRef]
- Aydınoğlu, D.; Karaca, N.; Ceylan, Ö. Natural Carrageenan/Psyllium Composite Hydrogels Embedded Montmorillonite and Investigation of Their Use in Agricultural Water Management. J. Polym. Environ. 2020, 29, 785–798. [Google Scholar] [CrossRef]
- Vundavalli, R.; Vundavalli, S.; Nakka, M.; Rao, D.S. Biodegradable Nano-Hydrogels in Agricultural Farming—Alternative Source for Water Resources. Procedia Mater. Sci. 2015, 10, 548–554. [Google Scholar] [CrossRef]
- de Vasconcelos, M.C.; Gomes, R.F.; Sousa, A.A.L.; Moreira, F.J.C.; Rodrigues, F.H.A.; Fajardo, A.R.; Neto, L.G.P. Superabsorbent Hydrogel Composite Based on Starch/Rice Husk Ash as a Soil Conditioner in Melon (Cucumis Melo, L.) Seedling Culture. J. Polym. Environ. 2020, 28, 131–140. [Google Scholar] [CrossRef]
- Soil Survey Staff—NRCS/USDA Keys to Soil Taxonomy. Soil Conserv. Serv. 2014, 12, 1–372.
- Schoonover, J.E.; Crim, J.F. An Introduction to Soil Concepts and the Role of Soils in Watershed Management. J. Contemp. Water Res. Educ. 2015, 154, 21–47. [Google Scholar] [CrossRef]
- Onwuka, B.; Mang, B. Effects of Soil Temperature on Some Soil Properties and Plant Growth. Adv. Plants Agric. Res. 2018, 8, 34–37. [Google Scholar] [CrossRef]
- Hairiah, K.; Widianto, W.; Suprayogo, D.; Van Noordwijk, M. Tree Roots Anchoring and Binding Soil: Reducing Landslide Risk in Indonesian Agroforestry. Land 2020, 9, 256. [Google Scholar] [CrossRef]
- Neina, D. The Role of Soil PH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 9. [Google Scholar] [CrossRef]
- EI-Kazzaz, K.A.; EI-Kazzaz, A.A. Soilless Agriculture a New and Advanced Method for Agriculture Development: An Introduction. Agric. Res. Technol. Access J. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Sikder, A.; Pearce, A.K.; Parkinson, S.J.; Napier, R.; O’Reilly, R.K. Recent Trends in Advanced Polymer Materials in Agriculture Related Applications. ACS Appl. Polym. Mater. 2021, 3, 1203–1217. [Google Scholar] [CrossRef]
- Zhang, Z.; Rod, M.; Hosseinian, F. A Comprehensive Review on Sustainable Industrial Vertical Farming Using Film Farming Technology. Sustain. Agric. Res. 2020, 10, 46. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Habibzadeh, F.; Ataei, R.; Hemmati, P.; Ebrahimian, E. Zeolite and Hydrogel Improve Yield of Greenhouse Cucumber in Soil-Less Medium under Water Limitation. Rhizosphere 2018, 6, 7–10. [Google Scholar] [CrossRef]
- Salam, A.; Ahmed Dewan, M.; Farabi Rahmat, U. Design of an Agro-Textile Cultivation Bed for Spread Rooted Plants with Hydrogel through Soilless Vertical Farming. Text. Today 2019. Available online: https://www.researchgate.net/profile/Md-Abdus-Salam-3/publication/344466183_Design_of_an_Agro-tex-tile_Cultivation_Bed_for_Spread_Rooted_Plants_with_Hydrogel_through_Soilless_Vertical_Farming/links/5f79dc71a6fdcc008655a0ca/Design-of-an-Agro-textile-Cultivation-Bed-for-Spread-Rooted-Plants-with-Hydrogel-through-Soilless-Vertical-Farming.pdf (accessed on 1 June 2022).
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Kalossaka, L.M.; Sena, G.; Barter, L.M.C.; Myant, C. Review: 3D Printing Hydrogels for the Fabrication of Soilless Cultivation Substrates. Appl. Mater. Today 2021, 24, 1–16. [Google Scholar] [CrossRef]
- Roy, S.; Kumar, P.; Kumari, R. Organic Farming Vs Conventional Farming—Paradigm Shift of Environmental Sustainability. GRD J. Eng. 2021, 6, 19–24. [Google Scholar]
- Killebrew, K.; Wolff, H. Environmental Impacts of Agricultural Technologies. In Evans School Policy Analysis and Research (EPAR); University of Washington: Seattle, WA, USA, 2010; pp. 1–18. [Google Scholar]
- Agaba, H.; Orikiriza, L.J.B.; Obua, J.; Kabasa, J.D.; Worbes, M.; Hüttermann, A. Hydrogel Amendment to Sandy Soil Reduces Irrigation Frequency and Improves the Biomass of Agrostis Stolonifera. Agric. Sci. 2011, 02, 544–550. [Google Scholar] [CrossRef]
- Narjary, B.; Aggarwal, P.; Singh, A.; Chakraborty, D.; Singh, R. Water Availability in Different Soils in Relation to Hydrogel Application. Geoderma 2012, 187–188, 94–101. [Google Scholar] [CrossRef]
- Hendrawan, H.; Khoerunnisa, F.; Sonjaya, Y.; Putri, A.D. Poly (Vinyl Alcohol)/Glutaraldehyde/Premna Oblongifolia Merr Extract Hydrogel for Controlled-Release and Water Absorption Application. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 12048. [Google Scholar] [CrossRef]
- Rozo, G.; Bohorques, L.; Santamaría, J. Controlled Release Fertilizer Encapsulated by a $κ$-Carrageenan Hydrogel. Polimeros 2019, 29, 1–12. [Google Scholar] [CrossRef]
- Rusu, M.; Rosu, E.; Bruma, I.S.; Florian, V.; Chitea, M.A. Organic Farming Versus Conventional Farming: Case Study, Dornelor Basin, Suceava County, Romania. Sci. Pap. Manag. Econ. Eng. Agric. Rural Dev. 2021, 21, 687–692. [Google Scholar]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef]
- Bratovcic, A.; Hikal, W.M.; Said-Al Ahl, H.A.H.; Tkachenko, K.G.; Baeshen, R.S.; Sabra, A.S.; Sany, H. Nanopesticides and Nanofertilizers and Agricultural Development: Scopes, Advances and Applications. Open, J. Ecol. 2021, 11, 301–316. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.H. Current and Future Perspectives on the Use of Nanofertilizers for Sustainable Agriculture: The Case of Phosphorus Nanofertilizer. 3 Biotech 2021, 11, 2–21. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Chu, H.; Li, J. Preparation and Characterization of Slow-Release and Water-Retention Fertilizer Based on Starch and Halloysite. Int. J. Biol. Macromol. 2019, 133, 1210–1218. [Google Scholar] [CrossRef]
- Naz, M.Y.; Sulaiman, S.A. Slow Release Coating Remedy for Nitrogen Loss from Conventional Urea: A Review. J. Control. Release 2016, 225, 109–120. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; Ghobashy, M.M. Controlled Release Fertilizers Using Superabsorbent Hydrogel Prepared by Gamma Radiation. Radiochim. Acta 2017, 105, 865–876. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A Mini-Review on Chitosan-Based Hydrogels with Potential for Sustainable Agricultural Applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Noppakundilograt, S.; Pheatcharat, N.; Kiatkamjornwong, S. Multilayer-Coated NPK Compound Fertilizer Hydrogel with Controlled Nutrient Release and Water Absorbency. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Narayanan, A.; Dhamodharan, R. Super Water-Absorbing New Material from Chitosan, EDTA and Urea. Carbohydr. Polym. 2015, 134, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Reyhani Tabar, A. Slow-Release NPK Fertilizer Encapsulated by Carboxymethyl Cellulose-Based Nanocomposite with the Function of Water Retention in Soil. Mater. Sci. Eng. C 2018, 90, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Lu, P.; Zhang, M. Preparation and Properties of Hydrogel Based on Sawdust Cellulose for Environmentally Friendly Slow Release Fertilizers. Green Process. Synth. 2020, 9, 139–152. [Google Scholar] [CrossRef]
- Ramli, R.A.; Lian, Y.M.; Nor, N.M.; Azman, N.I.Z. Synthesis, Characterization, and Morphology Study of Coco Peat-Grafted-Poly(Acrylic Acid)/NPK Slow Release Fertilizer Hydrogel. J. Polym. Res. 2019, 26. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Water Retention and Slow Release Studies of a Salep-Based Hydrogel Nanocomposite Reinforced with Montmorillonite Clay. New J. Chem. 2018, 42, 2758–2766. [Google Scholar] [CrossRef]
- Karunarathna, M.H.J.S.; Bailey, K.M.; Ash, B.L.; Matson, P.G.; Wildschutte, H.; Davis, T.W.; Midden, W.R.; Ostrowski, A.D. Nutrient Capture from Aqueous Waste and Photocontrolled Fertilizer Delivery to Tomato Plants Using Fe(III)-Polysaccharide Hydrogels. ACS Omega 2020, 5, 23009–23020. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.T.; Jiang, Z. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Park, M.; Lee, D.; Hyun, J. Nanocellulose-Alginate Hydrogel for Cell Encapsulation. Carbohydr. Polym. 2014, 116, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Agarwal, S.; Jain, A.; Khan, S. 3-Dimensional Cross Linked Hydrophilic Polymeric Network “Hydrogels”: An Agriculture Boom. Agric. Water Manag. 2021, 253. [Google Scholar] [CrossRef]
- Al-Mubaddel, F.S.; Aijaz, M.O.; Haider, S.; Haider, A.; Almasry, W.A.; Al-Fatesh, A.S. Synthesis of Chitosan Based Semi-IPN Hydrogels Using Epichlorohydrine as Crosslinker to Study the Adsorption Kinetics of Rhodamine, B. Desalin. Water Treat. 2015, 57, 17523–17536. [Google Scholar] [CrossRef]
- Van Tran, V.; Park, D.; Lee, Y.C. Hydrogel Applications for Adsorption of Contaminants in Water and Wastewater Treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.K.; Zhu, J.; Lim, J.Y.; Gao, Z.; Loh, C.S.; Li, J.; Ong, C.N. Use of Okara-Derived Hydrogel for Enhancing Growth of Plants by Minimizing Leaching and Locking Nutrients and Water in Growing Substrate. Ecol. Eng. 2020, 159, 106122. [Google Scholar] [CrossRef]
- Zhu, J.; Song, X.; Tan, W.K.; Wen, Y.; Gao, Z.; Ong, C.N.; Loh, C.S.; Swarup, S.; Li, J. Chemical Modification of Biomass Okara Using Poly(Acrylic Acid) through Free Radical Graft Polymerization. J. Agric. Food Chem. 2020, 68, 13241–13246. [Google Scholar] [CrossRef]
- Qayumova, L.S.; Bekmurodov, U.B.; Burieva, S.T.; Pulatov, G.E. Modern Technology of Irrigation of Garden Trees in Agriculture. Intern. J. Integr. Educ. 2021, 4, 223–224. [Google Scholar]
- Li, S.; Chen, G. Agricultural Waste-Derived Superabsorbent Hydrogels: Preparation, Performance, and Socioeconomic Impacts. J. Clean. Prod. 2019, 251. [Google Scholar] [CrossRef]
- Song, B.; Liang, H.; Sun, R.; Peng, P.; Jiang, Y.; She, D. Hydrogel Synthesis Based on Lignin/Sodium Alginate and Application in Agriculture. Int. J. Biol. Macromol. 2020, 144, 219–230. [Google Scholar] [CrossRef]
- Gan, S.; Zakaria, S.; Chia, C.H.; Chen, R.S.; Ellis, A.V.; Kaco, H. Highly Porous Regenerated Cellulose Hydrogel and Aerogel Prepared from Hydrothermal Synthesized Cellulose Carbamate. PLoS ONE 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Ma, J.; Yang, M.; Yu, F.; Zheng, J. Water-Enhanced Removal of Ciprofloxacin from Water by Porous Graphene Hydrogel. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.; Ambrose, R.P.K. Starch-Based Biodegradable Hydrogel as Seed Coating for Corn to Improve Early Growth under Water Shortage. J. Appl. Polym. Sci. 2020, 137, 1–12. [Google Scholar] [CrossRef]
- Rydlová, J.; Püschel, D. Arbuscular Mycorrhiza, but Not Hydrogel, Alleviates Drought Stress of Ornamental Plants in Peat-Based Substrate. Appl. Soil Ecol. 2019, 146, 103394. [Google Scholar] [CrossRef]
- Callaghan, S.E.; Williams, A.P.; Burgess, T.; White, D.; Keovorlajak, T.; Phitsanoukane, P.; Phantavong, S.; Vilavong, S.; Ireland, K.B.; Duckitt, G.S.; et al. First Report of Phytophthora Capsici in the Lao PDR. A. Australas. Plant Dis. Notes 2016, 11, 22. [Google Scholar] [CrossRef][Green Version]
- Versluys, M.; Tarkowski, Ł.P.; Ende, W. Van Den Fructans As DAMPs or MAMPs: Evolutionary Prospects, Cross-Tolerance, and Multistress Resistance Potential. Front. Plant Sci. 2017, 7, 2061. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhao, Y.; Lu, Z.; Xing, R.; Yao, X.; Jin, Z.; Wang, Y.; Yu, F. Citral-Loaded Chitosan/Carboxymethyl Cellulose Copolymer Hydrogel Microspheres with Improved Antimicrobial Effects for Plant Protection. Int. J. Biol. Macromol. 2020, 164, 986–993. [Google Scholar] [CrossRef]
- Adukwu, E.C.; Bowles, M.; Edwards-Jones, V.; Bone, H. Antimicrobial Activity, Cytotoxicity and Chemical Analysis of Lemongrass Essential Oil (Cymbopogon flexuosus) and Pure Citral. Appl. Microbiol. Biotechnol. 2016, 100, 9619–9627. [Google Scholar] [CrossRef]
- Mori, Y. New Agro-Technology (Imec) by Hydrogel Membrane. React. Funct. Polym. 2013, 73, 936–938. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Gels Horizons: From Science to Smart Materials. In Polymer Gels Science and Fundamentals; Springer: Heidelberg, Germany, 2018; ISBN 9789811060854. [Google Scholar]
- Li, Y.; Blank, K.G.; Sun, F.; Cao, Y. Editorial: Synthesis of Novel Hydrogels with Unique Mechanical Properties. Front. Chem. 2020, 8, 8–10. [Google Scholar] [CrossRef]
| Parameters | Hydroponic System | |||||
|---|---|---|---|---|---|---|
| Media Soilless System | Nutrient Solution System | Aeroponics | Aquaponics | |||
| Open | Closed | Open | Closed | |||
| % Irrigation water saving | 80 | 85 | 85 | 90 | 95 | %85–80 |
| % Fertilizer saving | 55 | 80 | 68 | 85 | 85 | %99–85 |
| % Productivity increase | 100 | 150 | 200 | 250 | 300 | %150–100 |
| % Water productivity | 1000 | 1600 | 2000 | 3500 | 8000 | 1000–1600 |
| Type of Fertiliser | Composition | Crosslinker | Technique | Rate of Release | Reference |
|---|---|---|---|---|---|
| Slow-Release | Sulfonated-carboxymethyl cellulose/ polyvinylpyrrolidone/silica/NPK | N,N′-methylene bisacrylamide (MBA) | In-situ graft polymerisation | Excellent slow-release properties | [98] |
| Sawdust cellulose- polyacrylic acid monomer (PAA)/ polyacrylamide(PAM)/Urea | N,N′-methylenebis acrylamide (MBA) | Graft Copolymerization | 210 g/g was the best, suitable | [99] | |
| Cocopeat/Poly Acrylic Acid/NPK | N,N′-methylene bisacrylamide (MBA) | In-situ graft polymerization | Slow release property is better than the commercial super absorbance polymer (CSAP) | [100] | |
| Controlled Release | Starch-g-(acrylic acid-co-methyl methacrylate), carbendazim-loaded hydrogels (CLHs) | N,N′-methylene bisacrylamide (MBA) | Polymerization | Good performance | [13] |
| Polyvinylpyrrolidone/ carboxylmethyl cellulose | - | Gamma radiation | Treatment of slow-release fertiliser shows better performance than the untreated soil | [94] | |
| κ-carrageenan-based hydrogel | Glycerol | Chemical Crosslinking | It may have potentials as a control released fertiliser | [87] |
| Plant | Factor | Number of Flowers | Diameter of Largest Flower | Shoot Dry Weight | Root Dry Weight | Plant Height/Total Length of Branches (P. peltatum) | Number of Branches | Total Leaf Area | Shoot P Concentration | Mycorrhizal Colonization |
|---|---|---|---|---|---|---|---|---|---|---|
| Gazania rigens | (1) watering | 20.2 *** ↘ | ns | 5.5 ** ↘ | ns | 4.5 * ↘ | — | 8.2 *** ↘ | 5.6 ** ↘ | 18.9 *** ↗ |
| (2) AMF | 31.1 *** ↗ | 86.5 *** ↗ | 20.0 *** ↗ | ns | 76.5 *** ↗ | — | 40.5 *** ↗ | 13.8 *** ↗ | — | |
| (3) gel | ns | 4.9 * ↘ | ns | ns | ns | — | ns | ns | ns | |
| 1 × 2 | ns | 3.4 * | 6.7 ** | ns | 14.2 *** | — | 5.5 ** | ns | — | |
| 1 × 3 | ns | ns | ns | ns | ns | — | ns | ns | ns | |
| 2 × 3 | ns | ns | ns | ns | ns | — | ns | 5.7 * | — | |
| 1 × 2 × 3 | ns | ns | ns | ns | ns | — | ns | ns | — | |
| Pelargonium peltatum | (1) watering | 6.6 ** ↗ | — | ns | ns | 4.6 * | ns | 7.0 ** ↗ | 3.8 * ↘ | 42.5 *** ↗ |
| (2) AMF | ns | — | 6.2 * ↗ | ns | 11.4 ** ↗ | ns | 25.0 *** ↗ | 14.5 *** ↗ | — | |
| (3) gel | 6.7 * ↘ | — | ns | ns | ns | ns | ns | ns | 10.4 ** ↘ | |
| 1 × 2 | 3.3 * | — | ns | ns | ns | ns | 3.9 * | ns | — | |
| 1 × 3 | ns | — | ns | ns | ns | ns | ns | ns | ns | |
| 2 × 3 | ns | — | ns | ns | 6.8 * | ns | 14.2 *** | ns | — | |
| 1 × 2 × 3 | ns | — | ns | ns | ns | ns | ns | ns | — | |
| Pelargonium zonale | (1) watering | ns | — | 11.5 *** ↘ | 24.4 *** ↘ | 13.4 *** | ns | 26.8 *** | 4.4 * ↗ | 12.2 *** ↗ |
| (2) AMF | 148.5 *** ↗ | — | 169.9 *** ↗ | 190.4 *** ↗ | 378.0 *** ↗ | 62.4 *** ↗ | 442.5 *** ↗ | 52.5 *** ↗ | — | |
| (3) gel | 7.3 ** ↘ | — | ns | ns | ns | ns | ns | ns | ns | |
| 1 × 2 | ns | — | ns | 7.5 *** | 9.3 *** | ns | 12.1 *** | ns | — | |
| 1 × 3 | ns | — | ns | ns | ns | ns | 5.6 ** | 3.6 * | 8.7 *** | |
| 2 × 3 | ns | — | ns | ns | 6.5 * | ns | ns | 7.9 ** | — | |
| 1 × 2 × 3 | ns | — | ns | 4.4 * | ns | ns | ns | ns | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palanivelu, S.D.; Armir, N.A.Z.; Zulkifli, A.; Hair, A.H.A.; Salleh, K.M.; Lindsey, K.; Che-Othman, M.H.; Zakaria, S. Hydrogel Application in Urban Farming: Potentials and Limitations—A Review. Polymers 2022, 14, 2590. https://doi.org/10.3390/polym14132590
Palanivelu SD, Armir NAZ, Zulkifli A, Hair AHA, Salleh KM, Lindsey K, Che-Othman MH, Zakaria S. Hydrogel Application in Urban Farming: Potentials and Limitations—A Review. Polymers. 2022; 14(13):2590. https://doi.org/10.3390/polym14132590
Chicago/Turabian StylePalanivelu, Swarna Devi, Nur Amira Zainul Armir, Amalia Zulkifli, Ainul Hafiza Abdul Hair, Kushairi Mohd Salleh, Keith Lindsey, Muhamad Hafiz Che-Othman, and Sarani Zakaria. 2022. "Hydrogel Application in Urban Farming: Potentials and Limitations—A Review" Polymers 14, no. 13: 2590. https://doi.org/10.3390/polym14132590
APA StylePalanivelu, S. D., Armir, N. A. Z., Zulkifli, A., Hair, A. H. A., Salleh, K. M., Lindsey, K., Che-Othman, M. H., & Zakaria, S. (2022). Hydrogel Application in Urban Farming: Potentials and Limitations—A Review. Polymers, 14(13), 2590. https://doi.org/10.3390/polym14132590