Crosslinked Polyethylene (XLPE) Recycling via Foams
Abstract
1. Introduction
2. Materials and Methods
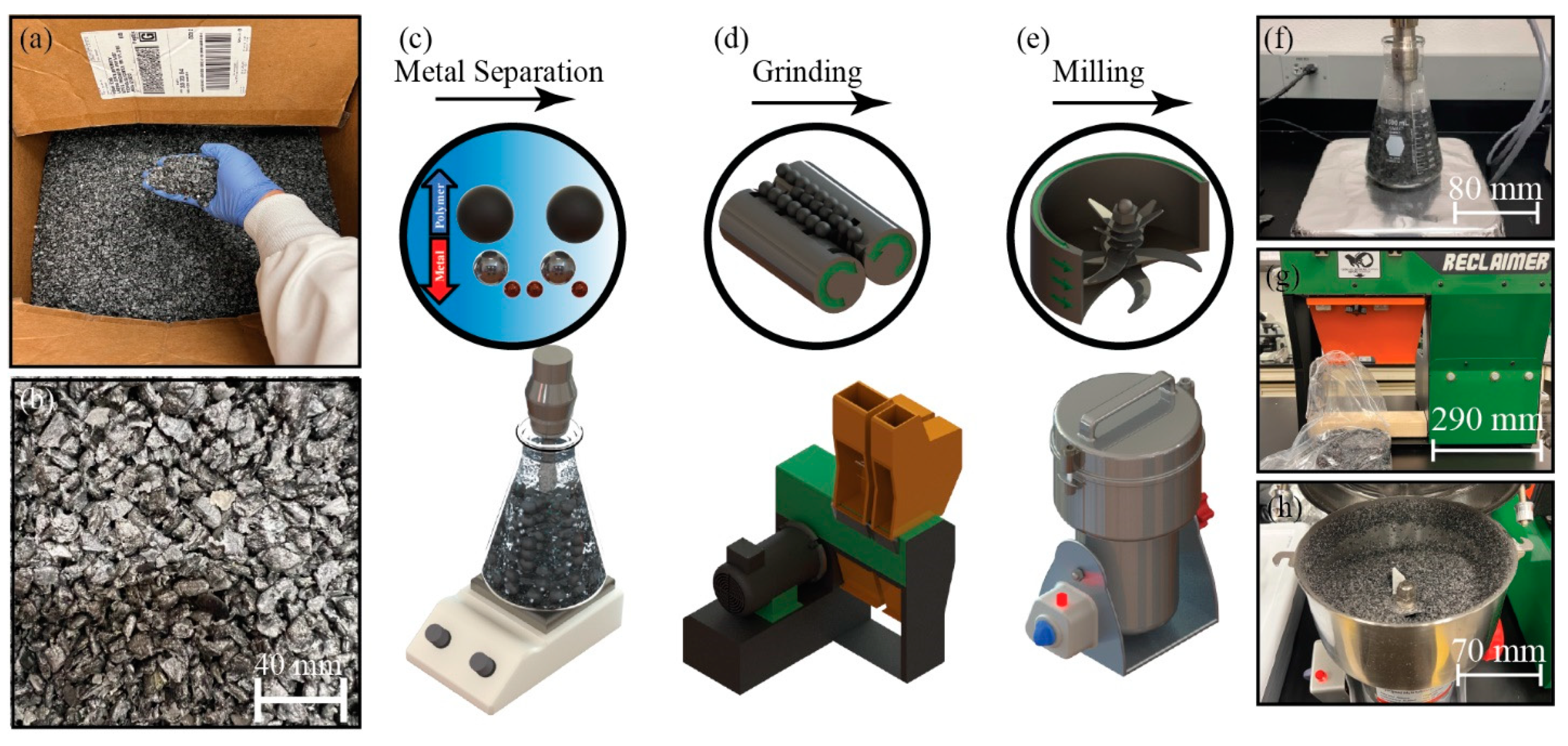
2.1. Materials and Foam Processing
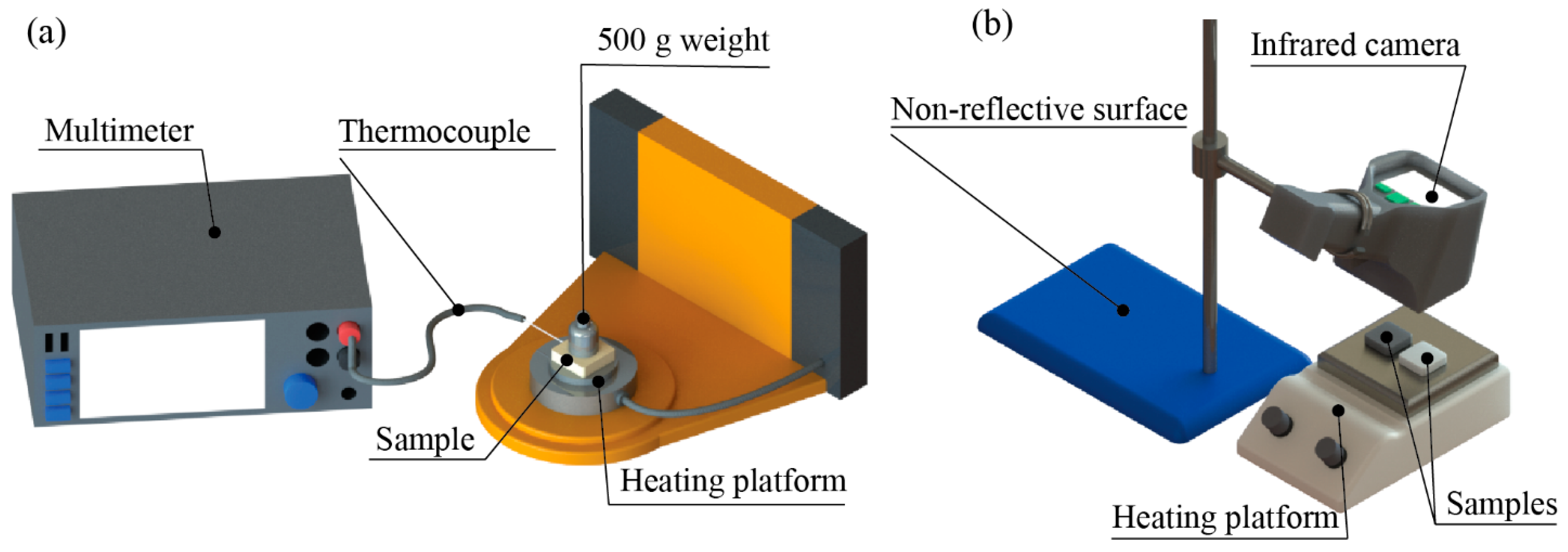
2.2. Characterization Methods
3. Results and Discussion
3.1. XLPE Particulate Fining
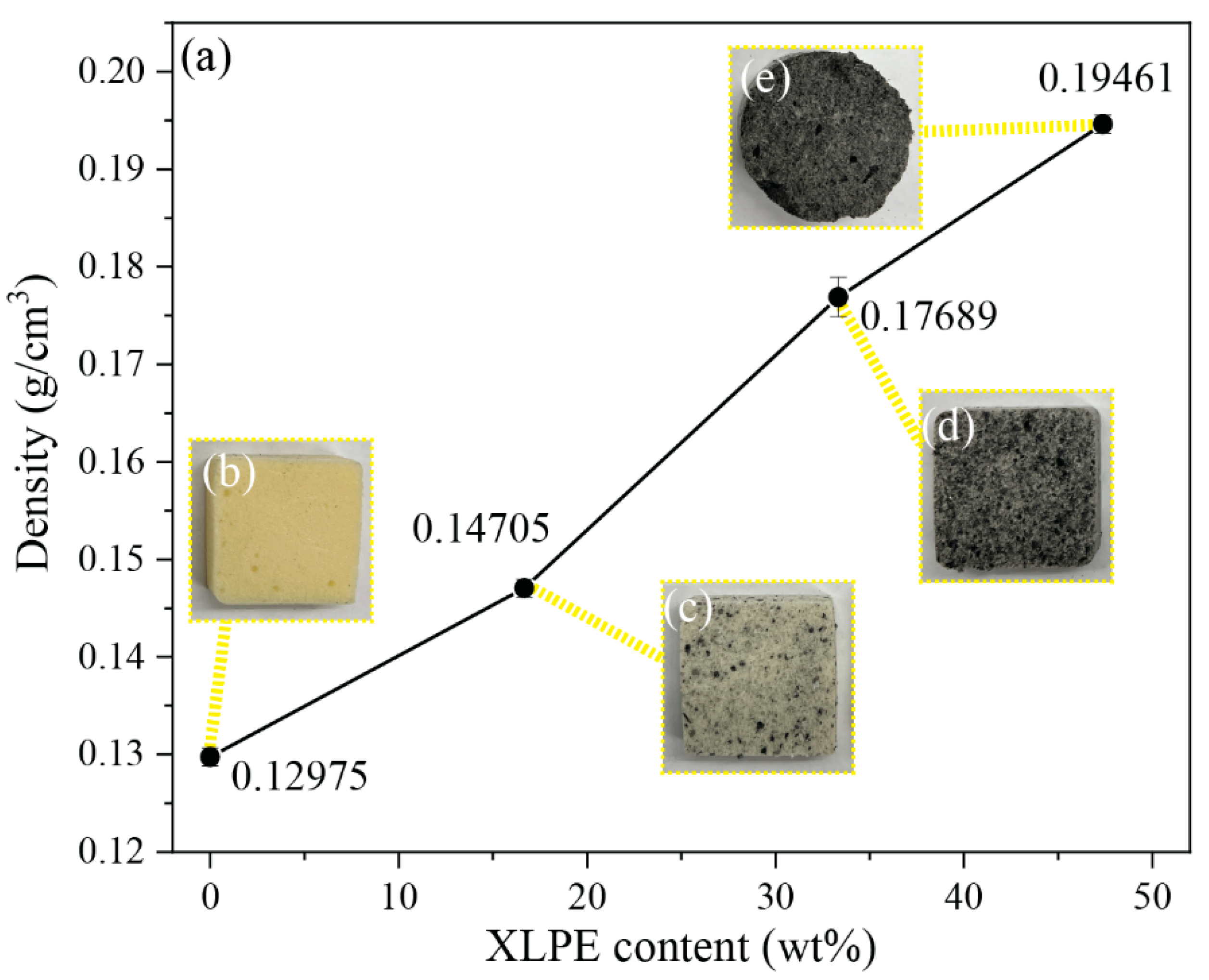
3.2. Foam Density
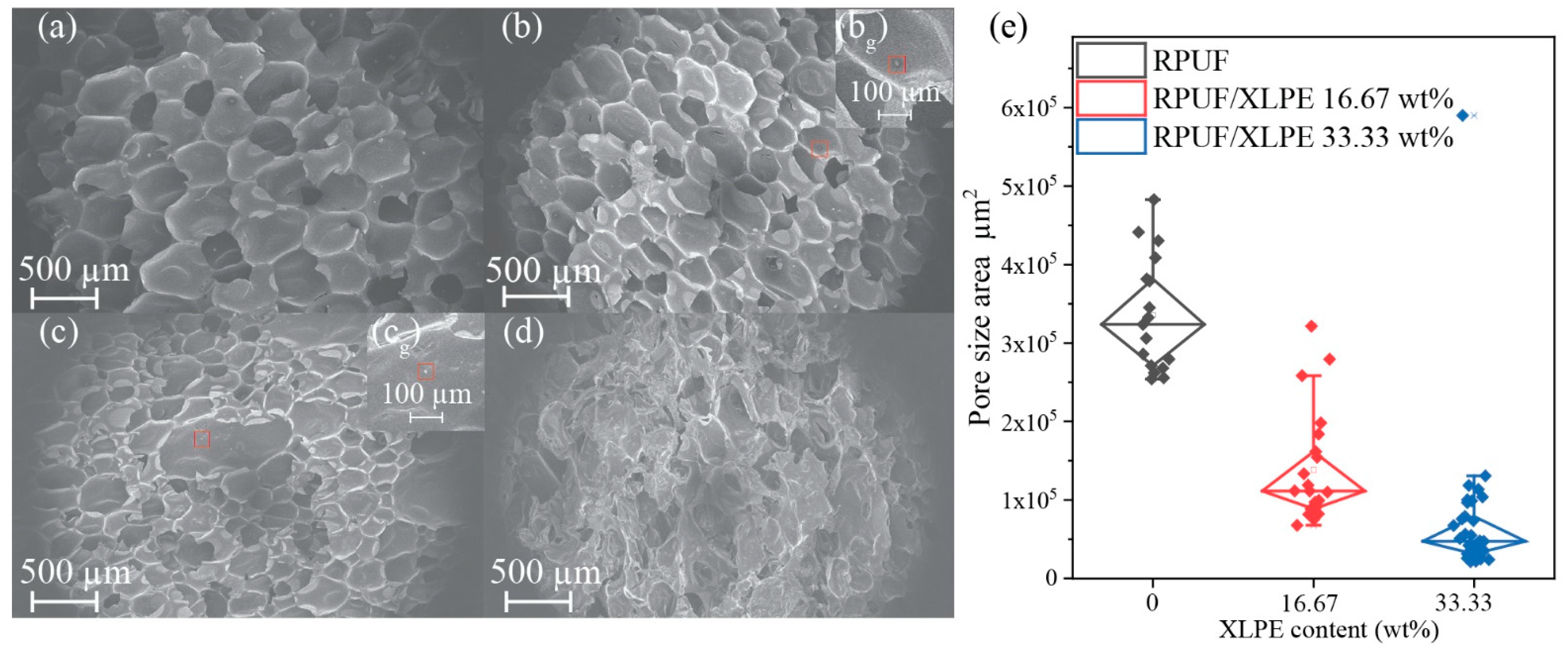
3.3. Foam Morphology
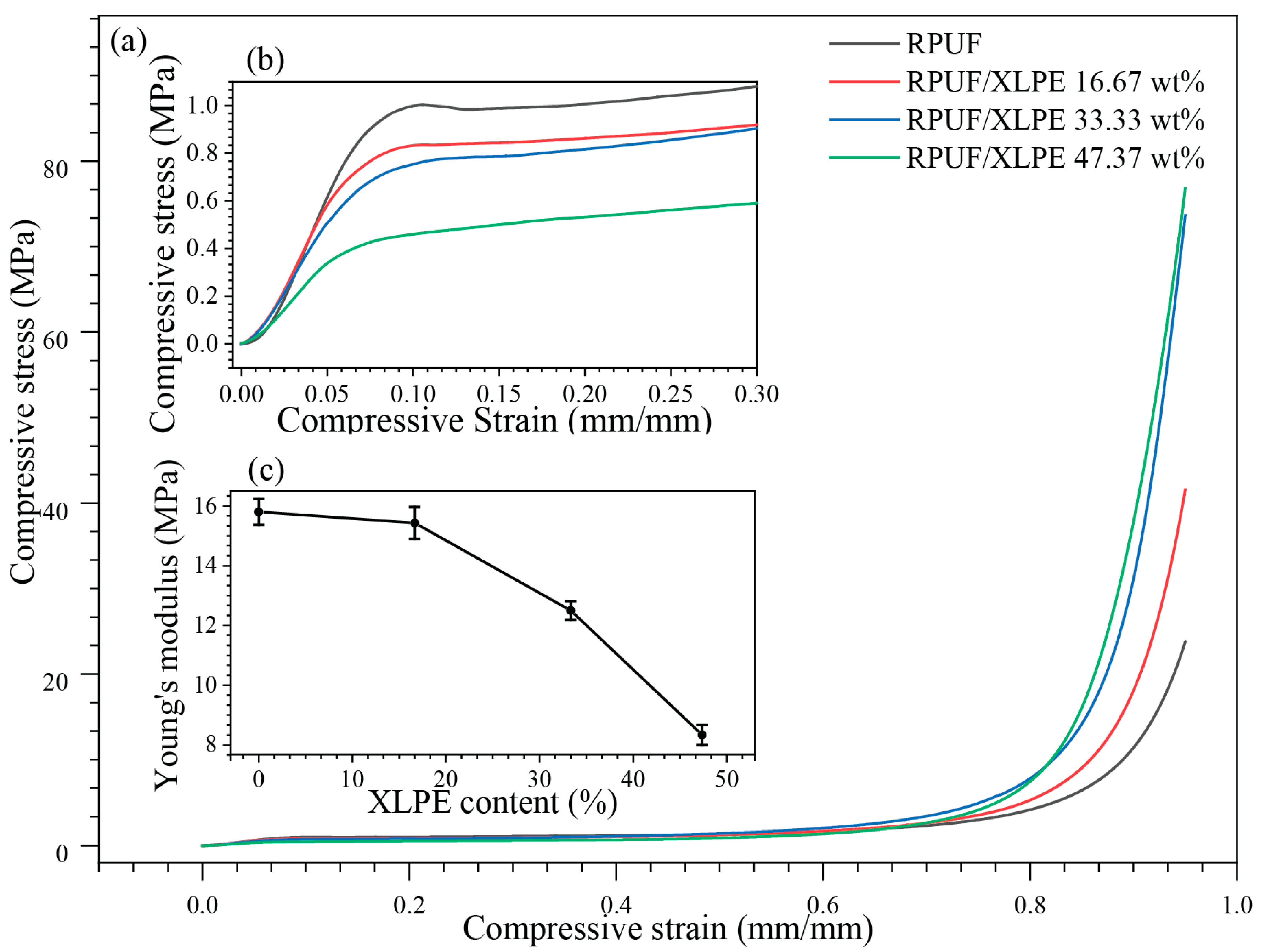
3.4. Mechanical Properties
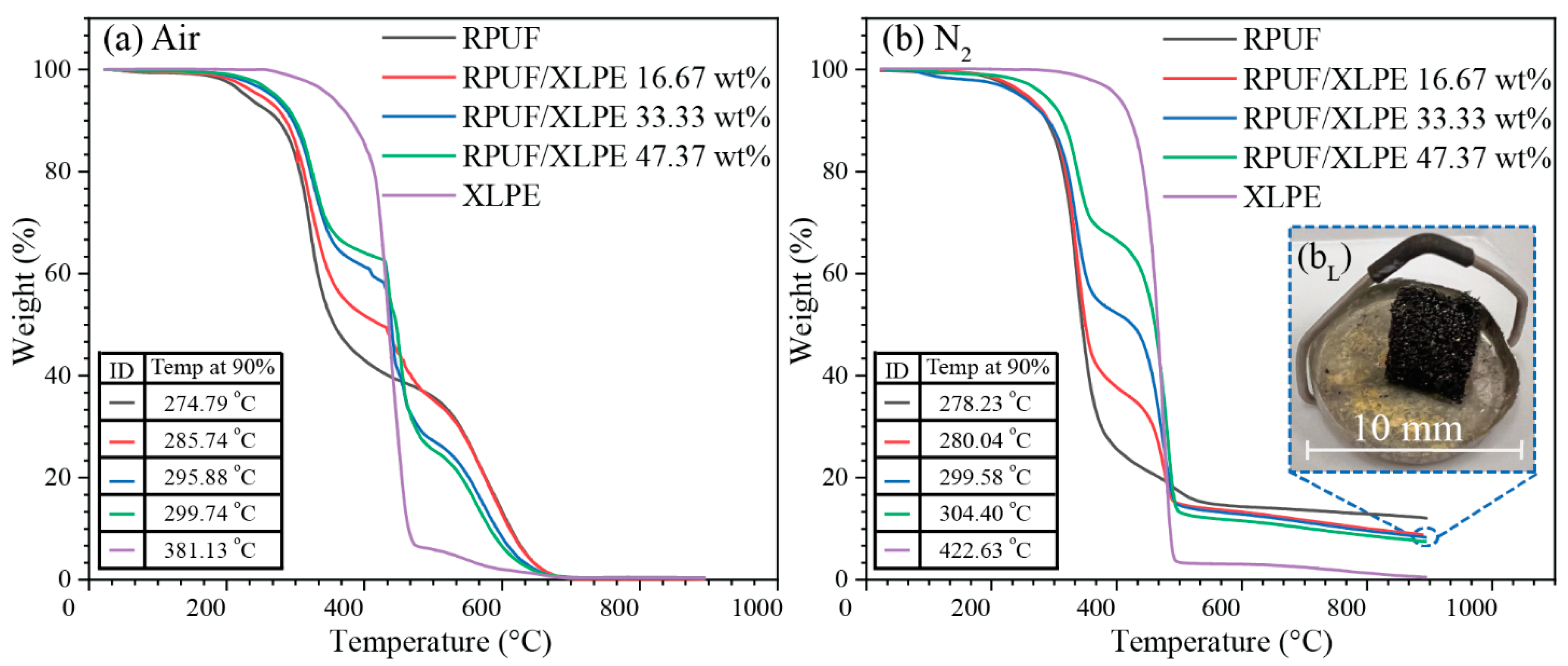
3.5. Degradation Points
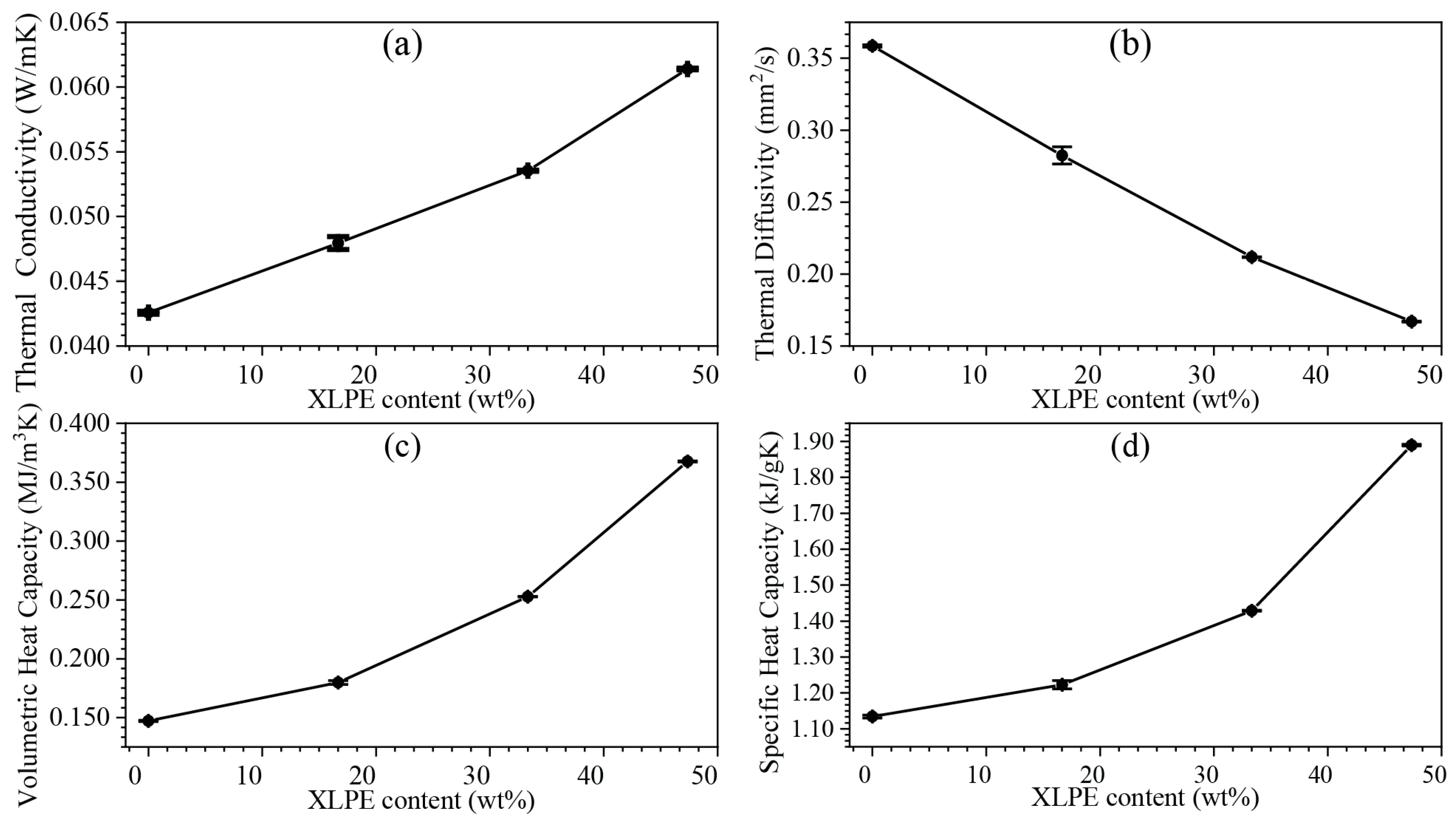
3.6. Thermal Conductivity and Diffusivity Properties
3.7. Thermal Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benavides, P.T.; Lee, U.; Zarè-Mehrjerdi, O. Life Cycle Greenhouse Gas Emissions and Energy Use of Polylactic Acid, Bio-Derived Polyethylene, and Fossil-Derived Polyethylene. J. Clean. Prod. 2020, 277, 124010. [Google Scholar] [CrossRef]
- Tamboli, S.M.; Mhaske, S.T.; Kale, D.D. Crosslinked Polyethylene. Indian J. Chem. Technol. 2004, 11, 853–864. [Google Scholar] [CrossRef]
- Formela, K.; Wołosiak, M.; Klein, M.; Wang, S. Characterization of Volatile Compounds, Structural, Thermal and Physico-Mechanical Properties of Cross-Linked Polyethylene Foams Degraded Thermo-Mechanically at Variable Times. Polym. Degrad. Stab. 2016, 134, 383–393. [Google Scholar] [CrossRef]
- Zéhil, G.P.; Assaad, J.J. Feasibility of Concrete Mixtures Containing Cross-Linked Polyethylene Waste Materials. Constr. Build. Mater. 2019, 226, 1–10. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Park, W.Y.; Hong, S.M.; Koo, C.M. Foaming of Recycled Crosslinked Polyethylenes via Supercritical Decrosslinking Reaction. J. Appl. Polym. Sci. 2012, 126, E21–E27. [Google Scholar] [CrossRef]
- Baek, B.K.; La, Y.H.; Lee, A.S.; Han, H.; Kim, S.H.; Hong, S.M.; Koo, C.M. Decrosslinking Reaction Kinetics of Silane-Crosslinked Polyethylene in Sub- and Supercritical Fluids. Polym. Degrad. Stab. 2016, 130, 103–108. [Google Scholar] [CrossRef]
- Al-Shammari, A. Post-Optimality Analysis of Production Levels in a Petrochemical Complex. Arab. J. Sci. Eng. 2015, 41, 2429–2438. [Google Scholar] [CrossRef]
- Stevenson, P. Foam Engineering Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2012; ISBN 9781119961093. [Google Scholar]
- Jin, F.-L.; Zhao, M.; Park, M.; Park, S.-J. Recent Trends of Foaming in Polymer Processing: A Review. Polymers 2019, 11, 953. [Google Scholar] [CrossRef]
- Nazeran, N.; Moghaddas, J. Synthesis and Characterization of Silica Aerogel Reinforced Rigid Polyurethane Foam for Thermal Insulation Application. J. Non-Cryst. Solids 2017, 461, 1–11. [Google Scholar] [CrossRef]
- Huang, L.; Ehsan, S.; Haghighi, B. Feasibility Study of Using Silica Aerogel as Insulation for Buildings; School Of Industrial Engineering And Management: Stockholm, Sweden, 2012; pp. 14–15, urn:nbn:se:kth:diva-96944. [Google Scholar]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology Limited: Shropshire, UK, 2005; Volume 26, ISBN 9781859575017. [Google Scholar]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef]
- Wang, S.K.; Sung, C.S.P. Fluorescence and IR Characterization of Cure in Polyurea, Polyurethane, and Polyurethane−Urea. Macromolecules 2002, 35, 883–888. [Google Scholar] [CrossRef]
- SAFETY DATA SHEET SDS No. 482A Foam-IT! 5 Rigid Polyurethane Foam. Available online: https://www.smooth-on.com/products/foam-it-5/ (accessed on 14 December 2021).
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.J.; Sanny, J.; Moebs, W. University Physics; Rice University: Houston, TX, USA, 2018; Volume 1, ISBN 978-1-947172-15-9. [Google Scholar]
- Dai, R.; Chandrasekaran, G.; Chen, J.; Jackson, C.; Liu, Y.; Nian, Q.; Kwon, B. Thermal Conductivity of Metal Coated Polymer Foam: Integrated Experimental and Modeling Study. Int. J. Therm. Sci. 2021, 169, 107045. [Google Scholar] [CrossRef]
- Theodore, L.B. Fundamentals of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Fourier, J. The Analytical Theory of Heat, Translated with Notes of A. FREE-MAN; Dover Publications Inc.: New York, NY, USA, 1955. [Google Scholar]
- Sauceau, M.; Fages, J.; Common, A.; Nikitine, C.; Rodier, E. New Challenges in Polymer Foaming: A Review of Extrusion Processes Assisted by Supercritical Carbon Dioxide. Prog. Polym. Sci. 2011, 36, 749–766. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Z.; Yang, H.; Wang, X.; Li, Q.; Turng, L.S. Microcellular Injection Molding of Polymers: A Review of Process Know-How, Emerging Technologies, and Future Directions. Curr. Opin. Chem. Eng. 2021, 33, 100694. [Google Scholar] [CrossRef]
- Maksimkin, A.V.; Senatov, F.S.; Anisimova, N.Y.; Kiselevskiy, M.V.; Zalepugin, D.Y.; Chernyshova, I.V.; Tilkunova, N.A.; Kaloshkin, S.D. Multilayer Porous UHMWPE Scaffolds for Bone Defects Replacement. Mater. Sci. Eng. C 2017, 73, 366–372. [Google Scholar] [CrossRef]
- Mucsi, G. A Review on Mechanical Activation and Mechanical Alloying in Stirred Media Mill. Chem. Eng. Res. Des. 2019, 148, 460–474. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.; Jiang, Y.; Chen, M.; Wan, C. Density Effect on Flame Retardancy, Thermal Degradation, and Combustibility of Rigid Polyurethane Foam Modified by Expandable Graphite or Ammonium Polyphosphate. Polymers 2019, 11, 668. [Google Scholar] [CrossRef]
- Nikolajevic, S.V. The Influence of the Water on Water Absorption and Density of XLPE Cable Insulation. IEEE Trans. Power Deliv. 1998, 13, 297–303. [Google Scholar] [CrossRef]
- Fan, D.; Li, M.; Qiu, J.; Xing, H.; Jiang, Z.; Tang, T. Novel Method for Preparing Auxetic Foam from Closed-Cell Polymer Foam Based on the Steam Penetration and Condensation Process. ACS Appl. Mater. Interfaces 2018, 10, 22669–22677. [Google Scholar] [CrossRef]
- Wu, G.; Xie, P.; Yang, H.; Dang, K.; Xu, Y.; Sain, M.; Turng, L.S.; Yang, W. A Review of Thermoplastic Polymer Foams for Functional Applications. Journal of Materials Science 2021, 56, 11579–11604. [Google Scholar] [CrossRef]
- Tsekmes, I.A.; Kochetov, R.; Morshuis, P.H.F.; Smit, J.J. Thermal Conductivity of Polymeric Composites: A Review. In Proceedings of the IEEE International Conference on Solid Dielectrics, ICSD, Bologna, Italy, 30 June–4 July 2013; pp. 678–681. [Google Scholar] [CrossRef]
- Wang, X.; Ho, V.; Segalman, R.A.; Cahill, D.G. Thermal Conductivity of High-Modulus Polymer Fibers. Macromolecules 2013, 46, 4937–4943. [Google Scholar] [CrossRef]
- Smaïli, A.; Masson, C.; Taleb, S.R.; Lamarche, L. Numerical Study of Thermal Behavior of a Wind Turbine Nacelle Operating in a Nordic Climate. Numer. Heat Transf. Part B Fundam. 2007, 50, 121–141. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, W.; Shi, H.; Li, B. Optimising the Design of Confined Laying Hen House Insulation Requirements in Cold Climates without Using Supplementary Heat. Biosyst. Eng. 2018, 174, 282–294. [Google Scholar] [CrossRef]
- Smaili, A.; Tahi, A.; Masson, C. Thermal Analysis of Wind Turbine Nacelle Operating in Algerian Saharan Climate. Energy Procedia 2012, 18, 187–196. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bawareth, M.; Xu, W.; Ravichandran, D.; Zhu, Y.; Jambhulkar, S.; Fonseca, N.; Miquelard-Garnier, G.; Camille, V.; Matthew, L.; Campbell, W.; et al. Crosslinked Polyethylene (XLPE) Recycling via Foams. Polymers 2022, 14, 2589. https://doi.org/10.3390/polym14132589
Bawareth M, Xu W, Ravichandran D, Zhu Y, Jambhulkar S, Fonseca N, Miquelard-Garnier G, Camille V, Matthew L, Campbell W, et al. Crosslinked Polyethylene (XLPE) Recycling via Foams. Polymers. 2022; 14(13):2589. https://doi.org/10.3390/polym14132589
Chicago/Turabian StyleBawareth, Mohammed, Weiheng Xu, Dharneedar Ravichandran, Yuxiang Zhu, Sayli Jambhulkar, Nathan Fonseca, Guillaume Miquelard-Garnier, Visnansky Camille, Lovelady Matthew, William Campbell, and et al. 2022. "Crosslinked Polyethylene (XLPE) Recycling via Foams" Polymers 14, no. 13: 2589. https://doi.org/10.3390/polym14132589
APA StyleBawareth, M., Xu, W., Ravichandran, D., Zhu, Y., Jambhulkar, S., Fonseca, N., Miquelard-Garnier, G., Camille, V., Matthew, L., Campbell, W., & Song, K. (2022). Crosslinked Polyethylene (XLPE) Recycling via Foams. Polymers, 14(13), 2589. https://doi.org/10.3390/polym14132589






