Exploration of the Crystal Structure and Thermal and Spectroscopic Properties of Monoclinic Praseodymium Sulfate Pr2(SO4)3
Abstract
:1. Introduction
2. Methods and Materials
3. Results and Discussion
3.1. Structural Properties
3.2. Electronic Properties
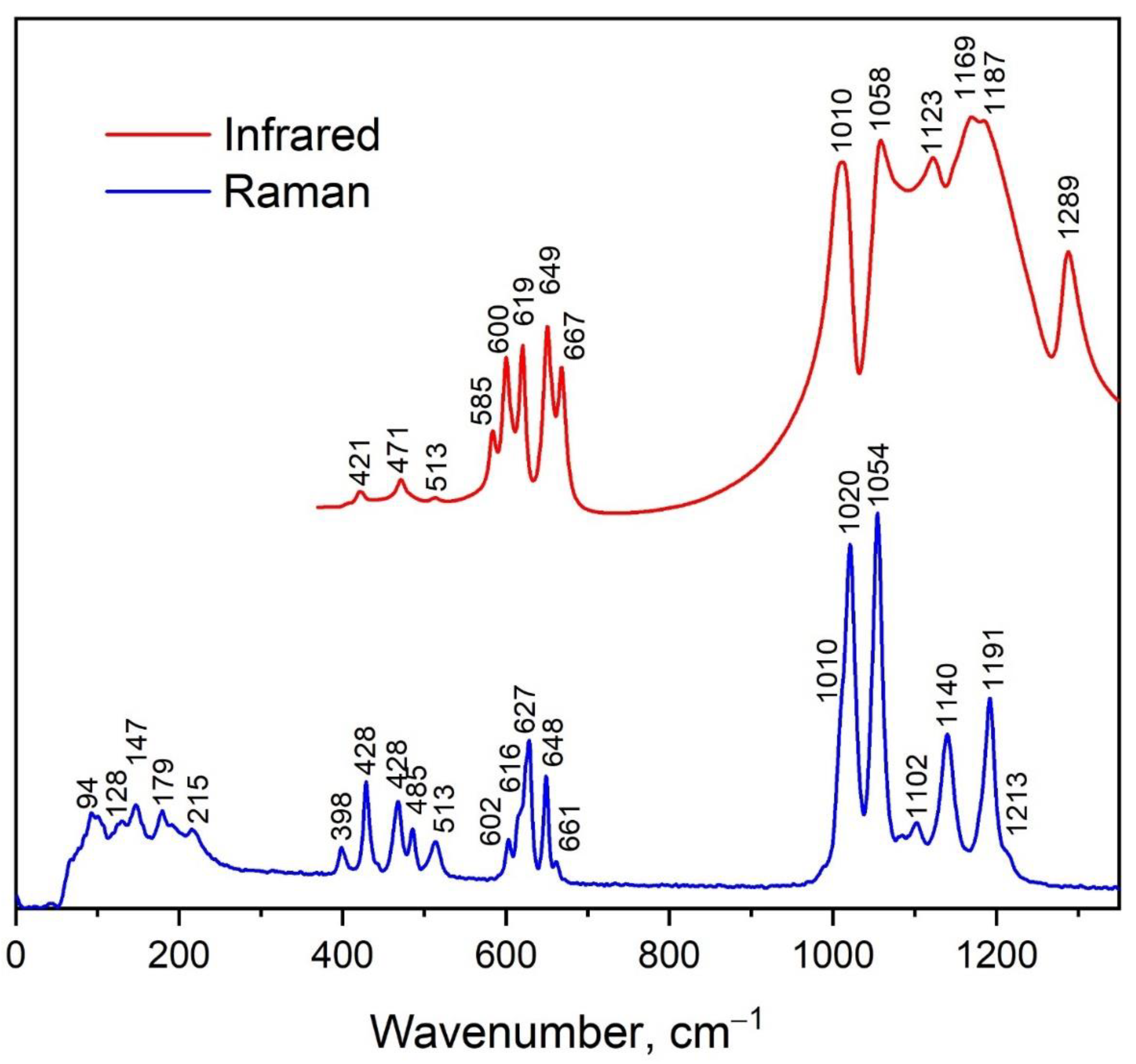
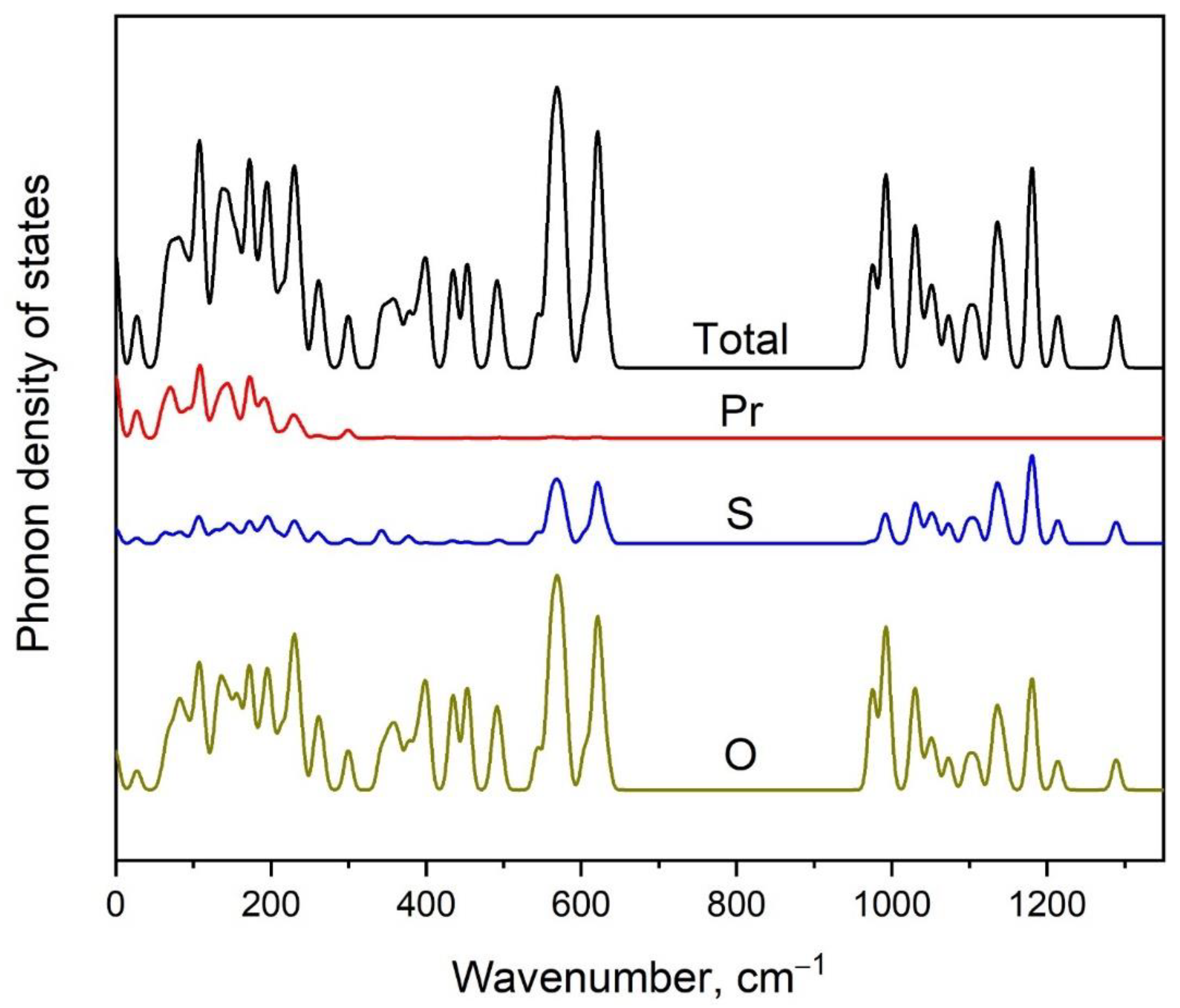
3.3. Vibrational Properties
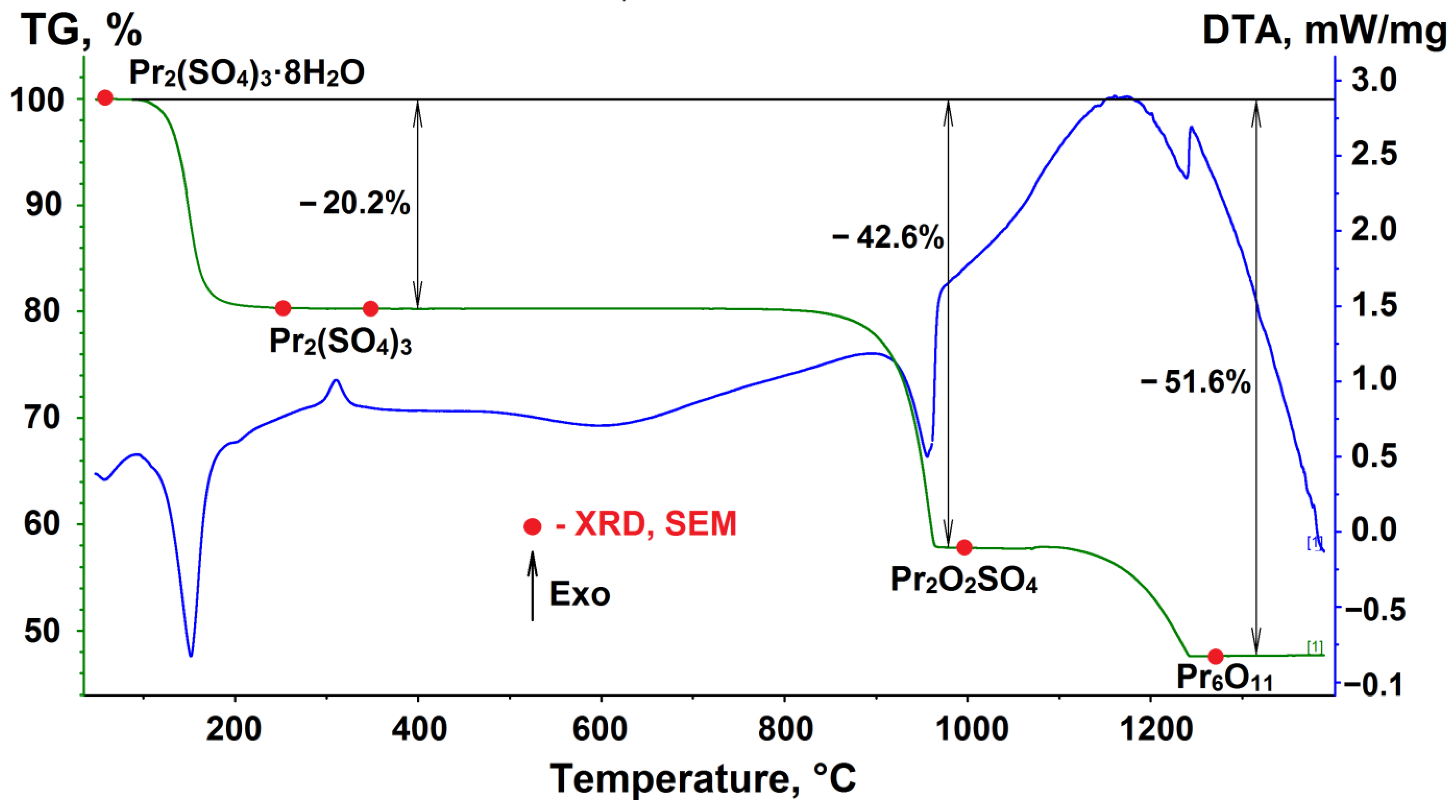
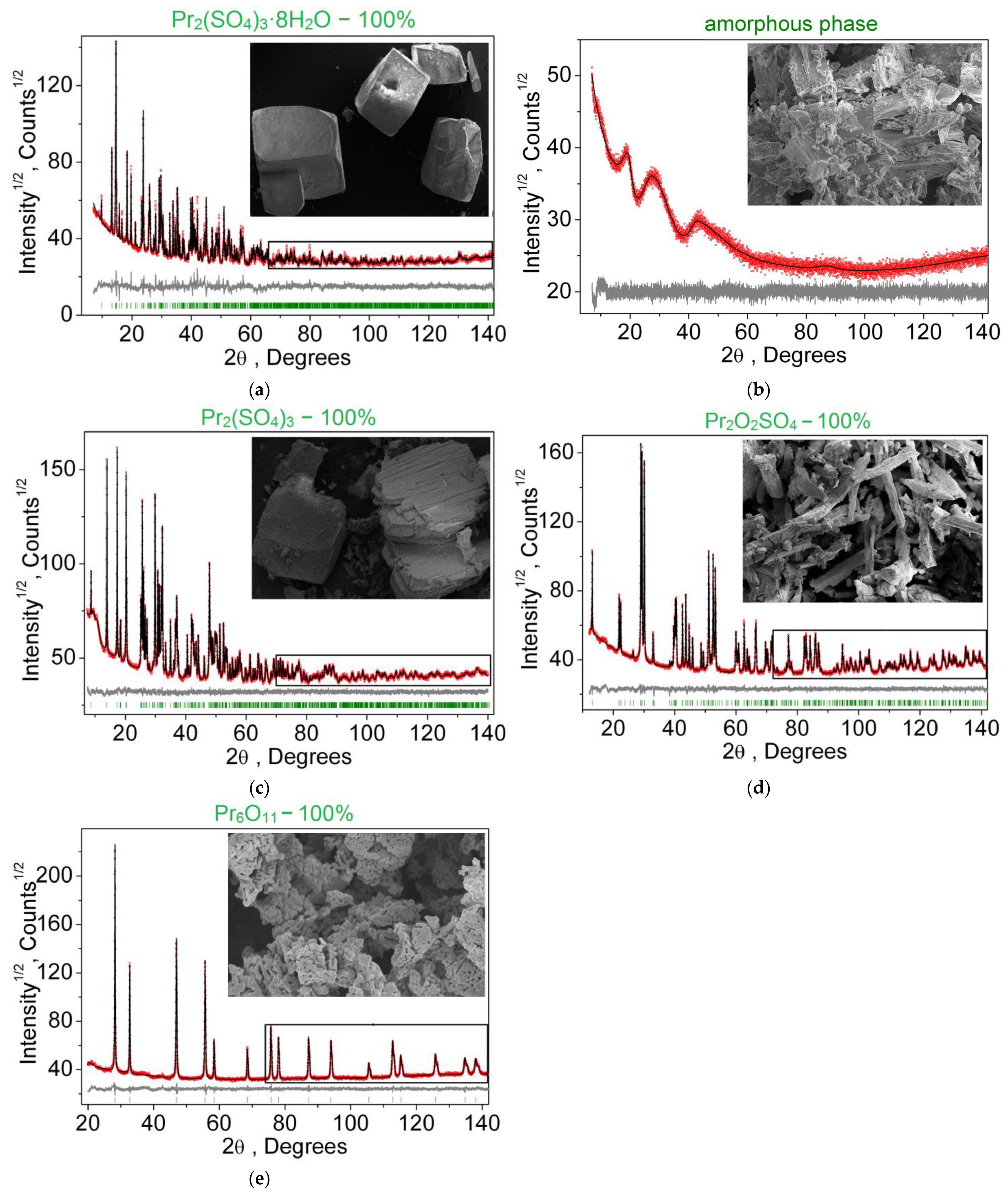
3.4. Thermal Properties
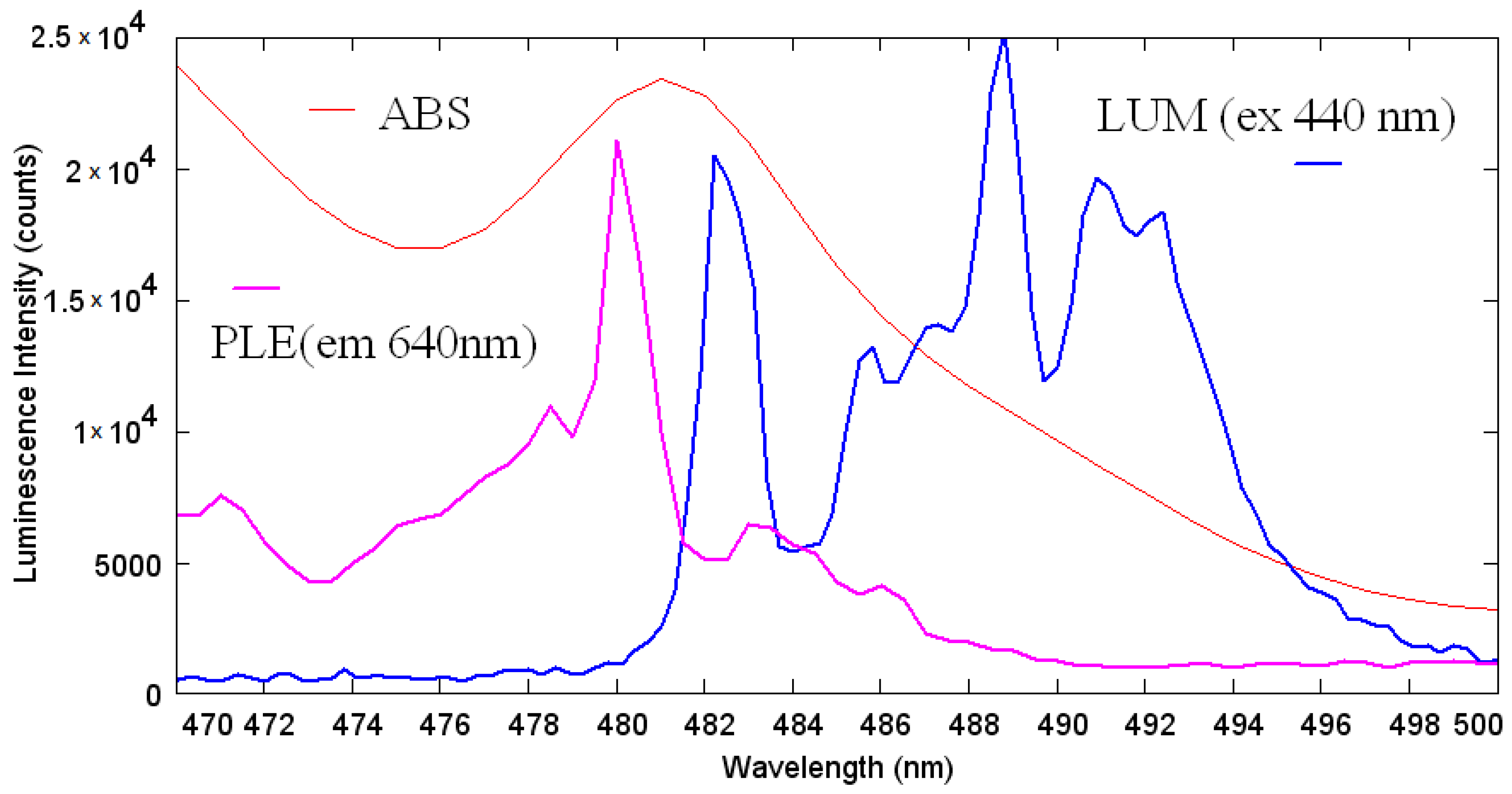
3.5. Luminescent Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating Rare Earth Element Availability: A Case with Revolutionary Demand from Clean Technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Baldi, L.; Peri, M.; Vandone, D. Clean energy industries and rare earth materials: Economic and financial issues. Energy Policy 2014, 66, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Sousa Filho, P.C.D.; de Lima, J.F.; Serra, O.A. From Lighting to Photoprotection: Fundamentals and Applications of Rare Earth Materials. J. Braz. Chem. Soc. 2015, 26, 2471–2495. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, Q. Progress in discovery and structural design of color conversion phosphors for LEDs. Prog. Mater. Sci. 2016, 84, 59–117. [Google Scholar] [CrossRef]
- Sugimoto, S. Current status and recent topics of rare-earth permanent magnets. J. Phys. D Appl. Phys. 2011, 44, 064001. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, Y.; Molokeev, M.S.; Atuchin, V.V. Structural and luminescence properties of yellow-emitting NaScSi2O6:Eu2+ phosphors: Eu2+ Ssite preference analysis and generation of red emission by codoping Mn2+ for white-light-emitting diode applications. J. Phys. Chem. C 2013, 117, 20847–20854. [Google Scholar] [CrossRef]
- Serres, J.M.; Mateos, X.; Loiko, P.; Yumashev, K.; Kuleshov, N.; Petrov, V.; Griebner, U.; Aguiló, M.; Díaz, F. Diode-pumped microchip Tm:KLu(WO4)2 laser with more than 3 W of output power. Opt. Lett. 2014, 39, 4247–4250. [Google Scholar] [CrossRef]
- Li, G.; Lin, C.C.; Chen, W.-T.; Molokeev, M.; Atuchin, V.V.; Chiang, C.-Y.; Zhou, W.; Wang, C.-W.; Li, W.-H.; Sheu, H.-S.; et al. Photoluminescence Tuning via Cation Substitution in Oxonitridosilicate Phosphors: DFT Calculations, Different Site Occupations, and Luminescence Mechanisms. Chem. Mater. 2014, 26, 2991–3001. [Google Scholar] [CrossRef]
- Mutailipu, M.; Xie, Z.; Su, X.; Zhang, M.; Wang, Y.; Yang, Z.; Janjua, M.R.S.A.; Pan, S. Chemical Cosubstitution-Oriented Design of Rare-Earth Borates as Potential Ultraviolet Nonlinear Optical Materials. J. Am. Chem. Soc. 2017, 139, 18397–18405. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Subanakov, A.K.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Dorzhieva, S.G.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; et al. Exploration of structural, thermal, vibrational and spectroscopic properties of new noncentrosymmetric double borate Rb3NdB6O12. Adv. Powder Technol. 2017, 28, 1309–1315. [Google Scholar] [CrossRef]
- Fischbacher, J.; Kovacs, A.; Gusenbauer, M.; Oezelt, H.; Exl, L.; Bance, S.; Schrefl, T. Micromagnetics of rare-earth efficient permanent magnets. J. Phys. D Appl. Phys. 2018, 51, 193002. [Google Scholar] [CrossRef]
- Razumkova, I.A.; Denisenko, Y.G.; Boyko, A.N.; Ikonnikov, D.A.; Aleksandrovsky, A.S.; Azarapin, N.O.; Andreev, O.V. Synthesis and upconversion luminescence in LaF3:Yb3+, Ho3+, GdF3: Yb3+, Tm3+ and YF3:Yb3+, Er3+ obtained from sulfide precursors. Z. Anorg. Allg. Chem. 2019, 645, 1393–1401. [Google Scholar] [CrossRef]
- Azarapin, N.O.; Aleksandrovsky, A.S.; Atuchin, V.V.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Mukherjee, S.; Oreshonkov, A.S.; Andreev, O.V. Synthesis, structural and spectroscopic properties of orthorhombic compounds BaLnCuS3 (Ln = Pr, Sm). J. Alloy. Compd. 2020, 832, 153134. [Google Scholar] [CrossRef]
- Lin, Z.-L.; Zeng, H.-J.; Zhang, G.; Xue, W.-Z.; Pan, Z.; Lin, H.; Loiko, P.; Liang, H.-C.; Petrov, V.; Mateos, X.; et al. Kerr-lens mode-locked Yb-SrLaAlO4 laser. Opt. Express 2021, 29, 42837–42943. [Google Scholar] [CrossRef]
- Lim, C.-S.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A.; Atuchin, V. Structural and spectroscopic effects of Li+ substitution for Na+ in LixNa1-xCaGd0.5Ho0.05Yb0.45(MoO4)3 scheelite-type upconversion phosphors. Molecules 2021, 26, 7357. [Google Scholar] [CrossRef]
- Shi, X.; Tudi, A.; Cheng, M.; Zhang, F.; Yang, Z.; Han, S.; Pan, S. Noncentrosymmetric rare-earth borate fluoride La2B5O9F3: A new ultraviolet nonlinear optical crystal with enhanced linear and nonlinear performance. ACS Appl. Mater. Interfaces 2022, 14, 18704–18712. [Google Scholar] [CrossRef]
- Atuchin, V.; Subanakov, A.; Aleksandrovsky, A.; Bazarov, B.; Bazarova, J.; Krylov, A.; Molokeev, M.; Oreshonkov, A.; Pugachev, A. New double nonlinear-optical borate Rb3SmB6O12: Synthesis, structure and spectroscopic properties. J. Alloy. Compd. 2022, 905, 164022. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcoganides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Pietrelli, L.; Bellomo, B.; Fontana, D.; Montereali, M. Rare earths recovery from NiMH spent batteries. Hydrometallurgy 2002, 66, 135–139. [Google Scholar] [CrossRef]
- Cascales, C.; Lor, B.G.; Puebla, E.G.; Iglesias, M.; Monge, M.A.; Valero, A.C.R.; Snejko, N. Catalytic Behavior of Rare-Earth Sulfates: Applications in Organic Hydrogenation and Oxidation Reactions. Chem. Mater. 2004, 16, 4144–4149. [Google Scholar] [CrossRef]
- Perles, J.; Fortes-Revilla, C.; Gutiérrez-Puebla, E.; Iglesias, M.; Monge, M.; Ruiz-Valero, A.C.; Snejko, N. Synthesis, Structure, and Catalytic Properties of Rare-Earth Ternary Sulfates. Chem. Mater. 2005, 17, 2701–2706. [Google Scholar] [CrossRef]
- Kul, M.; Topkaya, Y.; Karakaya, I. Rare earth double sulfates from pre-concentrated bastnasite. Hydrometallurgy 2008, 93, 129–135. [Google Scholar] [CrossRef]
- Deng, Z.; Bai, F.; Xing, Y.; Xing, N.; Xu, L. Reaction in situ found in the synthesis of a series of lanthanide sulfate complexes and investigation on their structure, spectra and catalytic activity. Open J. Inorg. Chem. 2013, 3, 76–99. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-W.; Long, Z.-Q.; Wang, L.; Feng, Z.-Y. Technology development for rare earth cleaner hydrometallurgy in China. Rare Met. 2015, 34, 215–222. [Google Scholar] [CrossRef]
- Zhu, Z.; Pranolo, Y.; Cheng, C.Y. Separation of uranium and thorium from rare earths for rare earth production—A review. Miner. Eng. 2015, 77, 185–196. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Diaz, L.A.; Lister, T.E.; Parkman, J.A.; Clark, G.G. Comprehensive process for the recovery of value and critical materials from electronic waste. J. Clean. Prod. 2016, 125, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Denisenko, Y.G.; Atuchin, V.V.; Molokeev, M.S.; Aleksandrovsky, A.S.; Krylov, A.S.; Oreshonkov, A.S.; Volkova, S.S.; Andreev, O.V. Structure, thermal stability, and spectroscopic properties of triclinic double sulfate AgEu(SO4)2 with isolated SO4 groups. Inorg. Chem. 2018, 57, 13279–13288. [Google Scholar] [CrossRef]
- Mikhailov, M.; Yuryev, S.; Lapin, A. Prospects for applying BaSO4 powders as pigments for spacecraft thermal control coatings. Acta Astronaut. 2019, 165, 191–194. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Lin, Z.; Li, Y.; Ding, Q.; Liu, Y.; Chen, Y.; Zhao, S.; Hong, M.; Luo, J. Pushing KTiOPO4-like Nonlinear Optical Sulfates into the Deep-Ultraviolet Spectral Region. Inorg. Chem. 2021, 60, 18950–18956. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Atuchin, V.V.; Molokeev, M.S.; Wang, N.; Jiang, X.; Aleksandrovsky, A.S.; Krylov, A.S.; Oreshonkov, A.S.; Sedykh, A.E.; Volkova, S.S.; et al. Negative thermal expansion in one-dimension of a new double sulfate AgHo(SO4)2 with isolated SO4 tetrahedra. J. Mater. Sci. Technol. 2021, 76, 111–121. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Zhao, S.; Liang, F.; Ding, Q.; Sun, J.; Lin, Z.; Hong, M.; Luo, J. A Deep-UV Nonlinear Optical Borosulfate with Incommensurate Modulations. Angew. Chem. Int. Ed. 2021, 60, 11457–11463. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, C.; Li, Z.; Li, M.; Liu, G. Synthesis, characterization and optical properties of three novel lanthanide sulfates. J. Solid State Chem. 2021, 303, 122481. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, Q.; Yang, F.; Huang, L.; Gao, D.; Bi, J.; Zou, G. Tin chloride sulfates A3Sn2(SO4)3−xCl1+2x (A = K, Rb, Cs; x = 0, 1) as multifunctional optical materials. Inorg. Chem. 2021, 60, 8322–8330. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Sedykh, A.E.; Basova, S.A.; Atuchin, V.V.; Molokeev, M.S.; Aleksandrovsky, A.S.; Krylov, A.S.; Orenshonkov, A.S.; Khritokhin, N.A.; Sal’nikova, E.I.; et al. Exploration of the structural, spectroscopic and thermal properties of double sulfate monohydrate NaSm(SO4)2·H2O and its thermal decomposition product NaSm(SO4)2. Adv. Powder Technol. 2021, 32, 3943–3953. [Google Scholar] [CrossRef]
- Song, Y.; Hao, X.; Lin, C.; Lin, D.; Luo, M.; Ye, N. Two Tellurium(IV)-Based Sulfates Exhibiting Strong Second Harmonic Generation and Moderate Birefringence as Promising Ultraviolet Nonlinear Optical Materials. Inorg. Chem. 2021, 60, 11412–11418. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, K.; He, C.; Wei, L.; Li, X.-F.; Zhang, S.; An, X.-T.; Li, J.-H.; Wang, G.-M. Linear and nonlinear optical properties of centrosymmetric Sb4O5SO4 and noncentrosymmetric Sb4O4(SO4)(OH)2 induced by lone pair stereoactivity. Inorg. Chem. 2021, 60, 11648–11654. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Molokeev, M.S.; Oreshonkov, A.S.; Krylov, A.S.; Aleksandrovsky, A.S.; Azarapin, N.O.; Andreev, O.V.; Razumkova, I.A.; Atuchin, V.V. Crystal structure, vibrational, spectroscopic and thermochemical properties of double sulfate crystalline hydrate [CsEu(H2O)3(SO4)2]·H2O and its thermal dehydration product CsEu(SO4)2. Crystals 2021, 11, 1027. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, X.; Xu, F.; Li, B.; Ye, N.; Luo, M. HgSO4: An excellent mid-infrared sulfate nonlinear optical crystal with wide band gap and strong second harmonic generation response. J. Alloy. Compd. 2022, 902, 163727. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, W.; Lin, X. Advances in second-order nonlinear optical sulfates. Coord. Chem. Rev. 2022, 459, 214443. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, X.; Hu, Y.; Jiang, C.; Wu, T.; Lin, Z.; Huang, Z.; Humphrey, M.G.; Zhang, C. A Lanthanum Ammonium Sulfate Double Salt with a Strong SHG Response and Wide Deep-UV Transparency. Angew. Chem. Int. Ed. 2021, 61, e202115855. [Google Scholar] [CrossRef]
- Sirotinkin, S.P.; Efremov, V.A.; Kovba, L.M.; Pokrovskij, A.N. Crystal structure of anhydrous neodymium sulfate Nd2(SO4)3. Kristallografiya 1977, 22, 1272–1273. [Google Scholar]
- Denisenko, Y.G.; Aleksandrovsky, A.S.; Atuchin, V.V.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Shestakov, N.P.; Andreev, O.V. Exploration of structural, thermal and spectroscopic properties of self-activated sulfate Eu2(SO4)3 with isolated SO4 groups. J. Ind. Eng. Chem. 2018, 68, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Wickleder, M.S. Wasserfreie sulfate der selten-erd-elemente: Synthese und kristallstruktur von Y2(SO4)3 und Sc2(SO4)3. Z. Anorg. Allg. Chem. 2000, 626, 1468–1472. [Google Scholar] [CrossRef]
- Wickleder, M.S. Sulfate und hydrogensulfate des erbiums: Er(HSO4)3-I, Er(HSO4)3-II, Er (SO4)(HSO4) und Er2(SO4)3. Z. Anorg. Allg. Chem. 1998, 624, 1347–1354. [Google Scholar] [CrossRef]
- Wickleder, M.S. Inorganic Lanthanide Compounds with Complex Anions. Chem. Rev. 2002, 102, 2011–2088. [Google Scholar] [CrossRef]
- Mills, S.J.; Petříček, V.; Kampf, A.R.; Herbst-Imer, R.; Raudsepp, M. The crystal structure of Yb2(SO4)3·3H2O and its decomposition product, β-Yb2(SO4)3. J. Solid State Chem. 2011, 184, 2322–2328. [Google Scholar] [CrossRef]
- Machida, M.; Kawano, T.; Eto, M.; Zhang, D.; Ikeue, K. Ln Dependence of the Large-Capacity Oxygen Storage/Release Property of Ln Oxysulfate/Oxysulfide Systems. Chem. Mater. 2007, 19, 954–960. [Google Scholar] [CrossRef]
- Liang, J.; Ma, R.; Geng, F.; Ebina, Y.; Sasaki, T. Ln2(OH)4SO4·nH2O (Ln = Pr to Tb; n ∼ 2): A new family of layered rare-earth hydroxides rigidly pillared by sulfate ions. Chem. Mater. 2010, 22, 6001–6007. [Google Scholar] [CrossRef]
- Atuchin, V.; Gavrilova, T.; Grivel, J.-C.; Kesler, V.; Troitskaia, I. Electronic structure of layered ferroelectric high-k titanate Pr2Ti2O7. J. Solid State Chem. 2012, 195, 125–131. [Google Scholar] [CrossRef]
- Dannenbauer, N.; Matthes, P.R.; Müller-Buschbaum, K. Luminescent coordination polymers for the VIS and NIR range constituting from LnCl3 and 1,2-bis(4-pyridyl)-ethane. Dalton Trans. 2016, 45, 6529–6540. [Google Scholar] [CrossRef] [PubMed]
- Dannenbauer, N.; Matthes, P.R.; Scheller, T.P.; Nitsch, J.; Zottnick, S.H.; Gernert, M.S.; Steffen, A.; Lambert, C.; Müller-Buschbaum, K. Near-infrared luminescence and inner filter effects of lanthanide coordination polymers with 1,2-Di(4-pyridyl)ethylene. Inorg. Chem. 2016, 55, 7396–7406. [Google Scholar] [CrossRef] [PubMed]
- Ridenour, J.A.; Carter, K.P.; Cahill, C.L. RE-p-halobenzoic acid–terpyridine complexes, part III: Structural and supramolecular trends in a series of p-iodobenzoic acid rare-earth hybrid materials. CrystEngComm 2017, 19, 1190–1203. [Google Scholar] [CrossRef]
- Sedykh, A.E.; Kurth, D.G.; Müller-Buschbaum, K. Two series of lanthanide coordination polymers and complexes with 4′-phenylterpyridine and their luminescence properties. Eur. J. Inorg. Chem. 2019, 2019, 4564–4571. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Sacco, O.; Sannino, D. Photocatalytic treatment of aqueous solutions at high dye concentration using praseodymium-doped ZnO catalysts. Appl. Catal. B Environ. 2017, 209, 621–630. [Google Scholar] [CrossRef]
- Jin, Q.; Shen, Y.; Zhu, S.; Liu, Q.; Li, X.; Yan, W. Effect of praseodymium additive on CeO2(ZrO2)/TiO2 for selective catalytic reduction of NO by NH3. J. Rare Earths 2016, 34, 1111–1120. [Google Scholar] [CrossRef]
- Najjar, H.; Batis, H.; Lamonier, J.-F.; Mentré, O.; Giraudon, J.-M. Effect of praseodymium and europium doping in La1−xLnxMnO3+δ (Ln: Pr or Eu, 0 ≤ x ≤ 1) perosvkite catalysts for total methane oxidation. Appl. Catal. A 2014, 469, 98–107. [Google Scholar] [CrossRef]
- Jin, Q.; Shen, Y.; Zhu, S. Praseodymium oxide modified CeO2/Al2O3 catalyst for selective catalytic reduction of NO by NH3. Chin. J. Chem. 2016, 34, 1283–1290. [Google Scholar] [CrossRef]
- Guillén-Hurtado, N.; García-García, A.; Bueno-López, A. Active oxygen by Ce–Pr mixed oxide nanoparticles outperform diesel soot combustion Pt catalysts. Appl. Catal. B 2015, 174, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Imamura, K.; Kawano, Y.; Miyahara, S.-I.; Yamamoto, T.; Matsumura, S.; Nagaoka, K. A low-crystalline ruthenium nano-layer supported on praseodymium oxide as an active catalyst for ammonia synthesis. Chem. Sci. 2017, 8, 674–679. [Google Scholar] [CrossRef] [Green Version]
- Bartl, M.H.; Gatterer, K.; Cavalli, E.; Speghini, A.; Bettinelli, M. Growth, optical spectroscopy and crystal field investigation of YAl3(BO3)4 single crystals doped with tripositive praseodymium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 1981–1990. [Google Scholar] [CrossRef]
- Guzik, M.; Aitasalo, T.; Szuszkiewicz, W.; Hölsä, J.; Keller, B.; Legendziewicz, J. Optical spectroscopy of yttrium double phosphates doped by cerium and praseodymium ions. J. Alloy. Compd. 2004, 380, 368–375. [Google Scholar] [CrossRef]
- Pisarski, W.A. Spectroscopic analysis of praseodymium and erbium ions in heavy metal fluoride and oxide glasses. J. Mol. Struct. 2005, 744–747, 473–479. [Google Scholar] [CrossRef]
- Utikal, T.; Eichhammer, E.; Petersen, L.; Renn, A.; Götzinger, S.; Sandoghdar, V. Spectroscopic detection and state preparation of a single praseodymium ion in a crystal. Nat. Commun. 2014, 5, 3627. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Feng, W.; Wang, K. Experimental and theoretical spectroscopic study of praseodymium(III) doped strontium aluminate phosphors. J. Alloy. Compd. 2015, 628, 343–346. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.-G.; Molokeev, M.S.; Zhu, Q.; Li, X.; Sun, X. Layered hydroxyl sulfate: Controlled crystallization, structure analysis, and green derivation of multi-color luminescent (La,RE) 2 O 2 SO 4 and (La,RE) 2 O 2 S phosphors (RE = Pr, Sm, Eu, Tb, and Dy). Chem. Eng. J. 2016, 302, 577–586. [Google Scholar] [CrossRef]
- Chen, H.-L.; Wei, L.-K.; Chang, Y.-S. Characterizations of Pr3+ Ion-Doped LaVO4 Phosphor Prepared Using a Sol–Gel Method. J. Electron. Mater. 2018, 47, 6649–6654. [Google Scholar] [CrossRef]
- Tian, X.; Li, J.; Sheng, H.; Li, T.; Guo, L.; Ji, C.; Huang, Z.; Wen, J.; Liu, X.; Li, C.; et al. Luminescence and optical thermometry based on silico-carnotite Ca3Y2Si3O12: Pr3+ phosphor. Ceram. Int. 2021, 48, 3860–3868. [Google Scholar] [CrossRef]
- Bruker. Bruker AXS TOPAS V4; Bruker: Karlsruhe, Germany, 2008. [Google Scholar]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Kristallogr. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Zunger, A. Self-interaction correction to density-functional approximations for many-electron systems. Phys. Rev. B 1981, 23, 5048–5079. [Google Scholar] [CrossRef] [Green Version]
- Ceperley, D.M.; Alder, B.J. Ground State of the Electron Gas by a Stochastic Method. Phys. Rev. Lett. 1980, 45, 566–569. [Google Scholar] [CrossRef] [Green Version]
- NETZSCH Proteus 6, Thermic Analyses e User’s and Software Manuals; NETZSCH: Selb, Germany, 2012.
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Bukovec, N.; Bukovec, P.; Šiftar, J. Kinetics of the thermal decomposition of Pr2(SO4)3 to Pr2O2SO4. Thermochim. Acta 1980, 35, 85–91. [Google Scholar] [CrossRef]
- Lyadov, A.S.; Kurilkin, V.V. Reduction specifics of rare-earth orthovanadates (REE = La, Nd, Sm, Dy, Ho, Er, Tm, Yb, and Lu). Russ. J. Inorg. Chem. 2016, 61, 86–92. [Google Scholar] [CrossRef]
- Llópiz, J.; Romero, M.; Jerez, A.; Laureiro, Y. Generalization of the kissinger equation for several kinetic models. Thermochim. Acta 1995, 256, 205–211. [Google Scholar] [CrossRef]
- Sirotinkin, S.P. Physicochemical Study of Double Sulfates of Lithium and Rare Earth Elements. D.Sc. Thesis, Moscow State University, Moscow, Russia, 1978. [Google Scholar]
- Favre-Nicolin, V.; Černý, R. FOX: Modular Approach to Crystal Structure Determination from Powder Diffraction. Mater. Sci. Forum 2004, 443–444, 35–38. [Google Scholar] [CrossRef]
- Favre-Nicolin, V.; Černý, R. FOX, free objects for crystallography: A modular approach to ab initio structure determination from powder diffraction. J. Appl. Crystallogr. 2002, 35, 734–743. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1985, B41, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Kroumova, E.; Aroyo, M.I.; Mato, J.M.P.; Kirov, A.; Capillas, C.; Ivantchev, S.; Wondratschek, H. Bilbao Crystallographic Server: Useful Databases and Tools for Phase-Transition Studies. Phase Transit. 2003, 76, 155–170. [Google Scholar] [CrossRef]
- Nakamoto, N. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Blatov, V.A.; Peskov, M.V. A comparative crystallochemical analysis of binary compounds and simple anhydrous salts containing pyramidal anions LO3 (L = S, Se, Te, Cl, Br, I). Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Poston, J.A., Jr.; Siriwardane, R.V.; Fisher, E.P.; Miltz, A.L. Thermal decomposition of the rare earth sulfates of cerium (III), cerium (IV), lanthanum (III) and samarium (III). Appl. Surf. Sci. 2003, 214, 83–102. [Google Scholar] [CrossRef]
- Andreev, O.V.; Denisenko, Y.G.; Osseni, S.A.; Bamburov, V.G.; Sal’nikova, E.I.; Khritokhin, N.A.; Andreev, P.O.; Polkovnikov, A.A. Sulfates and Oxysulfides of Rare-Earth Elements; UTMN Publishing: Tyumen, Russia, 2017. [Google Scholar]
- Stubblefield, C.T.; Eick, H.; Eyring, L. Praseodymium Oxides. III. The Heats of Formation of Several Oxides1. J. Am. Chem. Soc. 1956, 78, 3018–3020. [Google Scholar] [CrossRef]
- Eckman, J.R.; Rossini, F.D. The heat of formation of sulphur dioxide. Bur. Stand. J. Res. 1929, 3, 597–618. [Google Scholar] [CrossRef]
- Rossini, F.D. The heat of formation of water. Proc. Natl. Acad. Sci. USA 1930, 16, 694–699. [Google Scholar] [CrossRef] [Green Version]
- Denisenko, Y.; Khritokhin, N.; Andreev, O.; Basova, S.; Sal’Nikova, E.; Polkovnikov, A. Thermal decomposition of europium sulfates Eu2(SO4)3·8H2O and EuSO4. J. Solid State Chem. 2017, 255, 219–224. [Google Scholar] [CrossRef]
- Irtyugo, L.A.; Denisova, L.T.; Molokeev, M.S.; Denisov, V.M.; Aleksandrovsky, A.S.; Beletskii, V.V.; Sivkova, E.Y. Synthesis, Crystal Structure, and the Optical and Thermodynamic Properties of PrAlGe2O7. Russ. J. Phys. Chem. A 2021, 95, 1546–1550. [Google Scholar] [CrossRef]
- Denisova, L.T.; Molokeev, M.S.; Kargin, Y.F.; Gerasimov, V.P.; Krylov, A.S.; Aleksandrovskii, A.S.; Chumilina, L.G.; Denisov, V.M.; Vasil’ev, G.V. Synthesis, crystal structure, and physicochemical properties of Bi4–xPrxTi3O12 (x = 0.4, 0.8, 1.2, 1.6) solid solutions. Inorg. Mater. 2021, 57, 919–928. [Google Scholar] [CrossRef]
- Srivastava, A.M. Aspects of Pr3+ luminescence in solids. J. Lumin. 2016, 169, 445–449. [Google Scholar] [CrossRef]
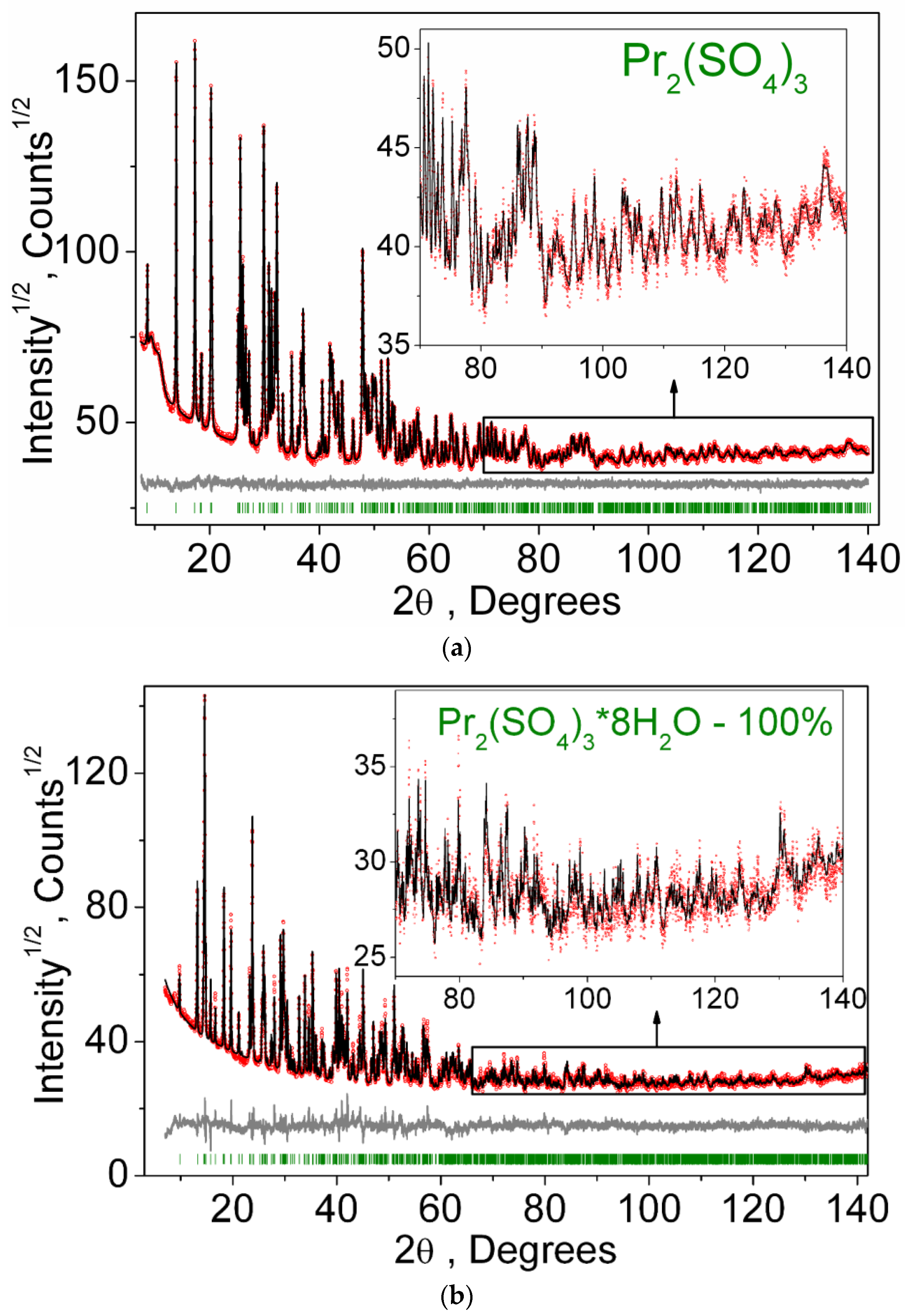


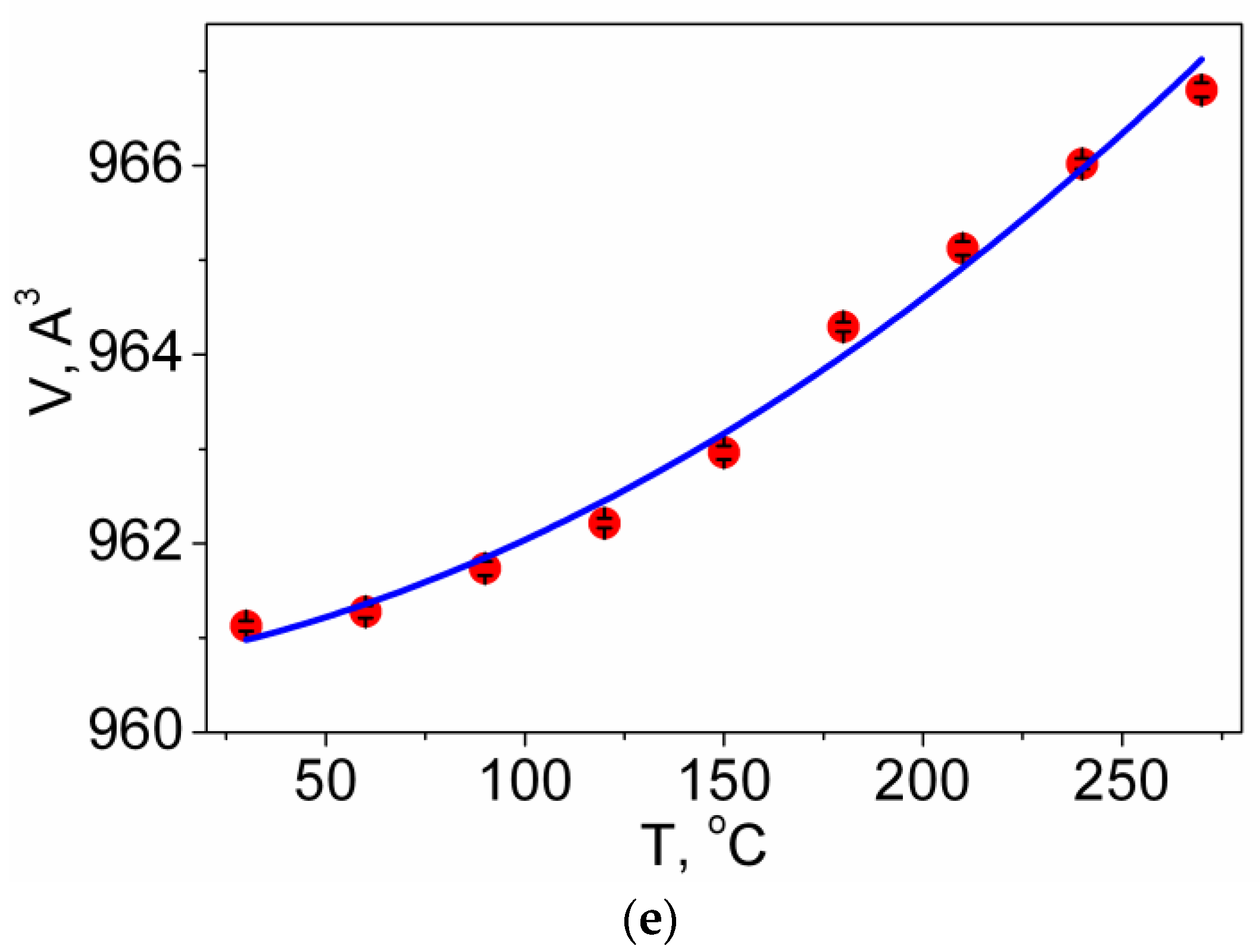
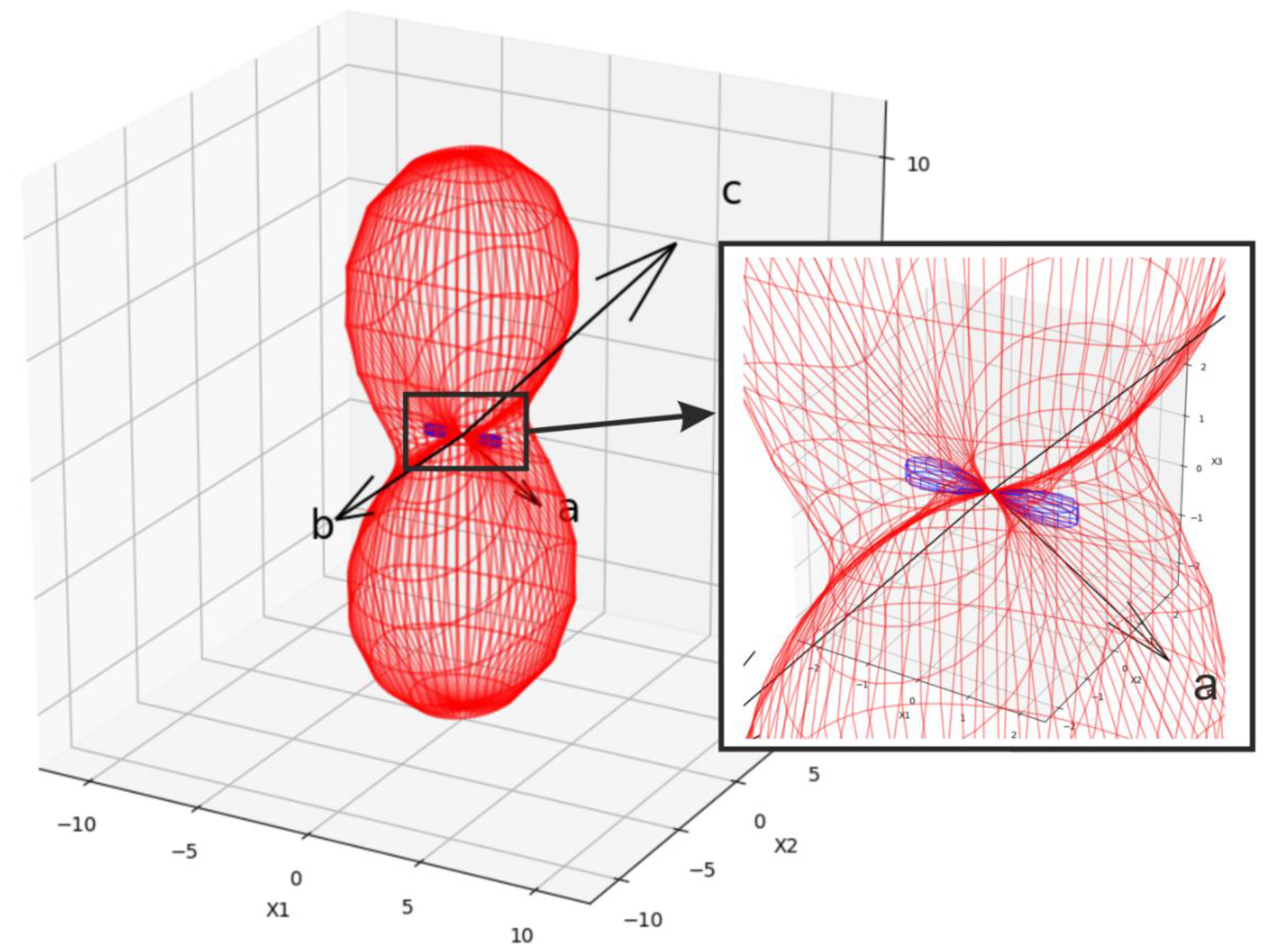
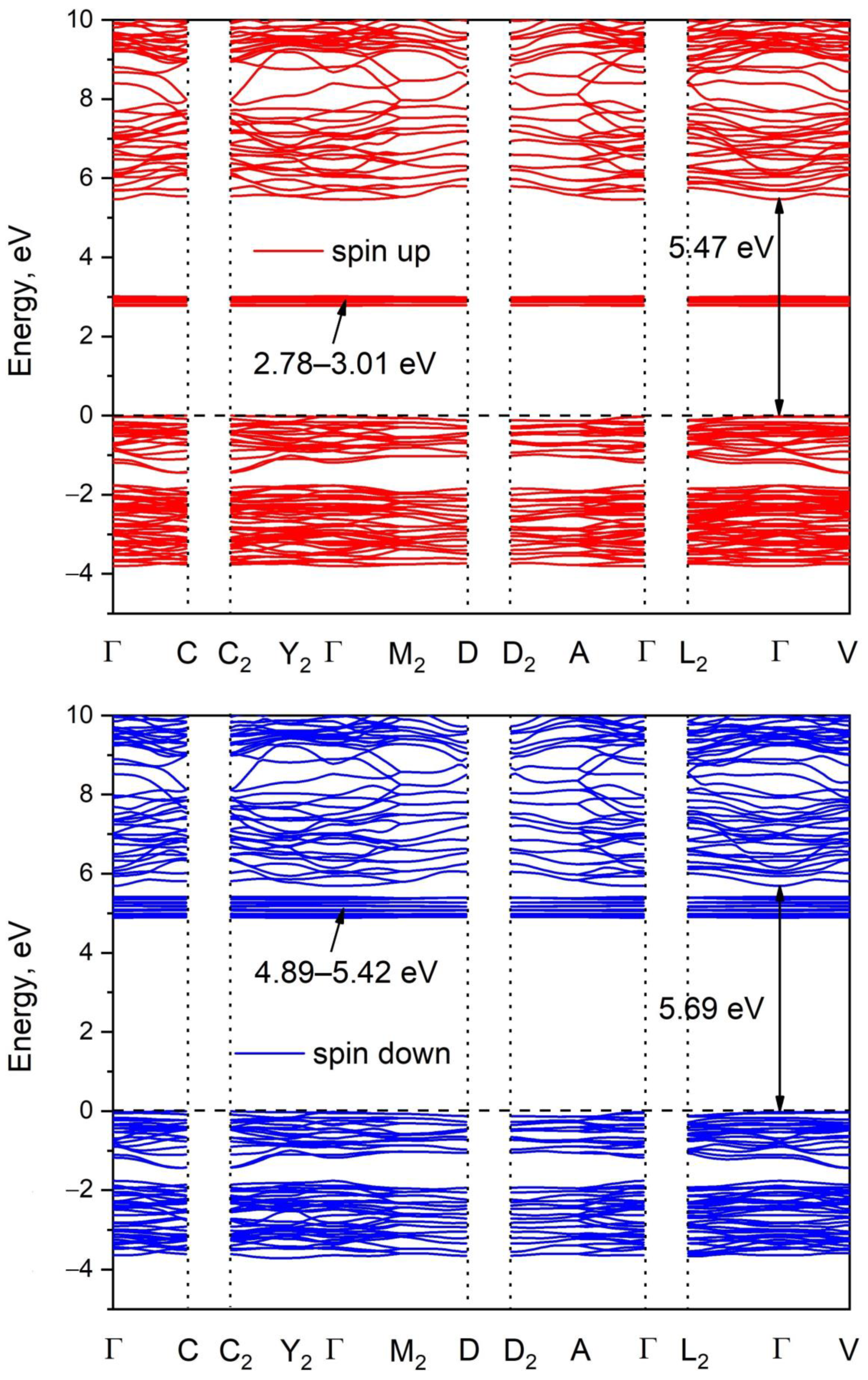


| Compound | Pr2(SO4)3 |
|---|---|
| Space group | C2/c |
| a, Å | 21.6052 (4) |
| b, Å | 6.7237 (1) |
| c, Å | 6.9777 (1) |
| β, ° | 107.9148 (7) |
| V, Å3 | 964.48 (3) |
| Z | 4 |
| 2θ-interval, ° | 7.5–140 |
| Tmeas. | 150 °C |
| Number of reflections | 922 |
| Number of refined parameters | 71 |
| Rwp, % | 2.75 |
| Rp, % | 2.16 |
| Rexp, % | 2.10 |
| χ2 | 1.31 |
| RB, % | 0.69 |
| Compound | Pr2(SO4)3·8H2O |
|---|---|
| Space group | C2/c |
| a, Å | 13.7058 (2) |
| b, Å | 6.8664 (1) |
| c, Å | 18.4702 (3) |
| β, ° | 102.816 (1) |
| V, Å3 | 1694.91 (5) |
| Z | 4 |
| 2θ-interval, ° | 7–144 |
| Tmeas. | 24 °C |
| Number of reflections | 1689 |
| Number of refined parameters | 48 |
| Rwp, % | 6.63 |
| Rp, % | 5.08 |
| Rexp, % | 2.95 |
| χ2 | 2.24 |
| RB, % | 3.65 |
| Wavenumber, cm−1 [81] | Td Free Molecule Symmetry | C1 Site Symmetry | C2h Unit Cell Symmetry |
|---|---|---|---|
| 983 | A1 (ν1) | A | Ag + Au + Bg + Bu |
| 450 | E (ν2) | 2A | 2Ag + 2Au + 2Bg + 2Bu |
| 1105 | F2 (ν3) | 3A | 3Ag + 3Au + 3Bg + 3Bu |
| 611 | F2 (ν4) | 3A | 3Ag + 3Au + 3Bg + 3Bu |
| Td Free molecule symmetry | C2 Site symmetry | C2h Unit cell symmetry | |
| 983 | A1 (ν1) | A | Ag + Au |
| 450 | E (ν2) | 2A | 2Ag + 2Au |
| 1105 | F2 (ν3) | A + 2B | Ag + Au + 2Bg + 2Bu |
| 611 | F2 (ν4) | A + 2B | Ag + Au + 2Bg + 2Bu |
| Compound | ∆H°f, kJ/mol |
|---|---|
| Pr2(SO4)3·8H2O | −5361.2 |
| Pr2(SO4)3 | −3317.9 |
| Pr2O2SO4 | −2224.3 |
| Number of Reaction | Chemical Equation | A | Ea, kJ/mol |
|---|---|---|---|
| 1 | Pr2(SO4)3·8H2O → Pr2(SO4)3 + 8H2O | 6 × 106 | 77 |
| 2 | Pr2(SO4)3 → Pr2O2SO4 + 2SO2 + O2 | 1 × 1010 | 303 |
| 3 | 6Pr2O2SO4 → 2Pr6O11 + 6SO2 + O2 | 2 × 108 | 323 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denisenko, Y.G.; Atuchin, V.V.; Molokeev, M.S.; Sedykh, A.E.; Khritokhin, N.A.; Aleksandrovsky, A.S.; Oreshonkov, A.S.; Shestakov, N.P.; Adichtchev, S.V.; Pugachev, A.M.; et al. Exploration of the Crystal Structure and Thermal and Spectroscopic Properties of Monoclinic Praseodymium Sulfate Pr2(SO4)3. Molecules 2022, 27, 3966. https://doi.org/10.3390/molecules27133966
Denisenko YG, Atuchin VV, Molokeev MS, Sedykh AE, Khritokhin NA, Aleksandrovsky AS, Oreshonkov AS, Shestakov NP, Adichtchev SV, Pugachev AM, et al. Exploration of the Crystal Structure and Thermal and Spectroscopic Properties of Monoclinic Praseodymium Sulfate Pr2(SO4)3. Molecules. 2022; 27(13):3966. https://doi.org/10.3390/molecules27133966
Chicago/Turabian StyleDenisenko, Yuriy G., Victor V. Atuchin, Maxim S. Molokeev, Alexander E. Sedykh, Nikolay A. Khritokhin, Aleksandr S. Aleksandrovsky, Aleksandr S. Oreshonkov, Nikolai P. Shestakov, Sergey V. Adichtchev, Alexey M. Pugachev, and et al. 2022. "Exploration of the Crystal Structure and Thermal and Spectroscopic Properties of Monoclinic Praseodymium Sulfate Pr2(SO4)3" Molecules 27, no. 13: 3966. https://doi.org/10.3390/molecules27133966
APA StyleDenisenko, Y. G., Atuchin, V. V., Molokeev, M. S., Sedykh, A. E., Khritokhin, N. A., Aleksandrovsky, A. S., Oreshonkov, A. S., Shestakov, N. P., Adichtchev, S. V., Pugachev, A. M., Sal’nikova, E. I., Andreev, O. V., Razumkova, I. A., & Müller-Buschbaum, K. (2022). Exploration of the Crystal Structure and Thermal and Spectroscopic Properties of Monoclinic Praseodymium Sulfate Pr2(SO4)3. Molecules, 27(13), 3966. https://doi.org/10.3390/molecules27133966











