The Interphase Gas-Solid Synthesis of Ammonium Alginate—The Comparison of Two Synthesis Methods and the Effect of Low Molecular Weight Electrolyte Presence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. 1H NMR Spectroscopy
2.3. Alginic Acid Preparation/Synthesis
2.4. Synthesis of Ammonium Alginate
2.5. FTIR ATR Analysis
2.6. Assessment of Alginic Acid Substitution by Ammonium Groups
2.7. UV–Vis Analysis
2.8. Analysis of Intrinsic Viscosity
2.9. Dynamic Lights Scattering (DLS)
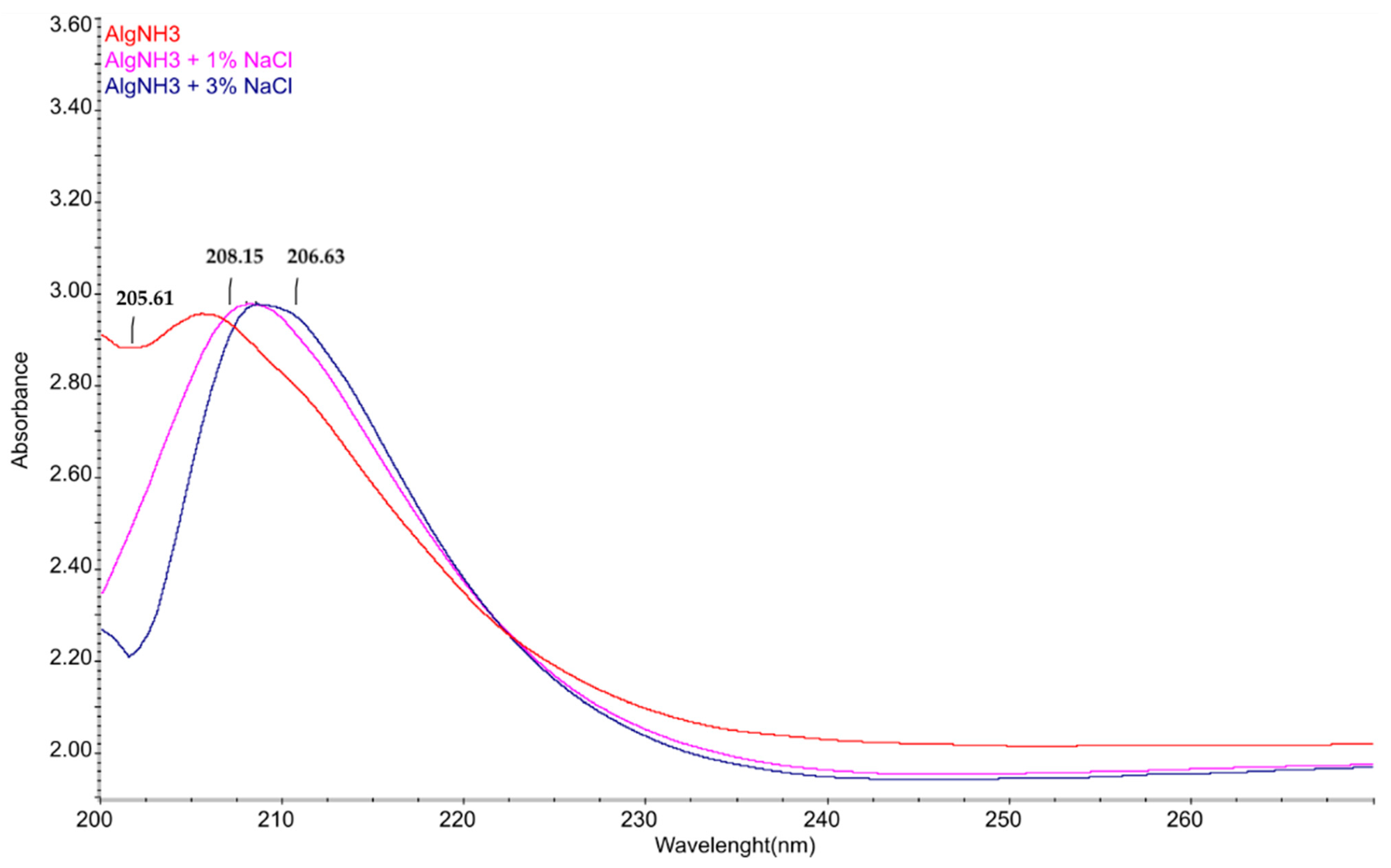
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Stephen, A.M.; Phillips, G.O.; Williams, P.A. Food Polysaccharides and Their Applications: Second Edition. In Food Polysaccharides and Their Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 291–295. [Google Scholar]
- Pegg, C.E.; Jones, G.H.; Athauda, T.J.; Ozer, R.R.; Chalker, J.M. Facile preparation of ammonium alginate-derived nanofibers carrying diverse therapeutic cargo. Chem. Commun. 2013, 50, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Eiselt, P.; Yeh, J.; Latvala, R.K.; Shea, L.D.; Mooney, D. Porous carriers for biomedical applications based on alginate hydrogels. Biomaterials 2000, 21, 1921–1927. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Ale, M.T.; Ajalloueian, F.; Meyer, A.S. Characterization of alginates from Ghanaian brown seaweeds: Sargassum spp. and Padina spp. Food Hydrocoll. 2017, 71, 236–244. [Google Scholar] [CrossRef]
- Jiao, W.; Chen, W.; Mei, Y.; Yun, Y.; Wang, B.; Zhong, Q.; Chen, H.; Chen, W. Effects of Molecular Weight and Guluronic Acid/Mannuronic Acid Ratio on the Rheological Behavior and Stabilizing Property of Sodium Alginate. Molecules 2019, 24, 4374. [Google Scholar] [CrossRef] [Green Version]
- Ramos, P.E.; Silva, P.; Alario, M.M.; Pastrana, L.M.; Teixeira, J.; Cerqueira, M.A.; Vicente, A.A. Effect of alginate molecular weight and M/G ratio in beads properties foreseeing the protection of probiotics. Food Hydrocoll. 2018, 77, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Dos Santos, F.A.; Iulianelli, G.C.V.; Tavares, M.I.B. The Use of Cellulose Nanofillers in Obtaining Polymer Nanocomposites: Properties, Processing, and Applications. Mater. Sci. Appl. 2016, 7, 257–294. [Google Scholar] [CrossRef] [Green Version]
- Ouwerx, C.; Velings, N.; Mestdagh, M.M.; Axelos, M.A.V. Physico-chemical properties and rheology of alginate gel beads formed with various divalent cations. Polym. Gels Networks 1998, 6, 393–408. [Google Scholar] [CrossRef]
- Shen, W.; Hsieh, Y.-L. Biocompatible sodium alginate fibers by aqueous processing and physical crosslinking. Carbohydr. Polym. 2013, 102, 893–900. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y. The gel swelling properties of alginate fibers and their applications in wound management Yimin. Polym. Adv. Technol. 2008, 19, 6–14. [Google Scholar] [CrossRef]
- Sun, F.; Guo, J.; Liu, Y.; Yu, Y. Preparation, characterizations and properties of sodium alginate grafted acrylonitrile/polyethylene glycol electrospun nanofibers. Int. J. Biol. Macromol. 2019, 137, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-Y.; Zhang, X.-C.; Zhou, F.-Z.; Zhang, X.-Z.; Cheng, S.-X.; Zhuo, R.-X. Sustained release of antineoplastic drugs from chitosan-reinforced alginate microparticle drug delivery systems. Int. J. Pharm. 2008, 357, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, B.K.; Rangaraj, S.; Subramani, K.; Srinivasan, S.; Aicher, W.K.; Venkatachalam, R. Biomimetic TiO2-chitosan/sodium alginate blended nanocomposite scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2020, 110, 110710. [Google Scholar] [CrossRef]
- Gao, F.; Liu, X.; Chen, W.; Guo, W.; Chen, L.; Li, D. Hydroxyl radical pretreatment for low-viscosity sodium alginate production from brown seaweed. Algal Res. 2018, 34, 191–197. [Google Scholar] [CrossRef]
- Xu, G.K.; Liu, L.; Yao, J.M. Fabrication and Characterization of Alginate Fibers by Wet-Spinning. Adv. Mater. Res. 2013, 796, 87–91. [Google Scholar] [CrossRef]
- Pabjańczyk-Wlazło, E.; Szparaga, G.; Król, P.; Skrzetuska, E.; Wojtasik, K.; Sieradzka, M.; Boguń, M.; Rabiej, S. Sodium Alginate Fibers Containing Nanosilver. Adv. Polym. Technol. 2014, 33. [Google Scholar] [CrossRef]
- McDowell, R.H. Properties of Alginates, 4th ed.; Alginate Industries: London, UK, 1977. [Google Scholar]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Cross-linker and non-gelling Na+ effects on multi-functional alginate dressings. Carbohydr. Polym. 2012, 87, 1796–1802. [Google Scholar] [CrossRef]
- Dumont, M.; Villet, R.; Guirand, M.; Montembault, A.; Delair, T.; Lack, S.; Barikosky, M.; Crepet, A.; Alcouffe, P.; Laurent, F.; et al. Processing and antibacterial properties of chitosan-coated alginate fibers. Carbohydr. Polym. 2018, 190, 31–42. [Google Scholar] [CrossRef]
- Pamies, R.; Schmidt, R.R.; Martínez, M.D.C.L.; de la Torre, J.G. The influence of mono and divalent cations on dilute and non-dilute aqueous solutions of sodium alginates. Carbohydr. Polym. 2010, 80, 248–253. [Google Scholar] [CrossRef]
- Dodero, A.; Vicini, S.; Alloisio, M.; Castellano, M. Rheological properties of sodium alginate solutions in the presence of added salt: An application of Kulicke equation. Rheol. Acta 2020, 59, 365–374. [Google Scholar] [CrossRef]
- Yethiraj, A. Liquid State Theory of Polyelectrolyte Solutions. J. Phys. Chem. B 2008, 113, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Nakajima, A. Anomalies in Light Scattering from Polyelectrolyte in Semi-Dilute Solution Region. Polym. J. 1980, 12, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zheng, H.; Zhang, Q.; Wang, J.; Konno, M. The interaction of sodium alginate with univalent cations. Biopolymers 1998, 46, 395–402. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Zhang, L.; Ci, M.; Zhang, X.; Jiang, Z.; Zhu, P. Preparation and characterization of polyvinyl alcohol/sodium alginate/Pyrovatex CP composite fibers. Ferroelectrics 2020, 562, 125. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Wet-spinning of Biomedical Polymers: From Single Fibers Production to Additive Manufacturing of 3D Scaffolds. Polym. Int. 2017, 66, 1690–1696. [Google Scholar] [CrossRef]
- Sa, V.; Kornev, K.G. A method for wet spinning of alginate fibers with a high concentration of single-walled carbon nanotubes. Carbon 2011, 49, 1859–1868. [Google Scholar] [CrossRef]
- Sztajnowski, S.; Krucińska, I.; Sulak, K.; Puchalski, M.; Wrzosek, H.; Bilska, J. Effects of the artificial weathering of biodegradable spun-bonded PLA nonwovens in respect to their application in agriculture. Fibres Text. East. Eur. 2012, 96, 89–95. [Google Scholar]
- Pals, D.T.F.; Hermans, J.J. New method for deriving the intrinsic viscosity of polyelectrolytes. J. Polym. Sci. 1950, 5, 733–734. [Google Scholar] [CrossRef]
- Huggins, M.L. The Viscosity of Dilute Solutions of Long-Chain Molecules. IV. Dependence on Concentration. J. Am. Chem. Soc. 1942, 64, 2716–2718. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Wang, J.; Guo, R.; Zhang, Q. The effect of ionic strength on the viscosity of sodium alginate solution. Polym. Adv. Technol. 2001, 12, 740–745. [Google Scholar] [CrossRef]
- Rinaudo, M.; Graebling, D. On the viscosity of sodium alginates in the presence of external salt. Polym. Bull. 1986, 15, 235–236. [Google Scholar] [CrossRef]
- Rinaudo, M. On the abnormal exponents a? and aD in Mark Houwink type equations for wormlike chain polysaccharides. Polym. Bull. 1992, 27, 585–589. [Google Scholar] [CrossRef]
- Ghimici, L.; Nichifor, M.; Eich, A.; Wolf, B.A. Intrinsic viscosities of polyelectrolytes in the absence and in the presence of extra salt: Consequences of the stepwise conversion of dextran into a polycation. Carbohydr. Polym. 2011, 87, 405–410. [Google Scholar] [CrossRef]
- Lopez, C.G.; Richtering, W. Viscosity of Semidilute and Concentrated Nonentangled Flexible Polyelectrolytes in Salt-Free Solution. J. Phys. Chem. B 2019, 123, 5626–5634. [Google Scholar] [CrossRef] [PubMed]
- Grasdalen, H. High-field, 1H-n.m.r. spectroscopy of alginate: Sequential structure and linkage conformations. Carbohydr. Res. 1983, 118, 255–260. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E.m.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef] [Green Version]
- Storz, H.; Müller, K.J.; Ehrhart, F.; Gómez, I.; Shirley, S.G.; Gessner, P.; Zimmermann, G.; Weyand, E.; Sukhorukov, V.L.; Forst, T.; et al. Physicochemical features of ultra-high viscosity alginates. Carbohydr. Res. 2009, 344, 985–995. [Google Scholar] [CrossRef]
- Penman, A.; Sanderson, G.R. A method for the determination of uronic acid sequence in alginates. Carbohydr. Res. 1972, 25, 273–282. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Sakugawa, K.; Ikeda, A.; Takemura, A.; Ono, H. Simplified method for estimation of composition of alginates by FTIR. J. Appl. Polym. Sci. 2004, 93, 1372–1377. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Critchley, A.; van de Velde, F.; Ribeiro-Claro, P. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef] [Green Version]
- Beltrán, F.R.; Climent-Pascual, E.; de la Orden, M.U.; Urreaga, J.M. Effect of solid-state polymerization on the structure and properties of mechanically recycled poly(lactic acid). Polym. Degrad. Stab. 2019, 171, 109045. [Google Scholar] [CrossRef]
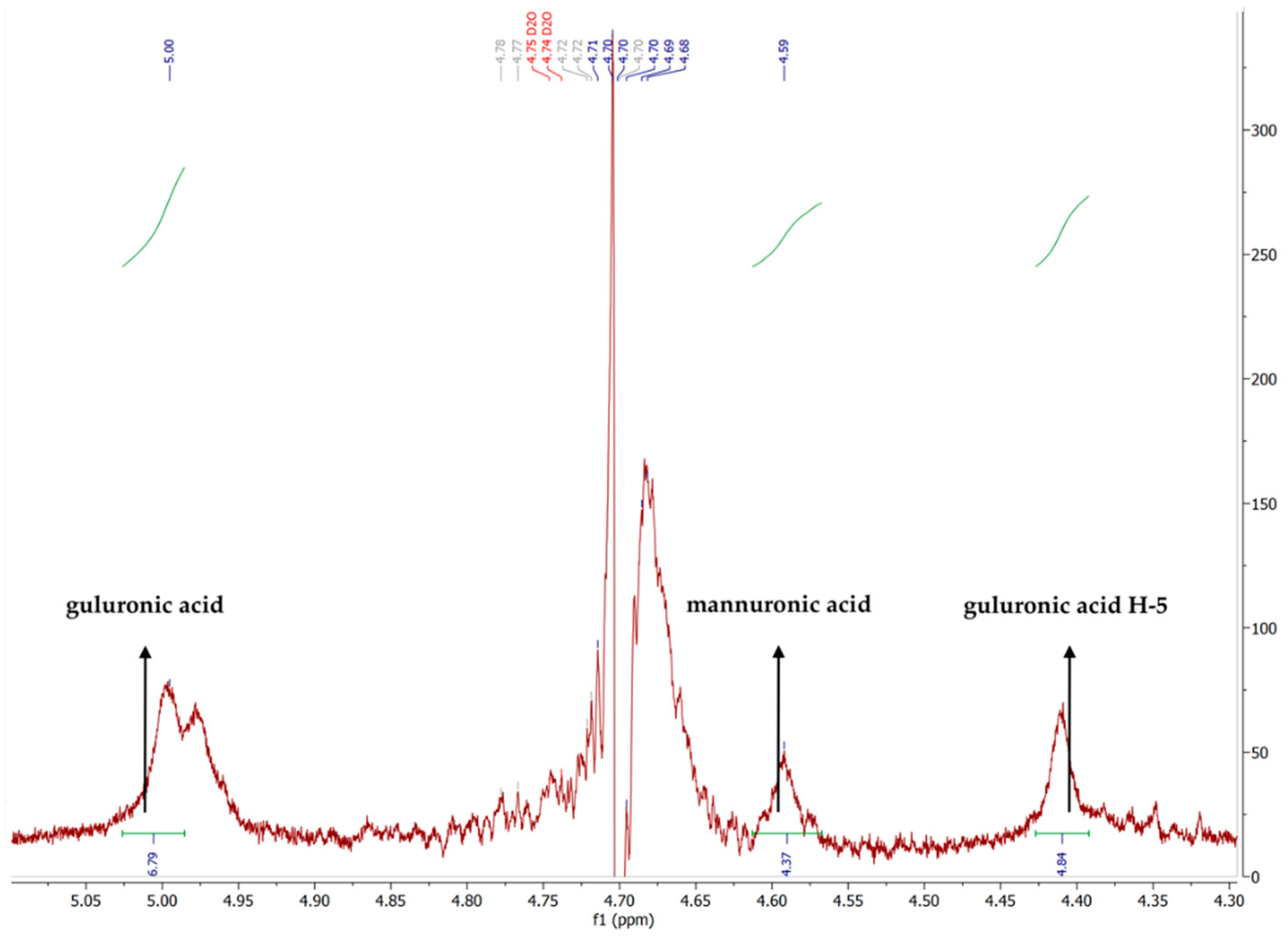
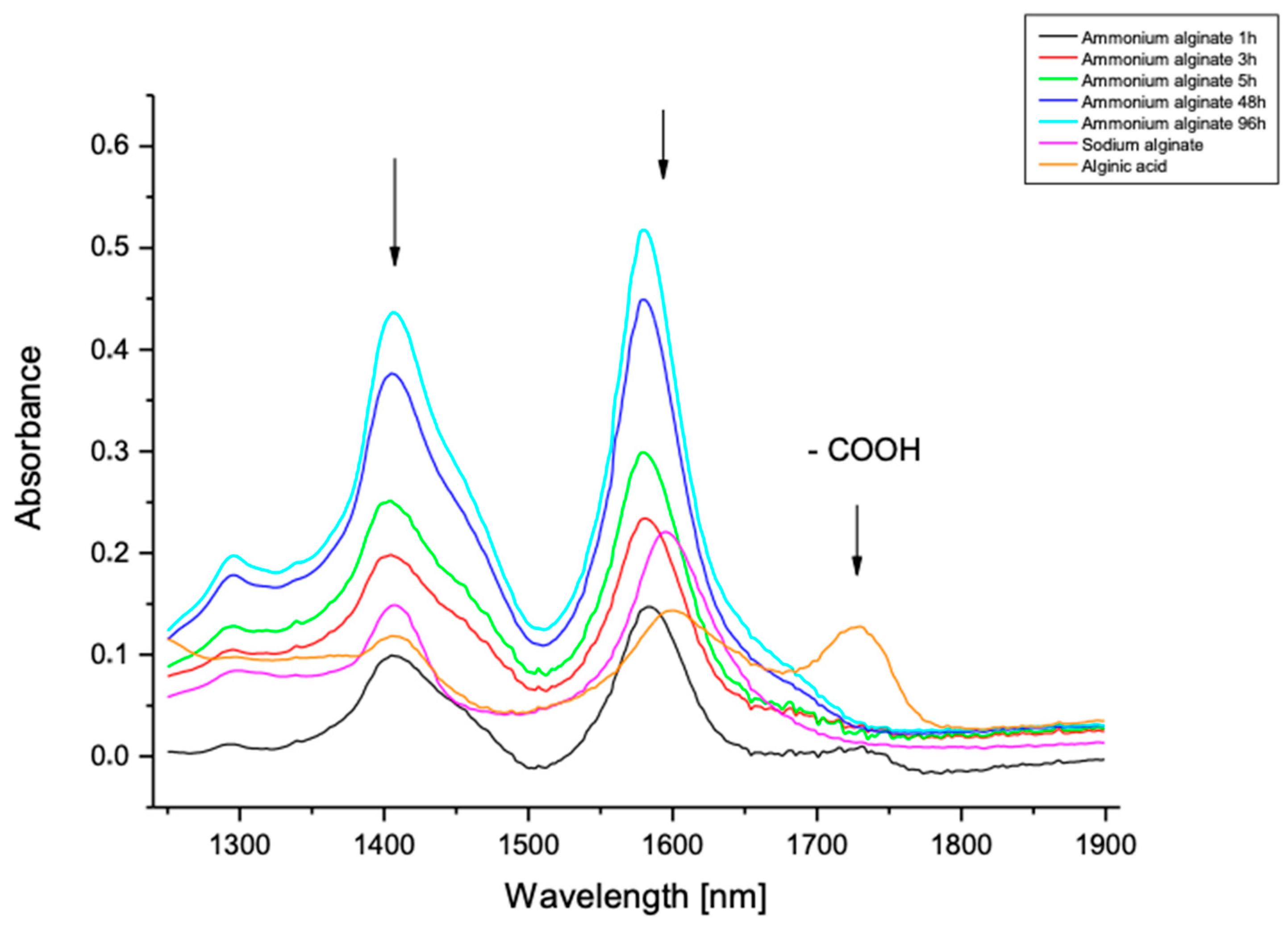
| FM | FG | M/G | FMM | FMG | FGM | FGG |
|---|---|---|---|---|---|---|
| 0.29 | 0.71 | 0.41 | 0.09 | 0.20 | 0.20 | 0.51 |
| Treatment Time (h) | Degree of Alginic Acid Substitution by -COONH4 (%) |
|---|---|
| 1 | 57.1 |
| 3 | 61.2 |
| 5 | 60.8 |
| 48 | 65.6 |
| 96 | 73.0 |
| 216 | 73.1 |
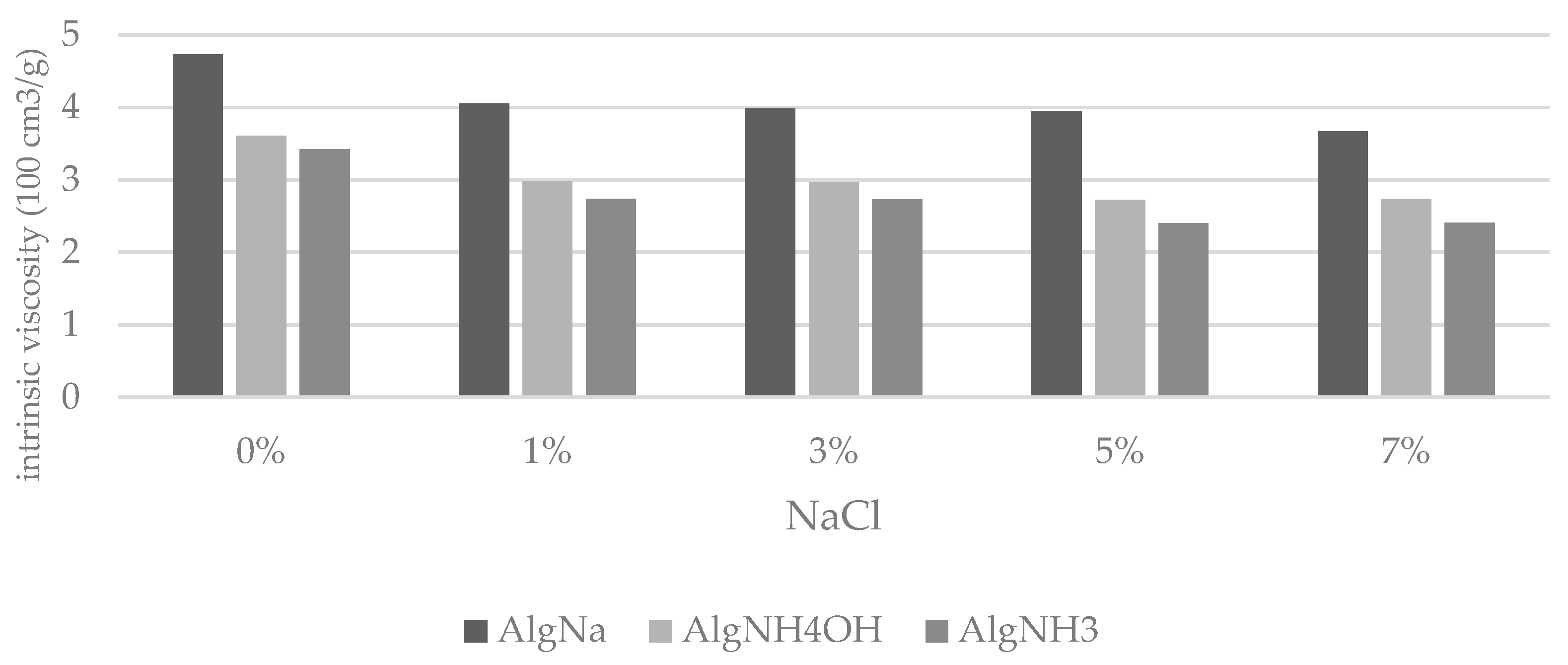
| Cs (mol/l) | ||||||
|---|---|---|---|---|---|---|
| 0 | 0.17 (1%) | 0.51 (3%) | 0.86 (5%) | 1.20 (7%) | ||
| Intrinsic Viscosity η (100 cm3/g) | ||||||
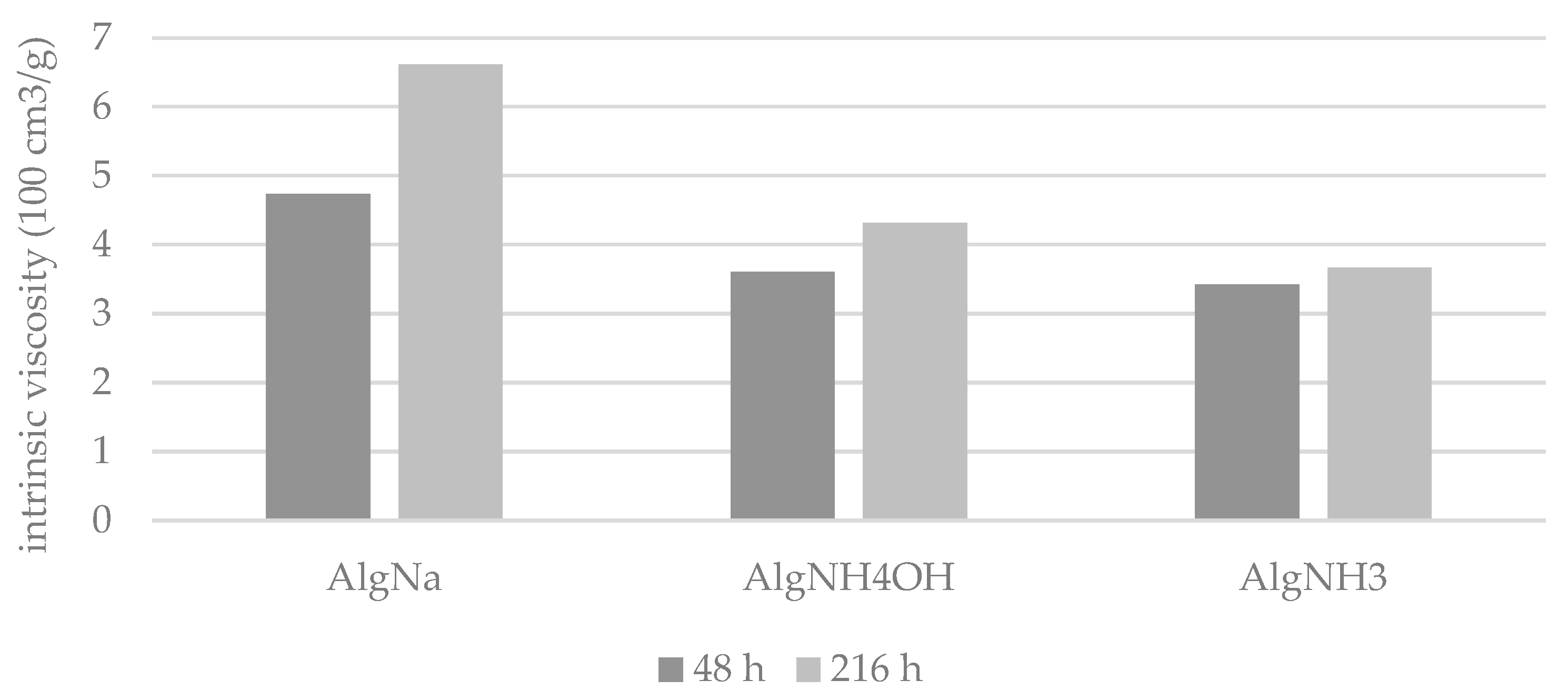
| AlgNa | 48 h | 4.7 ± 0.2 | 4.2 ± 0.1 | 4.1 ± 0.1 | 4.0 ± 0.2 | 3.7 ± 0.1 |
| 216 h | 6.6 ± 0.1 | 4.5 ± 0.1 | 4.1 ± 0.1 | 3.8 ± 0.2 | 3.5 ± 0.1 | |
| AlgNH4OH | 48 h | 3.6 ± 0.2 | 3.1 ± 0.2 | 3.0 ± 0.2 | 2.8 ± 0.1 | 2.8 ± 0.2 |
| 216 h | 4.3 ± 0.3 | 3.5 ± 0.2 | 3.3 ± 0.1 | 3.1 ± 0.1 | 2.9 ± 0.1 | |
| AlgHNH3 | 48 h | 3.4 ± 0.2 | 2.9 ± 0.2 | 2.8 ± 0.1 | 2.5 ± 0.2 | 2.5 ± 0.1 |
| 216 h | 3.7 ± 0.1 | 2.8 ± 0.1 | 2.7 ± 0.1 | 2.5 ± 0.1 | 2.3 ± 0.2 | |
| AlgNH4OH | AlgNH3 | AlgNa | |||||
|---|---|---|---|---|---|---|---|
| NaCl | Z-Ave (nm) | PDI | Z-Ave (nm) | PDI | Z-Ave (nm) | PDI | |
| 24 h | 0% | 421.3 | 0.569 | 248.1 | 0.484 | 558.4 | 0.633 |
| 1% | 787.1 | 0.775 | 330.2 | 0.525 | 281.6 | 0.722 | |
| 3% | 875.2 | 0.774 | 174.2 | 0.501 | 601.3 | 0.916 | |
| 5% | 1361 | 0.232 | 416.2 | 0.840 | 771.7 | 0.972 | |
| 7% | 1682 | 0.673 | 1014 | 0.751 | 849.3 | 0.923 | |
| 48 h | 0% | 298 | 0.562 | 239.3 | 0.501 | 621 | 0.650 |
| 1% | 417.2 | 0.575 | 173.4 | 0.531 | 231.5 | 0.946 | |
| 3% | 541.7 | 0.878 | 1684 | 0.929 | 547.2 | 0.874 | |
| 5% | 2006 | 0.305 | 2265 | 0.678 | 356.1 | 0.788 | |
| 7% | 2330 | 0.184 | 4489 | 0.578 | 529.5 | 0.774 | |
| 216 h | 0% | 244.2 | 0.540 | 260 | 0.490 | 602.1 | 0.829 |
| 1% | 504 | 0.607 | 127.3 | 0.701 | 203.2 | 1 | |
| 3% | 487.4 | 0.885 | 599.4 | 0.899 | 672.8 | 1 | |
| 5% | 896.1 | 0.944 | 4555 | 0.617 | 1345 | 1 | |
| 7% | 7063 | 0.342 | 5317 | 0.494 | 213.4 | 0.793 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarzynska, N.; Bednarowicz, A.; Pabjanczyk-Wlazlo, E.; Draczyński, Z. The Interphase Gas-Solid Synthesis of Ammonium Alginate—The Comparison of Two Synthesis Methods and the Effect of Low Molecular Weight Electrolyte Presence. Materials 2022, 15, 4321. https://doi.org/10.3390/ma15124321
Tarzynska N, Bednarowicz A, Pabjanczyk-Wlazlo E, Draczyński Z. The Interphase Gas-Solid Synthesis of Ammonium Alginate—The Comparison of Two Synthesis Methods and the Effect of Low Molecular Weight Electrolyte Presence. Materials. 2022; 15(12):4321. https://doi.org/10.3390/ma15124321
Chicago/Turabian StyleTarzynska, Nina, Anna Bednarowicz, Ewelina Pabjanczyk-Wlazlo, and Zbigniew Draczyński. 2022. "The Interphase Gas-Solid Synthesis of Ammonium Alginate—The Comparison of Two Synthesis Methods and the Effect of Low Molecular Weight Electrolyte Presence" Materials 15, no. 12: 4321. https://doi.org/10.3390/ma15124321
APA StyleTarzynska, N., Bednarowicz, A., Pabjanczyk-Wlazlo, E., & Draczyński, Z. (2022). The Interphase Gas-Solid Synthesis of Ammonium Alginate—The Comparison of Two Synthesis Methods and the Effect of Low Molecular Weight Electrolyte Presence. Materials, 15(12), 4321. https://doi.org/10.3390/ma15124321







