Quantitatively Unravelling the Impact of High Altitude on Oolong Tea Flavor from Camellia sinensis Grown on the Plateaus of Tibet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Reagents
2.2. Electronic Nose Measurements
2.3. Analysis of Volatile Compounds by GC-MS
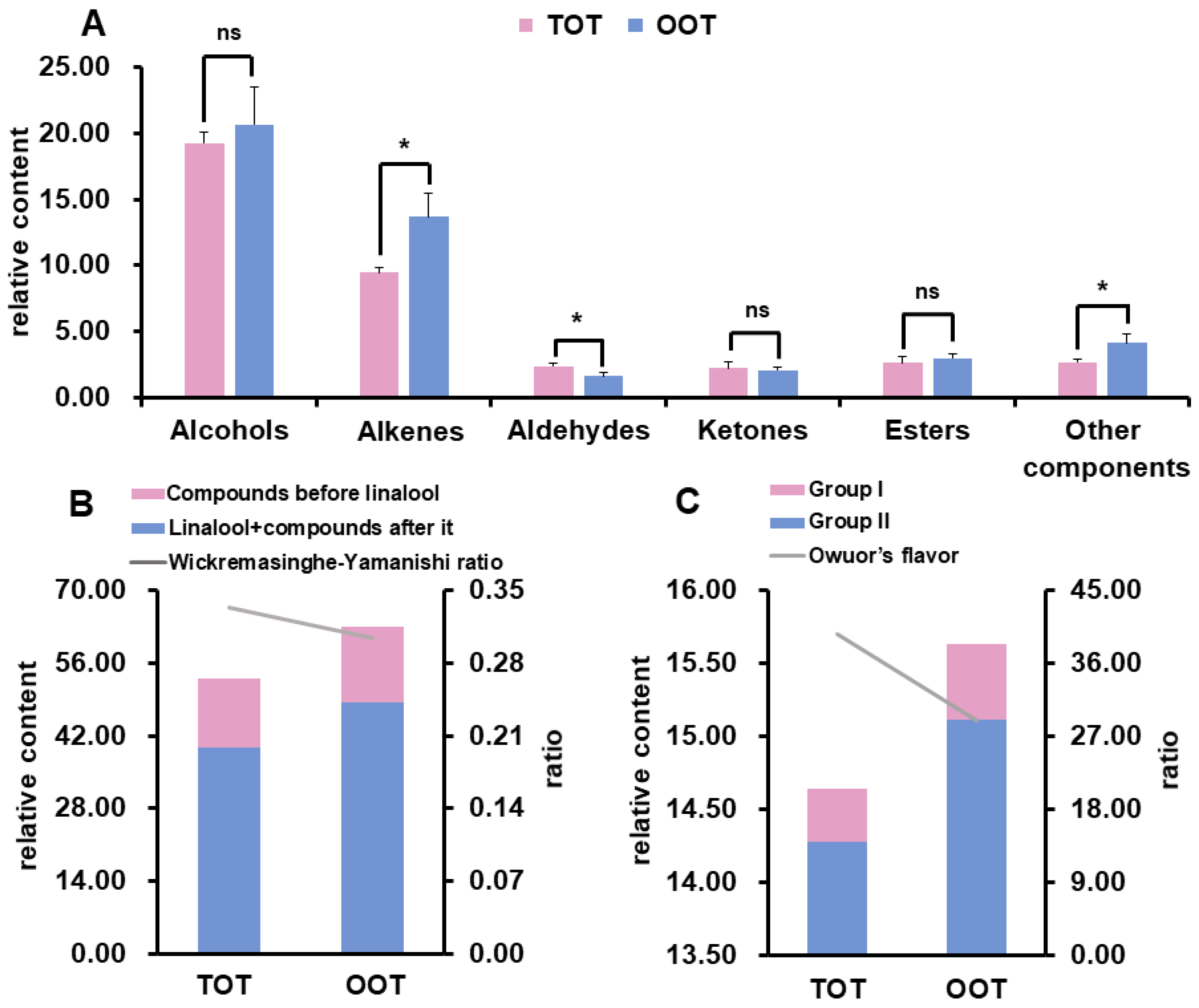
2.4. Calculation of Wickremasinghe-Yamanishi Ratio and Owuor’s Flavor Index
2.5. Determination of Water Extractable Components
2.6. Determination of Free Amino Acids
2.7. Quantification of Caffeine Contents
2.8. Determination of Tea Polyphenols
2.9. Quantification of Catechin Contents
2.10. Statistical Analysis
3. Results and Discussion
3.1. Aroma-Active Compounds Identified by an Electronic Nose
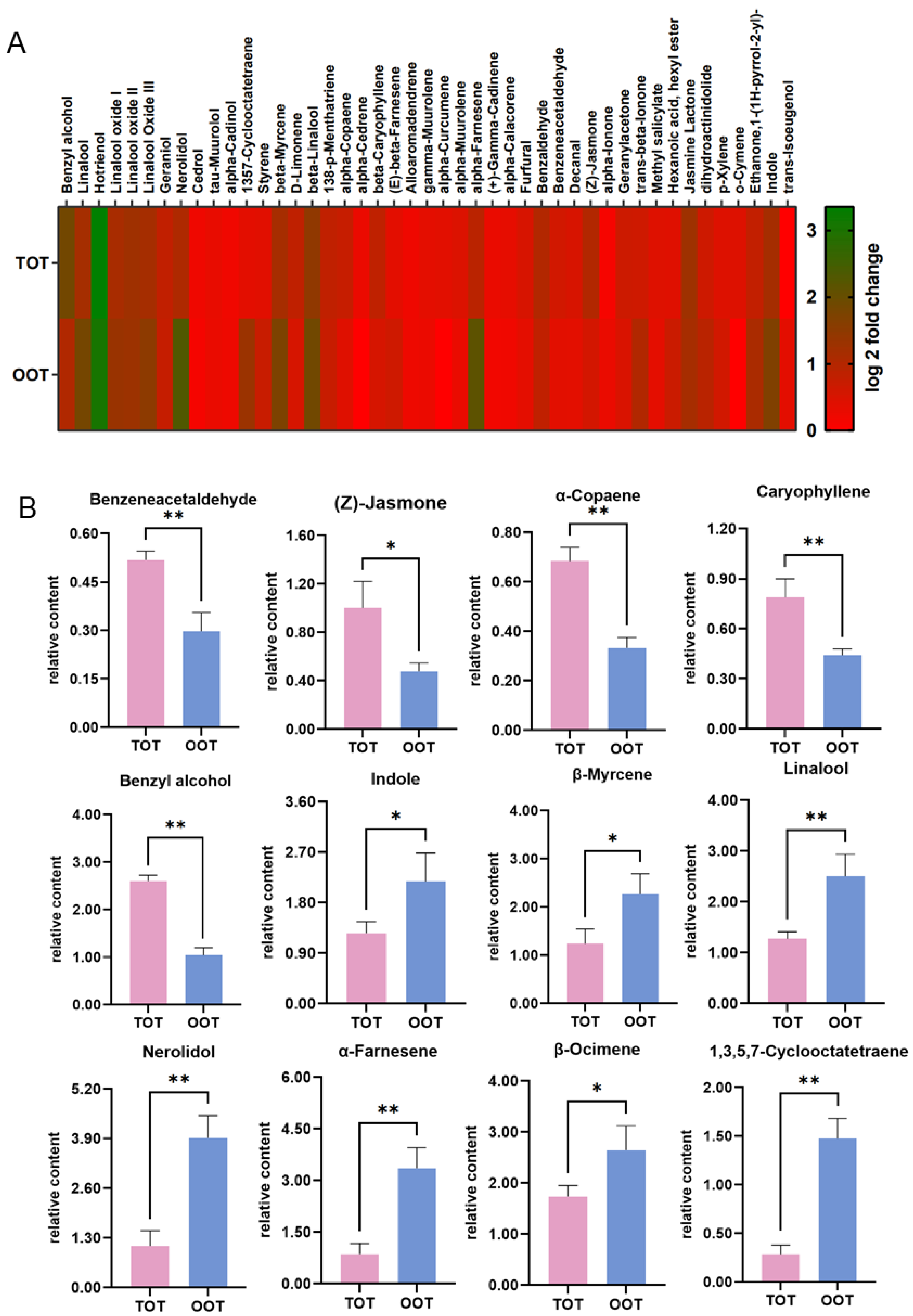
3.2. Volatile Organic Compounds Identified by GC-MS
3.3. Key Volatile Compounds Identified in OOT and TOT
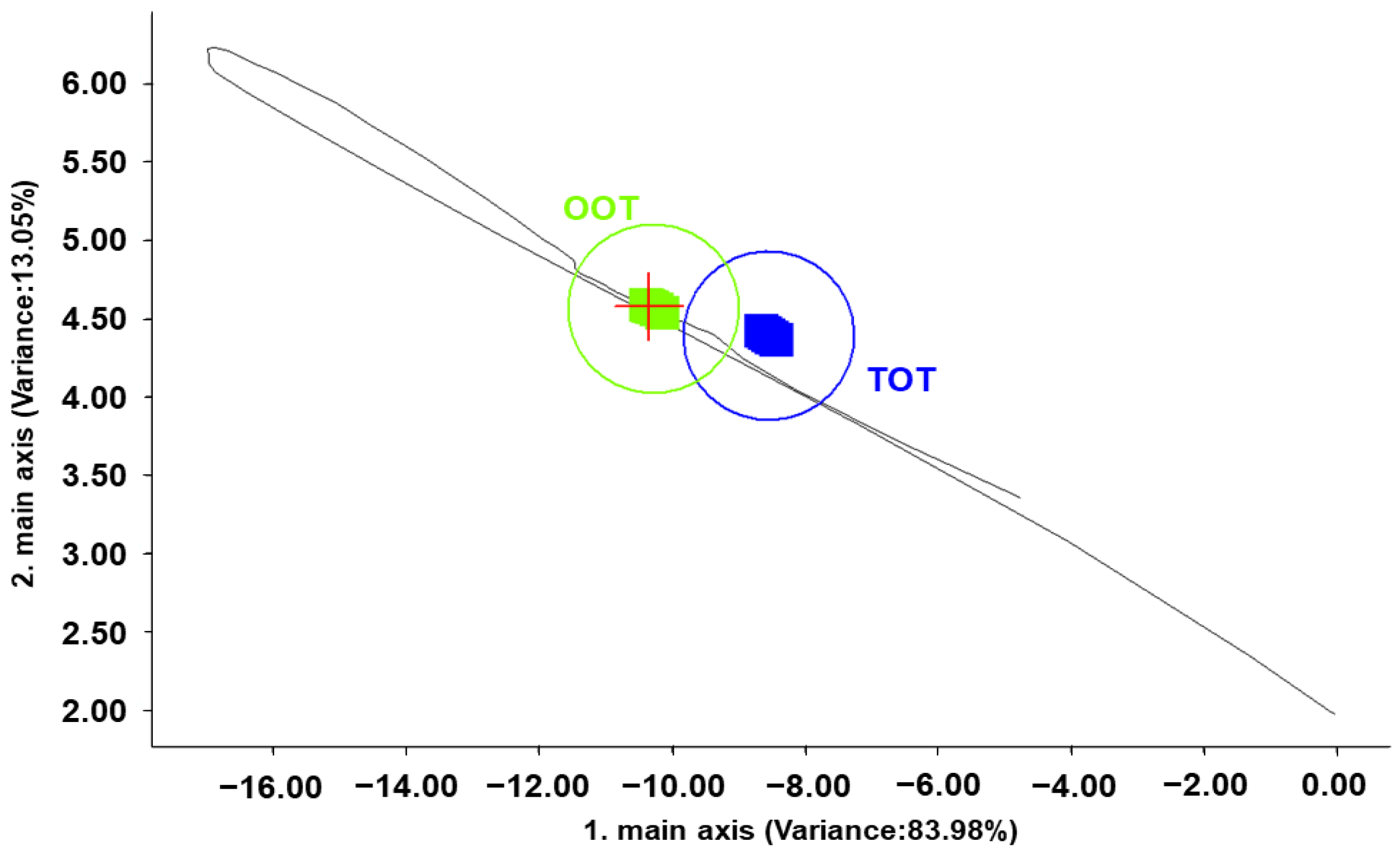
3.4. PCA of Volatile Compounds
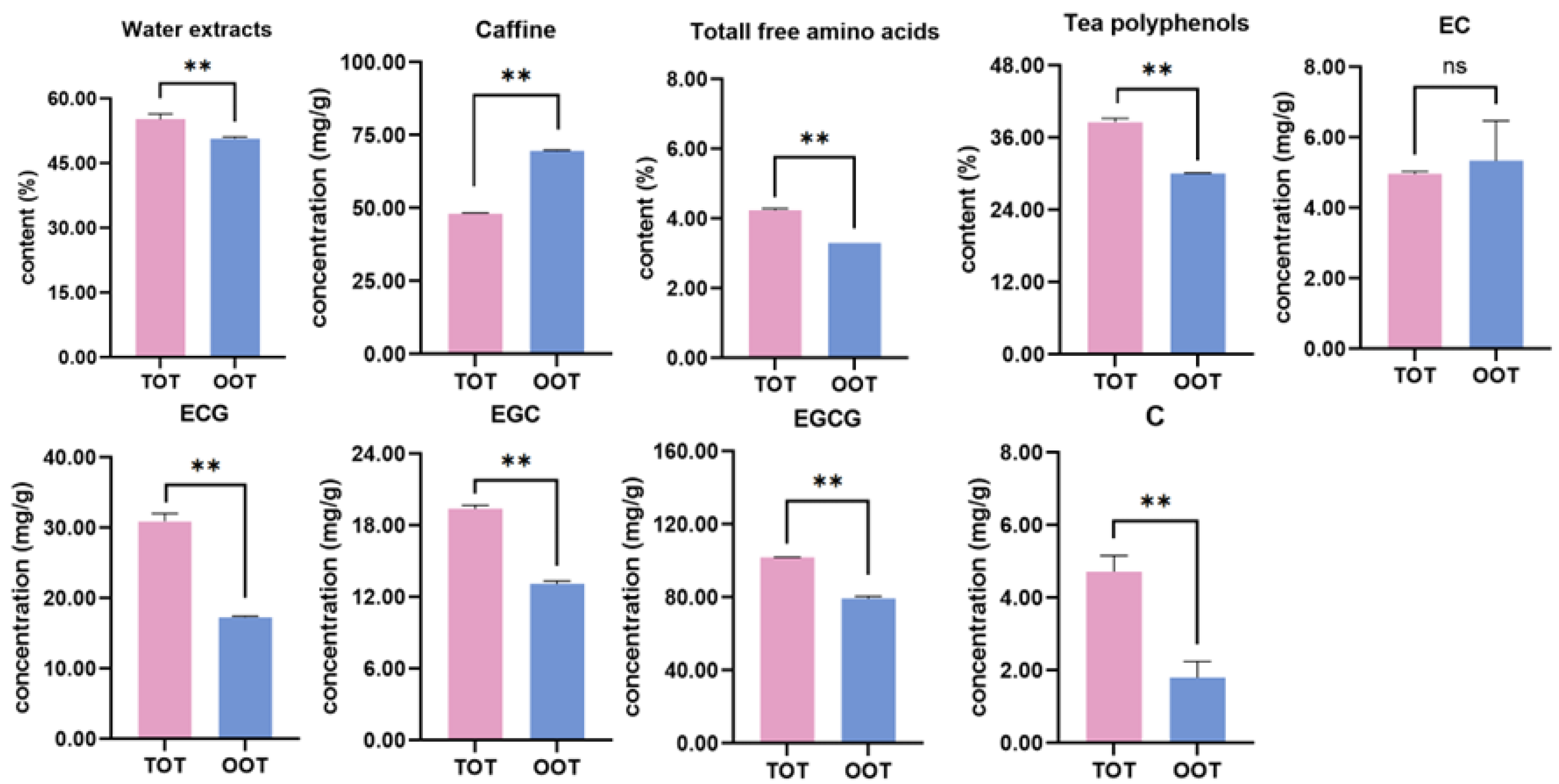
3.5. Taste Substances in TOT and OOT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deji, Q. Research on the Development of Agricultural Modernization in Tibet. South China Agric. 2021, 15, 25–26. [Google Scholar]
- Liao, Z.H.; Chen, Y.J.; Tzen, J.T.; Kuo, P.C.; Lee, M.R.; Mai, F.D.; Rairat, T.; Chou, C.C. Effect of teapot materials on the chemical composition of oolong tea infusions. J. Sci. Food Agric. 2018, 98, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Duan, J.; Jiang, Y.M.; Shi, J.; Peng, L.; Xue, S.; Kakuda, Y. Production, Quality, and Biological Effects of Oolong Tea (Camellia sinensis). Food Rev. Int. 2010, 27, 1–15. [Google Scholar] [CrossRef]
- Ning, J.; Li, D.; Luo, X.; Ding, D.; Song, Y.; Zhang, Z.; Wan, X. Stepwise Identification of Six Tea (Camellia sinensis (L.)) Categories Based on Catechins, Caffeine, and Theanine Contents Combined with Fisher Discriminant Analysis. Food Anal. Methods 2016, 9, 3242–3250. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, X.; Su, X.; Yang, Z. Chinese oolong tea: An aromatic beverage produced under multiple stresses. Trends Food Sci. Technol. 2020, 106, 242–253. [Google Scholar] [CrossRef]
- Hu, C.; Li, D.; Ma, Y.; Zhang, W.; Lin, C.; Zheng, X.; Liang, Y.; Lu, J. Formation mechanism of the oolong tea characteristic aroma during bruising and withering treatment. Food Chem. 2018, 269, 202–211. [Google Scholar] [CrossRef]
- Ye, X. Carefully irrigate the “hidden lotus” to open to the sun. Foshan Daily, 26 November 2021; T12. [Google Scholar]
- De Flaviis, R.; Mutarutwa, D.; Sacchetti, G.; Mastrocola, D. Quantitatively unravelling the effect of altitude of cultivation on the volatiles fingerprint of wheat by a chemometric approach. Food Chem. 2022, 370, 131296. [Google Scholar] [CrossRef]
- Alessandrini, M.; Gaiotti, F.; Belfiore, N.; Matarese, F.; D’Onofrio, C.; Tomasi, D. Influence of vineyard altitude on Glera grape ripening (Vitis vinifera L.): Effects on aroma evolution and wine sensory profile. J. Sci. Food Agric. 2017, 97, 2695–2705. [Google Scholar] [CrossRef]
- Bertrand, B.; Boulanger, R.; Dussert, S.; Ribeyre, F.; Berthiot, L.; Descroix, F.; Joeet, T. Climatic factors directly impact the volatile organic compound fingerprint in green Arabica coffee bean as well as coffee beverage quality. Food Chem. 2012, 135, 2575–2583. [Google Scholar] [CrossRef]
- Zou, G.; Wang, W.; Wang, M.; Xiao, Y.; Zhang, H. Quality inspection of Xinyang Maojian tea with electronic nose/tongue fusion technology. Food Sci. 2019, 40, 279–284. [Google Scholar]
- Qiao, D.; Mi, X.; An, Y.; Xie, H.; Cao, K.; Chen, H.; Chen, M.; Liu, S.; Chen, J.; Wei, C. Integrated metabolic phenotypes and gene expression profiles revealed the effect of spreading on aroma volatiles formation in postharvest leaves of green tea. Food Res. Int. 2021, 149, 110680. [Google Scholar] [CrossRef]
- Yamanishi, T.; Wickremasinghe, R.L.; Perera, K.P.W.C. Studies on flavour and quality of tea. 3. Gas chromatographic analysis of aroma complex. Tea Q. 1978, 39, 81–86. [Google Scholar]
- Owuor, P.O. Comparison of gas chromatographic volatile profiling methods for assessing the flavour quality of kenyan black teas. J. Sci. Food Agric. 1992, 59, 189–197. [Google Scholar] [CrossRef]
- GBT8305-2013; Tea-Determination of Water Extracts Content. China Standards Press: Beijing, China, 2013.
- GB/T 8314-2013; Determination of the Total Amount of Free Amino Acids in Tea. China Standards Press: Beijing, China, 2013.
- Mei, S.; Yu, Z.; Chen, J.; Zheng, P.; Sun, B.; Guo, J.; Liu, S. The Physiology of Postharvest Tea (Camellia sinensis) Leaves, According to Metabolic Phenotypes and Gene Expression Analysis. Molecules 2022, 27, 1708. [Google Scholar] [CrossRef]
- GB/T 8313-2018; A Method for Detecting the Content of Tea Polyphenols and Catechins in Tea Leaves. China Standards Press: Beijing, China, 2018.
- Li, J.; Liu, S.; Chen, P.; Cai, J.; Tang, S.; Yang, W.; Cao, F.; Zheng, P.; Sun, B. Systematic Analysis of the R2R3-MYB Family in Camellia sinensis: Evidence for Galloylated Catechins Biosynthesis Regulation. Front. Plant Sci. 2022, 12, 782220. [Google Scholar] [CrossRef] [PubMed]
- Baldermann, S.; Yang, Z.; Katsuno, T.; Tu, V.A.; Mase, N.; Nakamura, Y.; Watanabe, N. Discrimination of Green, Oolong, and Black Teas by GC-MS Analysis of Characteristic Volatile Flavor Compounds. Am. J. Anal. Chem. 2014, 5, 620–632. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Wang, J.; Ye, L. Classification and prediction of rice wines with different marked ages by using a voltammetric electronic tongue. Biosens. Bioelectron. 2011, 26, 4767–4773. [Google Scholar] [CrossRef]
- Vestergaard, J.S.; Martens, M.; Turkki, P. Application of an electronic nose system for prediction of sensory quality changes of a meat product (pizza topping) during storage. LWT-Food Sci. Technol. 2007, 40, 1095–1101. [Google Scholar] [CrossRef]
- Wan, X.; Xia, T. Tea Plant Secondary Metabolism; Science Press: Beijing, China, 2015; pp. 125–131. [Google Scholar]
- Lv, S.; Wu, Y.; Jiang, Y.; Meng, Q. Aroma characteristics and difference analysis of oolong tea in different production areas. Food Sci. 2014, 35, 146–153. [Google Scholar]
- Lu, D.; Chao, J.; Yin, F.; Liu, F.; Ke, Z.; Ye, H.; Huang, J.; Liu, Z. Basal analysis of aroma substances of Dancong Oolong tea. Food Sci. 2022, 43, 288–296. [Google Scholar]
- Xiao, Z.; Wang, H.; Niu, Y.; Liu, Q.; Zhu, J.; Chen, H.; Ma, N. Characterization of aroma compositions in different Chinese congou black teas using GC-MS and GC-O combined with partial least squares regression. Flavour Frag. J. 2017, 32, 265–276. [Google Scholar] [CrossRef]
- Katsuno, T.; Kasuga, H.; Kusano, Y.; Yaguchi, Y.; Tomomura, M.; Cui, J.; Yang, Z.; Baldermann, S.; Nakamura, Y.; Ohnishi, T.; et al. Characterisation of odorant compounds and their biochemical formation in green tea with a low temperature storage process. Food Chem. 2014, 148, 388–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, H.N. Green Tea Composition, Consumption, and Polyphenol Chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Guo, X.; Ho, C.; Schwab, W.; Wan, X. Effect of the roasting degree on flavor quality of large-leaf yellow tea. Food Chem. 2021, 347, 129016. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Lu, A.; Ouyang, K.; Meng, Q.; Tong, H. GC-MS combined with stoichiometry is used to explore the differences in aroma formation of the six major teas. Food Ferment. Ind. 2021, 47, 260–270. [Google Scholar]
- Shi, M.; Gong, S. Research progress on tea aroma. J. Tea 2012, 38, 19–23. [Google Scholar]
- Wang, K.; Liu, F.; Liu, Z.; Huang, J.; Xu, Z.; Li, Y.; Chen, J.; Gong, Y.; Yang, X. Analysis of chemical components in oolong tea in relation to perceived quality. Int. J. Food Sci. Technol. 2010, 45, 913–920. [Google Scholar] [CrossRef]
- Ma, C.; Li, J.; Chen, W.; Wang, W.; Qi, D.; Pang, S.; Miao, A. Study of the aroma formation and transformation during the manufacturing process of oolong tea by solid-phase micro-extraction and gas chromatography–mass spectrometry combined with chemometrics. Food Res. Int. 2018, 108, 413–422. [Google Scholar] [CrossRef]
- Shi, J.; Chen, D.; Huang, W.; Chen, Z.; Wu, H. Comparative analysis of aroma composition of Fenghuang Dancong oolong tea based on HS-SPME-GC-MS technology. Food Sci. 2016, 37, 111–117. [Google Scholar] [CrossRef]
- Tang, H.; Tang, J.; Cao, J.; Zhou, B.; Li, J.; Cai, J. Analysis of altitude interval differences in the quality of Fenghuang Dancong tea. Agric. Sci. Bull. 2015, 31, 143–151. [Google Scholar]
- Zhong, Q.; Lin, Z.; Chen, C.; Chen, Z.; You, X.; Chen, R. Effect of roasting temperature on the biochemical quality of Jiulongpao oolong tea. J. Tea Sci. 2014, 34, 9–20. [Google Scholar] [CrossRef]
- Ao, C.; Tang, D.; Gong, S.; Liu, L.; Gu, Z. Effects of different fresh leaf spreading treatments on aroma quality of summer and autumn tea. J. Tea Sci. 2010, 30, 384–392. [Google Scholar]
- Zhang, X.; Wang, M.; Han, B. Analysis of the similarities and differences in the aroma composition of Longjing tea in 3 different regions. J. Tea Sci. 2014, 34, 344–354. [Google Scholar]
- Liu, S. Manual of Synthetic Fragrance Technology; China Light Industry Press: Beijing, China, 2009. [Google Scholar]
- Wang, P.; Yu, J.; Jin, S.; Chen, S.; Yue, C.; Wang, W.; Gao, S.; Cao, H.; Zheng, Y.; Gu, M.; et al. Genetic basis of high aroma and stress tolerance in the oolong tea cultivar genome. Hortic Res.-Engl. 2021, 8, 107. [Google Scholar] [CrossRef]
- Liao, S.; Tong, H. Analysis of fatty acid and glycoside aroma precursors of different tea varieties. J. Southwest Univ. (Nat. Sci. Ed.) 2008, 8, 62–66. [Google Scholar]
- Li, Z.; Wang, J. Identification and similarity analysis of aroma substances in main types of Fenghuang Dancong tea. PLoS ONE 2020, 15, e244224. [Google Scholar] [CrossRef]
- Ruan, Y.; Wang, Y. The biochemical basis of green tea’s mellow, fresh and strong taste. J. Tea Commun. 1987, 1–4. [Google Scholar]
- Liang, M.; Xia, L.; Chen, L.; Fang, C.; Duan, Z.; Chen, J.; Yang, S.; Sun, R. Study on the Changes of Physical and Chemical Indexes During the Fermentation Process of Pu-erh Tea. Chin. Agric. Sci. Bull. 2006, 321–325. [Google Scholar]
- Fu, D.; Liu, Z.; Huang, J.; Hao, F.; Wang, F.; Lei, Y. Changes of main chemical components during processing of Fuzhuan tea. Food Sci. 2008, 64–67. [Google Scholar]

| No. | Sensor Name | Selectivity |
|---|---|---|
| 1 | W1C | Aromatic components (e.g., benzene) |
| 2 | W5S | Nitrogen oxides |
| 3 | W3C | Aromatic compounds, ammonia |
| 4 | W6S | Hydrides |
| 5 | W5C | Short chain alkane, aromatic compounds |
| 6 | W1S | Methane |
| 7 | W1W | Inorganic sulfides |
| 8 | W2S | Alcohols, aldehydes and ketones |
| 9 | W2W | Aromatic compounds, organic sulfides |
| 10 | W3S | Long chain alkanes |
| No. | Compounds | Relative Contents a | Aroma Description b | RI (Cal) c/ RI (Ref) d | I Method e | |
|---|---|---|---|---|---|---|
| TOT | OOT | |||||
| Alcohols | ||||||
| 1 | Benzyl alcohol | 2.60 ± 0.10 | 1.04 ± 0.13 | Sweet, floral, rose-like, caramel-like 1 | 1035/1034 | MS, RI |
| 2 | Linalool oxide I | 1.19 ± 0.12 | 1.52 ± 0.23 | Sweet, floral, creamy 1 | 1075/1078 | MS, RI |
| 3 | Linalool oxide II | 1.38 ± 0.15 | 1.46 ± 0.30 | Floral 2 | 1090/1094 | MS, RI |
| 4 | Linalool | 1.27 ± 0.12 | 2.51 ± 0.36 | Floral, sweet, grape-like, woody 1 | 1101/1101 | MS, RI |
| 5 | Hotrienol | 9.22 ± 0.79 | 7.26 ± 1.07 | Fresh, floral, fruity 1 | 1107/1107 | MS, RI |
| 6 | Linalool oxide III | 1.26 ± 0.14 | 1.82 ± 0.24 | Musty, wood, sweet, tea-like, citrus 1 | 1171/1178 | MS, RI |
| 7 | Geraniol | 0.75 ± 0.05 | 0.69 ± 0.09 | Rose-like, sweet, honey-like 1 | 1255/1255 | MS, RI |
| 8 | Nerolidol | 1.09 ± 0.33 | 3.92 ± 0.47 | Floral, apple, green 1 | 1567/1535 | MS, RI |
| 9 | Cedrol | 0.15 ± 0.08 | 0.09 ± 0.02 | Wood, floral 1 | 1609/1593 | MS, RI |
| 10 | τ-Muurolol | 0.23 ± 0.08 | 0.21 ± 0.00 | Herb, weak spice 2 | 1648/1640 | MS, RI |
| 11 | α-Cadinol | 0.14 ± 0.05 | 0.16 ± 0.01 | Herb, wood 2 | 1660/1663 | MS, RI |
| Total alcohols | 19.28 ± 0.83 | 20.68 ± 2.88 | ||||
| Alkene | ||||||
| 1 | 1,3,5,7-Cyclooctatetraene | 0.28 ± 0.08 | 1.48 ± 0.17 | Solvent-like 1 | -/- | MS |
| 2 | Styrene | 0.31 ± 0.06 | 0.65 ± 0.11 | Balsamic, gasoline 2 | 913/898 | MS, RI |
| 3 | β-Myrcene | 1.24 ± 0.25 | 2.28 ± 0.34 | Wood, resinous, musty 1 | 992/988 | MS, RI |
| 4 | D-Limonene | 0.78 ± 0.16 | 0.45 ± 0.03 | Citrus, lemon, orange-like, green 1 | 1029/1030 | MS, RI |
| 5 | β-Ocimene | 1.74 ± 0.18 | 2.64 ± 0.39 | Warm, floral, herbal, sweet 1 | 1049/1046 | MS, RI |
| 6 | 1,3,8-p-Menthatriene | 0.77 ± 0.03 | 0.66 ± 0.09 | Turpentine 2 | 1131/1119 | MS, RI |
| 7 | α-Copaene | 0.68 ± 0.05 | 0.33 ± 0.03 | Wood, spice 2 | 1379/1367 | MS, RI |
| 8 | α-Cedrene | 0.19 ± 0.02 | - | Wood, spice 4 | 1418/1408 | MS, RI |
| 9 | β-Caryophyllene | 0.79 ± 0.09 | 0.44 ± 0.03 | Fried, Spice, wood 3 | 1424/1417 | MS, RI |
| 10 | (E)-β-Farnesene | 0.28 ± 0.08 | 0.56 ± 0.07 | Wood, citrus, sweet 2 | 1458/1443 | MS, RI |
| 11 | Alloaromadendrene | 0.15 ± 0.06 | 0.16 ± 0.01 | Wood 2 | 1466/1457 | MS, RI |
| 12 | γ-Muurolene | 0.27 ± 0.06 | 0.19 ± 0.01 | Herb, wood, spice 2 | 1481/1474 | MS, RI |
| 13 | α-Curcumene | 0.30 ± 0.06 | - | Herbal 2 | 1486/1481 | MS, RI |
| 14 | α-Muurolene | 0.37 ± 0.09 | 0.20 ± 0.01 | Wood 1 | 1504/1497 | MS, RI |
| 15 | α-Farnesene | 0.86 ± 0.25 | 3.36 ± 0.49 | Wood, green, floral, herbal 1 | 1511/1507 | MS, RI |
| 16 | γ-Cadinene | 0.20 ± 0.05 | 0.12 ± 0.00 | Wood 2 | 1519/1511 | MS, RI |
| 17 | α-Calacorene | 0.25 ± 0.06 | 0.14 ± 0.00 | Wood 1 | 1548/1542 | MS, RI |
| Total alkene | 9.46 ± 0.39 | 13.66 ± 1.76 | ||||
| Aldehydes | ||||||
| 1 | Furfural | 0.43 ± 0.00 | 0.27 ± 0.06 | Sweet, bready, caramel-like 1 | -/835 | MS |
| 2 | Benzaldehyde | 1.00 ± 0.06 | 0.77 ± 0.13 | Almond-like, fruity, cherry-like, nutty 1 | 963/961 | MS, RI |
| 3 | Benzene acetaldehyde | 0.52 ± 0.02 | 0.30 ± 0.05 | Floral, rose, cherry-like 1 | 1044/1043 | MS, RI |
| 4 | Decanal | 0.43 ± 0.11 | 0.29 ± 0.02 | Fatty, floral, green, orange peel 1 | 1206/1206 | MS, RI |
| Total aldehydes | 2.38 ± 0.19 | 1.63 ± 0.25 | ||||
| Ketones | ||||||
| 1 | (Z)-Jasmone | 1.00 ± 0.18 | 0.48 ± 0.06 | Sweet, flower 4 | 1402/1396 | MS, RI |
| 2 | α-Ionone | 0.08 ± 0.02 | 0.20 ± 0.01 | Floral, violet-like, powdery, berry-like 1 | 1400/1421 | MS, RI |
| 3 | Geranylacetone | 0.59 ± 0.14 | 0.41 ± 0.04 | Magnolia, green 2 | 1454/1452 | MS, RI |
| 4 | (E)-β-Ionone | 0.55 ± 0.14 | 1.02 ± 0.13 | Seaweed, violet, flower, raspberry 2 | 1489/1469 | MS, RI |
| Total ketones | 2.22 ± 0.48 | 2.11 ± 0.23 | ||||
| Esters | ||||||
| 1 | Methyl salicylate | 0.34 ± 0.04 | 0.27 ± 0.03 | Fresh, floral, fruity 1 | 1196/1187 | MS, RI |
| 2 | Hexanoic acid, hexyl ester | 0.33 ± 0.05 | 0.62 ± 0.08 | Fruity, brandy, ether 4 | 1387/1385 | MS, RI |
| 3 | Jasmine Lactone | 1.41 ± 0.28 | 1.24 ± 0.16 | Fruit 3 | 1486/- | MS |
| 4 | dihydroactinidiolide | 0.57 ± 0.15 | 0.88 ± 0.07 | Wood, fruit 4 | 1536/1525 | MS, RI |
| Total esters | 2.65 ± 0.52 | 3.01 ± 0.33 | ||||
| Other components | ||||||
| 1 | p-Xylene | 0.36 ± 0.02 | 0.52 ± 0.03 | Plastic, green, pungent 1 | -/870 | MS, RI |
| 2 | o-Cymene | 0.36 ± 0.06 | - | Aromatic 1 | 1025/1021 | MS, RI |
| 3 | Ethanone,1-(1H-pyrrol-2-yl)- | 0.74 ± 0.02 | 1.17 ± 0.20 | Toast 4 | 1064/1063 | MS, RI |
| 4 | Indole | 1.26 ± 0.17 | 2.18 ± 0.41 | Floral, animal-like 1 | 1296/1293 | MS, RI |
| 5 | (E)-Isoeugenol | - | 0.28 ± 0.05 | Flower 2 | 1452/1453 | MS, RI |
| Total others | 2.72 ± 0.20 | 4.15 ± 0.70 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Cai, J.; Zheng, P.; Yuan, Y.; Tsewang, W.; Chen, Y.; Xiao, X.; Liao, J.; Sun, B.; Liu, S. Quantitatively Unravelling the Impact of High Altitude on Oolong Tea Flavor from Camellia sinensis Grown on the Plateaus of Tibet. Horticulturae 2022, 8, 539. https://doi.org/10.3390/horticulturae8060539
Chen P, Cai J, Zheng P, Yuan Y, Tsewang W, Chen Y, Xiao X, Liao J, Sun B, Liu S. Quantitatively Unravelling the Impact of High Altitude on Oolong Tea Flavor from Camellia sinensis Grown on the Plateaus of Tibet. Horticulturae. 2022; 8(6):539. https://doi.org/10.3390/horticulturae8060539
Chicago/Turabian StyleChen, Peifen, Jiarong Cai, Peng Zheng, Yugui Yuan, Wugyan Tsewang, Yuchun Chen, Xi Xiao, Jinmei Liao, Binmei Sun, and Shaoqun Liu. 2022. "Quantitatively Unravelling the Impact of High Altitude on Oolong Tea Flavor from Camellia sinensis Grown on the Plateaus of Tibet" Horticulturae 8, no. 6: 539. https://doi.org/10.3390/horticulturae8060539
APA StyleChen, P., Cai, J., Zheng, P., Yuan, Y., Tsewang, W., Chen, Y., Xiao, X., Liao, J., Sun, B., & Liu, S. (2022). Quantitatively Unravelling the Impact of High Altitude on Oolong Tea Flavor from Camellia sinensis Grown on the Plateaus of Tibet. Horticulturae, 8(6), 539. https://doi.org/10.3390/horticulturae8060539





