Manual 3D Control of an Assistive Robotic Manipulator Using Alpha Rhythms and an Auditory Menu: A Proof-of-Concept
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
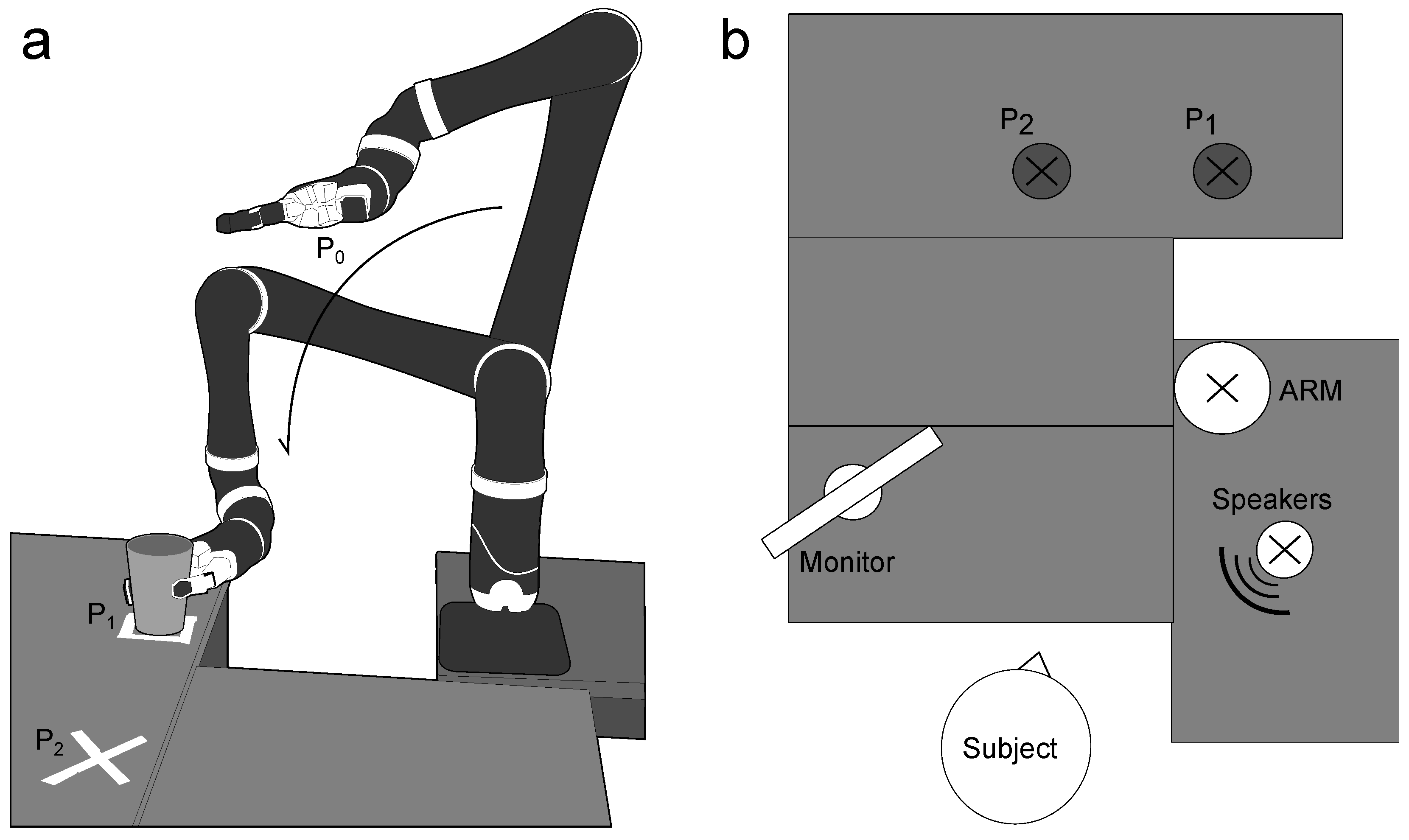
2.2. Data Acquisition
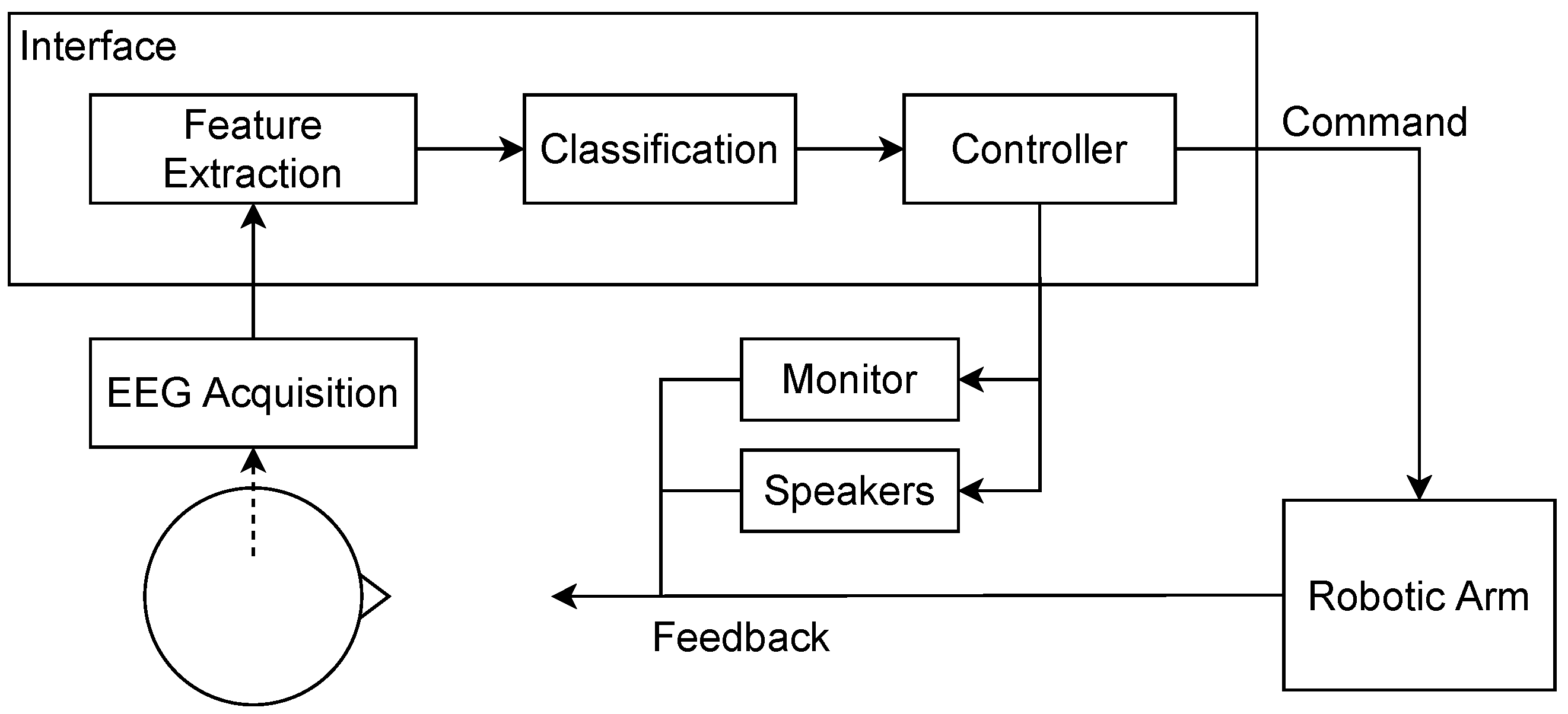
2.3. System Implementation
2.3.1. Signal Preprocessing
2.3.2. Feature Extraction
2.3.3. Classification
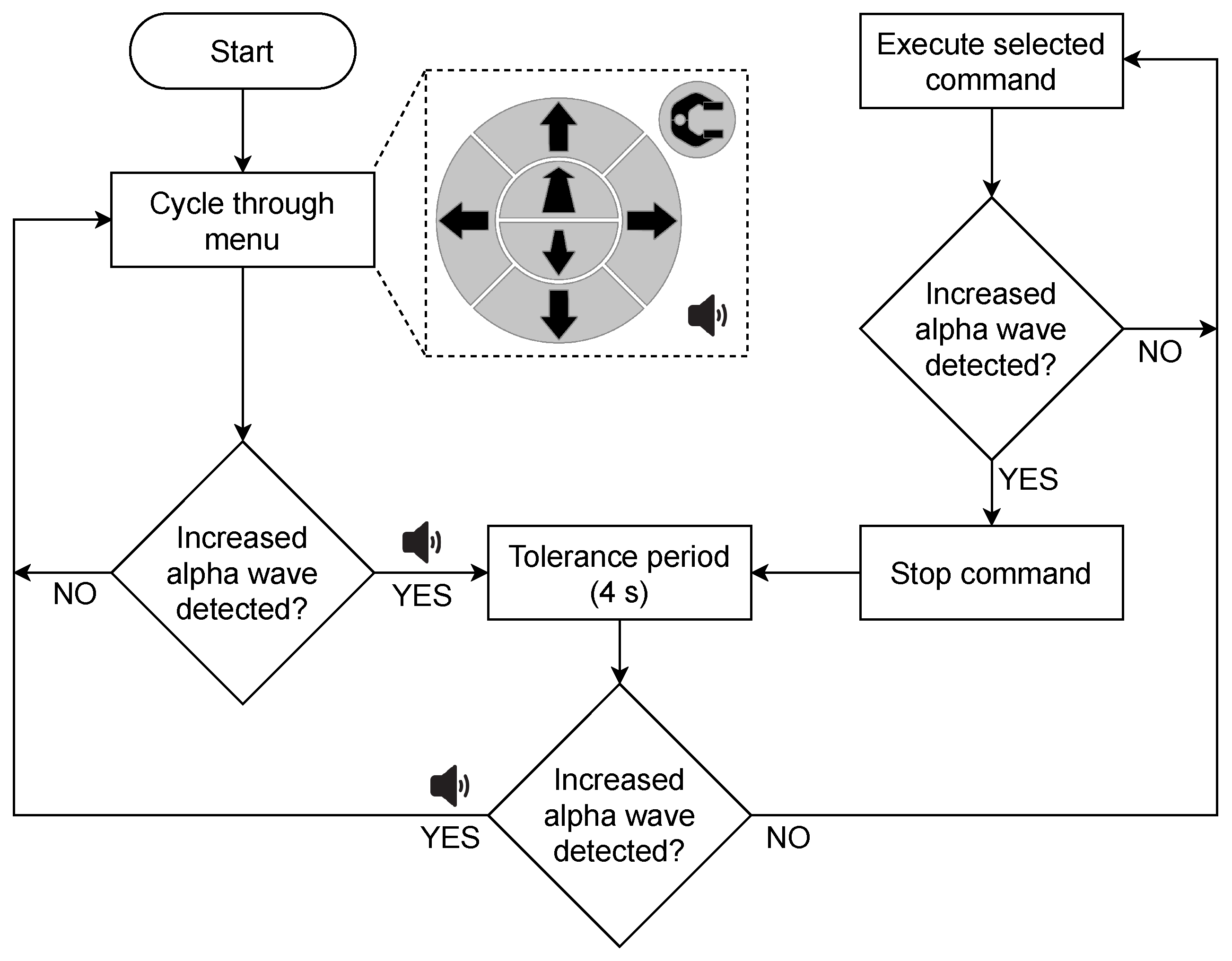
2.3.4. Online Control Loop
2.4. Experimental Procedure
2.4.1. Training Session
2.4.2. Testing Sessions
2.5. Performance Metrics
3. Results
4. Discussion
4.1. Task Performance
4.2. System Improvements
4.3. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCI | Brain-computer interface |
| ALS | Amyotrophic lateral sclerosis |
| AT | Assistive technologies |
| EEG | Electroencephalography |
| SSVEP | Steady-state visual evoked potentials |
| MI | Motor Imagery |
| ARM | Assistive robotic manipulator |
| EOG | Electrooculography |
References
- Abraham, A.; Drory, V.E. Fatigue in motor neuron diseases. Neuromuscul. Disord. 2012, 22, S198–S202. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.; Piemonte, M.E.P.; Callegaro, D.; Da Silva, H.C.A. Fatigue in amyotrophic lateral sclerosis: Frequency and associated factors. Amyotroph. Lateral Scler. Off. Publ. World Fed. Neurol. Res. Group Mot. Neuron Dis. 2008, 9, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Silva-Moraes, M.H.; Bispo-Torres, A.C.; Barouh, J.L.; Lucena, P.H.; Armani-Franceschi, G.; Dórea-Bandeira, I.; Vieira, F.; Miranda-Scippa, Â.; Quarantini, L.C.; Lucena, R.; et al. Suicidal behavior in individuals with Amyotrophic Lateral Sclerosis: A systematic review. J. Affect. Disord. 2020, 277, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Paganoni, S.; McDonnell, E.; Schoenfeld, D.; Yu, H.; Deng, J.; Atassi, H.; Sherman, A.; Yerramilli-Rao, P.; Cudkowicz, M.; Atassi, N. Functional Decline is Associated with Hopelessness in Amyotrophic Lateral Sclerosis (ALS). J. Neurol. Neurophysiol. 2017, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Kübler, A. The history of BCI: From a vision for the future to real support for personhood in people with locked-in syndrome. Neuroethics 2020, 13, 163–180. [Google Scholar] [CrossRef]
- Eicher, C.; Kiselev, J.; Brukamp, K.; Kiemel, D.; Spittel, S.; Maier, A.; Meyer, T.; Oleimeulen, U.; Greuèl, M. Experiences with assistive technologies and devices (ATD) in patients with amyotrophic lateral sclerosis (ALS) and their caregivers. Technol. Disabil. 2019, 31, 203–215. [Google Scholar] [CrossRef]
- Ward, A.L.; Hammond, S.; Holsten, S.; Bravver, E.; Brooks, B.R. Power Wheelchair Use in Persons with Amyotrophic Lateral Sclerosis: Changes Over Time. Assist. Technol. 2015, 27, 238–245. [Google Scholar] [CrossRef]
- Basha, S.G.; Venkatesan, M. Design of joystick controlled electrical wheelchair. J. Adv. Res. Dyn. Control Syst. 2018, 10, 1990–1994. [Google Scholar]
- Zhang, H.; Agrawal, S.K. An Active Neck Brace Controlled by a Joystick to Assist Head Motion. IEEE Robot. Autom. Lett. 2018, 3, 37–43. [Google Scholar] [CrossRef]
- Andreasen Struijk, L.N.S.; Egsgaard, L.L.; Lontis, R.; Gaihede, M.; Bentsen, B. Wireless intraoral tongue control of an assistive robotic arm for individuals with tetraplegia. J. NeuroEng. Rehabil. 2017, 14, 110. [Google Scholar] [CrossRef] [Green Version]
- Andreasen Struijk, L.N.S.; Lontis, E.R.; Gaihede, M.; Caltenco, H.A.; Lund, M.E.; Schioeler, H.; Bentsen, B. Development and functional demonstration of a wireless intraoral inductive tongue computer interface for severely disabled persons. Disabil. Rehabil. Assist. Technol. 2017, 12, 631–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotariu, C.; Costin, H.; Bozomitu, R.G.; Petroiu-Andruseac, G.; Ursache, T.I.; Doina Cojocaru, C. New assistive technology for communicating with disabled people based on gaze interaction. In Proceedings of the 2019 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 21–23 November 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Saha, D.; Sayyed, A.Q.M.S.; Saif, A.F.M.; Shahnaz, C.; Fattah, S.A. Eye Gaze Controlled Immersive Video Navigation System for Disabled People. In Proceedings of the 2019 IEEE R10 Humanitarian Technology Conference (R10-HTC)(47129), Depok, Indonesia, 12–14 November 2019; pp. 30–35. [Google Scholar] [CrossRef]
- Shih, J.J.; Krusienski, D.J.; Wolpaw, J.R. Brain-computer interfaces in medicine. Mayo Clin. Proc. 2012, 87, 268–279. [Google Scholar] [CrossRef] [Green Version]
- Millán, J.D.R.; Rupp, R.; Mueller-Putz, G.; Murray-Smith, R.; Giugliemma, C.; Tangermann, M.; Vidaurre, C.; Cincotti, F.; Kubler, A.; Leeb, R.; et al. Combining Brain–Computer Interfaces and Assistive Technologies: State-of-the-Art and Challenges. Front. Neurosci. 2010, 4, 161. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, U.; Birbaumer, N.; Ramos-Murguialday, A. Brain–Computer interfaces for communication and rehabilitation. Nat. Rev. Neurol. 2016, 12, 513–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas-Alonso, L.F.; Gomez-Gil, J. Brain computer interfaces, a review. Sensors 2012, 12, 1211–1279. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Jo, S. A Low-Cost EEG System-Based Hybrid Brain-Computer Interface for Humanoid Robot Navigation and Recognition. PLoS ONE 2013, 8, e74583. [Google Scholar] [CrossRef]
- Spataro, R.; Chella, A.; Allison, B.; Giardina, M.; Sorbello, R.; Tramonte, S.; Guger, C.; La Bella, V. Reaching and grasping a glass of water by locked-In ALS patients through a BCI-controlled humanoid robot. Front. Hum. Neurosci. 2017, 11, 68. [Google Scholar] [CrossRef] [Green Version]
- Leeb, R.; Perdikis, S.; Tonin, L.; Biasiucci, A.; Tavella, M.; Creatura, M.; Molina, A.; Al-Khodairy, A.; Carlson, T.; Millan, J.D. Transferring brain-computer interfaces beyond the laboratory: Successful application control for motor-disabled users. Artif. Intell. Med. 2013, 59, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, A.M.; Herrmann, C.S. Frequency-modulated steady-state visual evoked potentials: A new stimulation method for brain–computer interfaces. J. Neurosci. Methods 2015, 241, 1–9. [Google Scholar] [CrossRef]
- Stawicki, P.; Gembler, F.; Rezeika, A.; Volosyak, I. A Novel Hybrid Mental Spelling Application Based on Eye Tracking and SSVEP-Based BCI. Brain Sci. 2017, 7, 35. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Lu, J.; Li, P. A Hybrid BCI Based on SSVEP and EOG for Robotic Arm Control. Front. Neurorobot. 2020, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Wan, F.; Wong, C.M.; da Cruz, J.N.; Hu, Y. Objective evaluation of fatigue by EEG spectral analysis in steady-state visual evoked potential-based brain-computer interfaces. Biomed. Eng. Online 2014, 13, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padfield, N.; Zabalza, J.; Zhao, H.; Masero, V.; Ren, J. EEG-Based Brain-Computer Interfaces Using Motor-Imagery: Techniques and Challenges. Sensors 2019, 19, 1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherer, R.; Vidaurre, C. Chapter 8—Motor imagery based brain–computer interfaces. In Smart Wheelchairs and Brain-Computer Interfaces; Diez, P., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 171–195. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, Y.; Wu, C.; Song, A.; Liu, J.; Ji, P.; Xu, B.; Zhu, L.; Li, H.; Wen, P. Closed-Loop Hybrid Gaze Brain-Machine Interface Based Robotic Arm Control with Augmented Reality Feedback. Front. Neurorobot. 2017, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousseta, R.; El Ouakouak, I.; Gharbi, M.; Regragui, F. EEG Based Brain Computer Interface for Controlling a Robot Arm Movement through Thought. IRBM 2018, 39, 129–135. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, C.; Shu, X.; Gui, K.; Bezsudnova, Y.; Sheng, X.; Zhang, D. Shared control of a robotic arm using non-invasive brain–computer interface and computer vision guidance. Robot. Auton. Syst. 2019, 115, 121–129. [Google Scholar] [CrossRef]
- Xu, R.; Dosen, S.; Jiang, N.; Yao, L.; Farooq, A.; Jochumsen, M.; Mrachacz-Kersting, N.; Dremstrup, K.; Farina, D. Continuous 2D control via state-machine triggered by endogenous sensory discrimination and a fast brain switch. J. Neural Eng. 2019, 16, 056001. [Google Scholar] [CrossRef]
- Ron-Angevin, R.; Velasco-Álvarez, F.; Fernández-Rodríguez, A.; Díaz-Estrella, A.; Blanca-Mena, M.J.; Vizcaíno-Martín, F.J. Brain-Computer Interface application: Auditory serial interface to control a two-class motor-imagery-based wheelchair. J. NeuroEng. Rehabil. 2017, 14, 49. [Google Scholar] [CrossRef]
- Garrison, H.; McCullough, A.; Yu, Y.C.; Gabel, L.A. Feasibility study of EEG signals for asynchronous BCI system applications. In Proceedings of the 2015 41st Annual Northeast Biomedical Engineering Conference (NEBEC), Troy, NY, USA, 17–19 April 2015; pp. 1–2. [Google Scholar] [CrossRef]
- Geller, A.S.; Burke, J.F.; Sperling, M.R.; Sharan, A.D.; Litt, B.; Baltuch, G.H.; Lucas, T.H.; Kahana, M.J. Eye closure causes widespread low-frequency power increase and focal gamma attenuation in the human electrocorticogram. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2014, 125, 1764–1773. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wu, X.; Guo, X.; Liu, J.; Zhou, B. Design and Implementation of an Asynchronous BCI System with Alpha Rhythm and SSVEP. IEEE Access 2019, 7, 146123–146143. [Google Scholar] [CrossRef]
- Bi, L.; He, T.; Fan, X. A driver-vehicle interface based on ERD/ERS potentials and alpha rhythm. In Proceedings of the 2014 IEEE International Conference on Systems, Man, and Cybernetics (SMC), San Diego, CA, USA, 5–8 October 2014; pp. 1058–1062. [Google Scholar] [CrossRef]
- Korovesis, N.; Kandris, D.; Koulouras, G.; Alexandridis, A. Robot Motion Control via an EEG-Based Brain–Computer Interface by Using Neural Networks and Alpha Brainwaves. Electronics 2019, 8, 1387. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Zhang, S.; Bekyo, A.; Olsoe, J.; Baxter, B.; He, B. Noninvasive Electroencephalogram Based Control of a Robotic Arm for Reach and Grasp Tasks. Sci. Rep. 2016, 6, 38565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, F.; Li, M.; Zhao, S.N.; Xu, Q.; Xu, J.; Wu, H. Control of a Robotic Arm with an Optimized Common Template-Based CCA Method for SSVEP-Based BCI. Front. Neurorobot. 2022, 16, 855825. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, B.; Wang, Y.; Xu, S.; Gao, X. Control of a 7-DOF Robotic Arm System With an SSVEP-Based BCI. Int. J. Neural Syst. 2018, 28, 1850018. [Google Scholar] [CrossRef]
- Han, X.; Lin, K.; Gao, S.; Gao, X. A novel system of SSVEP-based human–robot coordination. J. Neural Eng. 2018, 16, 016006. [Google Scholar] [CrossRef] [PubMed]
- Lillo, P.D.; Arrichiello, F.; Vito, D.D.; Antonelli, G. BCI-controlled assistive manipulator: Developed architecture and experimental results. IEEE Trans. Cogn. Dev. Syst. 2020, 13, 91–104. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, B.; Wang, Y.; Gao, X. Combination of high-frequency SSVEP-based BCI and computer vision for controlling a robotic arm. J. Neural Eng. 2019, 16, 026012. [Google Scholar] [CrossRef]
- Xu, B.; Li, W.; Liu, D.; Zhang, K.; Miao, M.; Xu, G.; Song, A. Continuous Hybrid BCI Control for Robotic Arm Using Noninvasive Electroencephalogram, Computer Vision, and Eye Tracking. Mathematics 2022, 10, 618. [Google Scholar] [CrossRef]
- Ying, R.; Weisz, J.; Allen, P.K. Grasping with Your Brain: A Brain-Computer Interface for Fast Grasp Selection. In Robotics Research: Volume 1; Bicchi, A., Burgard, W., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 325–340. [Google Scholar] [CrossRef]
- Kim, D.; Hazlett-Knudsen, R.; Culver-Godfrey, H.; Rucks, G.; Cunningham, T.; Portee, D.; Bricout, J.; Wang, Z.; Behal, A. How Autonomy Impacts Performance and Satisfaction: Results from a Study with Spinal Cord Injured Subjects Using an Assistive Robot. IEEE Trans. Syst. Man Cybern.—Part A Syst. Hum. 2012, 42, 2–14. [Google Scholar] [CrossRef]
- Muelling, K.; Venkatraman, A.; Valois, J.S.; Downey, J.E.; Weiss, J.; Javdani, S.; Hebert, M.; Schwartz, A.B.; Collinger, J.L.; Bagnell, J.A. Autonomy infused teleoperation with application to brain computer interface controlled manipulation. Auton. Robot. 2017, 41, 1401–1422. [Google Scholar] [CrossRef]
- Xu, B.; Song, A. Pattern Recognition of Motor Imagery EEG using Wavelet Transform. J. Biomed. Sci. Eng. 2008, 1, 64–67. [Google Scholar] [CrossRef] [Green Version]
- León, M.; Orellana, D.; Chuquimarca, L.; Acaro, X. Study of Feature Extraction Methods for BCI Applications. In Advances in Emerging Trends and Technologies; Advances in Intelligent Systems and Computing; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; Chapter 2; pp. 13–23. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Käthner, I.; Wriessnegger, S.C.; Müller-Putz, G.R.; Kübler, A.; Halder, S. Effects of mental workload and fatigue on the P300, alpha and theta band power during operation of an ERP (P300) brain–computer interface. Biol. Psychol. 2014, 102, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.D.; Waytowich, N.R.; Cox, D.J.; Krusienski, D.J. Extending the discrete selection capabilities of the P300 speller to goal-oriented robotic arm control. In Proceedings of the 2010 3rd IEEE RAS EMBS International Conference on Biomedical Robotics and Biomechatronics, Tokyo, Japan, 26–29 September 2010; pp. 572–575. [Google Scholar] [CrossRef]
- Sharma, K.; Jain, N.; Pal, P.K. Detection of eye closing/opening from EOG and its application in robotic arm control. Biocybern. Biomed. Eng. 2020, 40, 173–186. [Google Scholar] [CrossRef]
- Ishii, R.; Canuet, L.; Ishihara, T.; Aoki, Y.; Ikeda, S.; Hata, M.; Katsimichas, T.; Gunji, A.; Takahashi, H.; Nakahachi, T.; et al. Frontal midline theta rhythm and gamma power changes during focused attention on mental calculation: An MEG beamformer analysis. Front. Hum. Neurosci. 2014, 8, 406. [Google Scholar] [CrossRef] [Green Version]
- Magosso, E.; De Crescenzio, F.; Ricci, G.; Piastra, S.; Ursino, M. EEG Alpha Power Is Modulated by Attentional Changes during Cognitive Tasks and Virtual Reality Immersion. Comput. Intell. Neurosci. 2019, 2019, 7051079. [Google Scholar] [CrossRef] [Green Version]
- Katahira, K.; Yamazaki, Y.; Yamaoka, C.; Ozaki, H.; Nakagawa, S.; Nagata, N. EEG Correlates of the Flow State: A Combination of Increased Frontal Theta and Moderate Frontocentral Alpha Rhythm in the Mental Arithmetic Task. Front. Psychol. 2018, 9, 300. [Google Scholar] [CrossRef] [Green Version]
- Fatimah, B.; Javali, A.; Ansar, H.; Harshitha, B.G.; Kumar, H. Mental Arithmetic Task Classification using Fourier Decomposition Method. In Proceedings of the 2020 International Conference on Communication and Signal Processing (ICCSP), Chennai, India, 28–30 July 2020; pp. 0046–0050. [Google Scholar] [CrossRef]
- So, W.K.Y.; Wong, S.W.H.; Mak, J.N.; Chan, R.H.M. An evaluation of mental workload with frontal EEG. PLoS ONE 2017, 12, e0174949. [Google Scholar] [CrossRef]
- Nuamah, J.K.; Seong, Y.; Yi, S. Electroencephalography (EEG) classification of cognitive tasks based on task engagement index. In Proceedings of the 2017 IEEE Conference on Cognitive and Computational Aspects of Situation Management (CogSIMA), Savannah, GA, USA, 27–31 March 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Becker, S.; Dhindsa, K.; Mousapour, L.; Al Dabagh, Y. BCI Illiteracy: It’s Us, Not Them. Optimizing BCIs for Individual Brains. In Proceedings of the 2022 10th International Winter Conference on Brain-Computer Interface (BCI), Gangwon-do, Korea, 21–23 February 2022; pp. 1–3. [Google Scholar] [CrossRef]
- Mierau, A.; Klimesch, W.; Lefebvre, J. State-dependent alpha peak frequency shifts: Experimental evidence, potential mechanisms and functional implications. Neuroscience 2017, 360, 146–154. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A.; Tsunoda, T. Subject-Specific-Frequency-Band for Motor Imagery EEG Signal Recognition Based on Common Spatial Spectral Pattern. In Proceedings of the PRICAI 2019: Trends in Artificial Intelligence; Nayak, A.C., Sharma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 712–722. [Google Scholar]
- Delisle-Rodriguez, D.; Cardoso, V.; Gurve, D.; Loterio, F.; Romero-Laiseca, M.A.; Krishnan, S.; Bastos-Filho, T. System based on subject-specific bands to recognize pedaling motor imagery: Towards a BCI for lower-limb rehabilitation. J. Neural Eng. 2019, 16, 056005. [Google Scholar] [CrossRef] [Green Version]
- Lazurenko, D.; Shepelev, I.; Shaposhnikov, D.; Saevskiy, A.; Kiroy, V. Discriminative Frequencies and Temporal EEG Segmentation in the Motor Imagery Classification Approach. Appl. Sci. 2022, 12, 2736. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, R.; Yao, L.; Ye, X.; Xu, K. Neurofeedback Training of Alpha Relative Power Improves the Performance of Motor Imagery Brain-Computer Interface. Front. Hum. Neurosci. 2022, 16, 831995. [Google Scholar] [CrossRef] [PubMed]
- Huggins, J.E.; Wren, P.A.; Gruis, K.L. What would brain-computer interface users want? Opinions and priorities of potential users with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2011, 12, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, R.A.; Vasilakos, A.V. Brain computer interface: Control signals review. Neurocomputing 2017, 223, 26–44. [Google Scholar] [CrossRef]


| SUB | TD (s) | RMT (s) | PE (%) | AST |
|---|---|---|---|---|
| 1 | 312.5 ± 76.9 | 40.6 ± 8.3 | 84.2 + 13.5 | 10 ± 2 |
| 2 | 278.8 ± 38.8 | 36.6 ± 2.4 | 96.1 + 4.9 | 8 ± 0 |
| 3 | 324.4 ± 119.8 | 35.4 ± 7.4 | 86.9 ± 11.2 | 10 ± 3 |
| 4 | 677.0 ± 289.1 | 33.7 ± 3.1 | 93.0 ± 8.6 | 12 ± 4 |
| 5 | 309.1 ± 42.7 | 36.8 ± 2.9 | 87.0 ± 6.7 | 9 ± 2 |
| 6 | 415.1 ± 120.6 | 37.4 ± 2.4 | 86.2 ± 5.6 | 10 ± 2 |
| 7 | 367.7 ± 118.8 | 34.5 ± 3.1 | 101.6 ± 8.1 | 8 ± 1 |
| 8 | 833.0 ± 214.2 | 36.8 ± 4.6 | 86.2 ± 8.3 | 12 ± 3 |
| Mean ± SD | 439.7 ± 203.3 | 36.5 ± 2.1 | 90.2 ± 6.1 | 9.4 ± 1.2 |
| SUB | Day 1 | Day 2 | ||||
|---|---|---|---|---|---|---|
| Success 1 | Failure | Success 1 | Failure | |||
| Type 1 | Type 2 | Type 1 | Type 2 | |||
| 1 | 10 (90.9) | 0 | 1 | 13 (100.0) | 0 | 0 |
| 2 | 12 (85.7) | 1 | 1 | 13 (92.9) | 1 | 0 |
| 3 | 10 (100.0) | 0 | 0 | 12 (92.3) | 0 | 1 |
| 4 | 3 (50.0) | 1 | 2 | 0 (0.0) | 7 | 0 |
| 5 | 10 (83.3) | 1 | 1 | 10 (71.4) | 4 | 0 |
| 6 | 8 (88.9) | 1 | 0 | 8 (88.9) | 0 | 1 |
| 7 | 13 (92.9) | 0 | 1 | 9 (100.0) | 0 | 0 |
| 8 | 4 (80.0) | 0 | 1 | 2 (50.0) | 2 | 0 |
| SUB | YI | ACC | VS 1 | VSCI 1 | ISC 1 | ISNC 1 |
|---|---|---|---|---|---|---|
| 1 | 0.95 | 0.98 | 247 (94) | 0 (0) | 14 (5) | 1 (0) |
| 2 | 0.97 | 0.98 | 224 (93) | 0 (0) | 18 (7) | 0 (0) |
| 3 | 0.89 | 0.93 | 222 (74) | 5 (2) | 71 (24) | 0 (0) |
| 4 | 0.45 | 0.69 | 112 (26) | 57 (13) | 242 (55) | 28 (6) |
| 5 | 0.91 | 0.96 | 235 (91) | 1 (0) | 23 (9) | 0 (0) |
| 6 | 0.77 | 0.87 | 175 (57) | 28 (9) | 99 (32) | 3 (1) |
| 7 | 0.89 | 0.95 | 192 (82) | 2 (1) | 40 (17) | 0 (0) |
| 8 | 0.42 | 0.58 | 104 (23) | 14 (3) | 331 (72) | 8 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos Cardoso, A.S.; Kæseler, R.L.; Jochumsen, M.; Andreasen Struijk, L.N.S. Manual 3D Control of an Assistive Robotic Manipulator Using Alpha Rhythms and an Auditory Menu: A Proof-of-Concept. Signals 2022, 3, 396-409. https://doi.org/10.3390/signals3020024
Santos Cardoso AS, Kæseler RL, Jochumsen M, Andreasen Struijk LNS. Manual 3D Control of an Assistive Robotic Manipulator Using Alpha Rhythms and an Auditory Menu: A Proof-of-Concept. Signals. 2022; 3(2):396-409. https://doi.org/10.3390/signals3020024
Chicago/Turabian StyleSantos Cardoso, Ana S., Rasmus L. Kæseler, Mads Jochumsen, and Lotte N. S. Andreasen Struijk. 2022. "Manual 3D Control of an Assistive Robotic Manipulator Using Alpha Rhythms and an Auditory Menu: A Proof-of-Concept" Signals 3, no. 2: 396-409. https://doi.org/10.3390/signals3020024
APA StyleSantos Cardoso, A. S., Kæseler, R. L., Jochumsen, M., & Andreasen Struijk, L. N. S. (2022). Manual 3D Control of an Assistive Robotic Manipulator Using Alpha Rhythms and an Auditory Menu: A Proof-of-Concept. Signals, 3(2), 396-409. https://doi.org/10.3390/signals3020024






