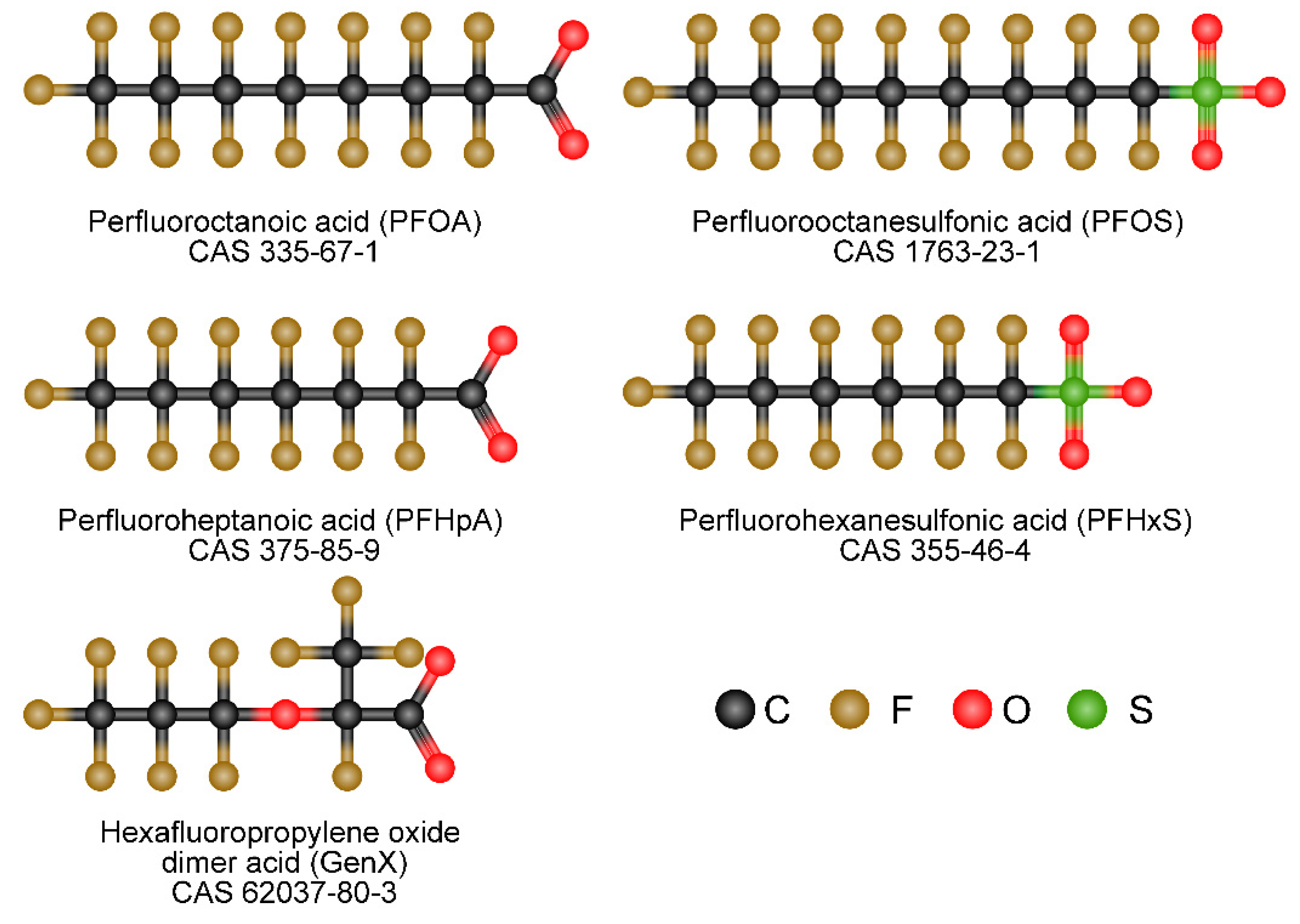
Toward a Mechanistic Understanding of Poly- and Perfluoroalkylated Substances and Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
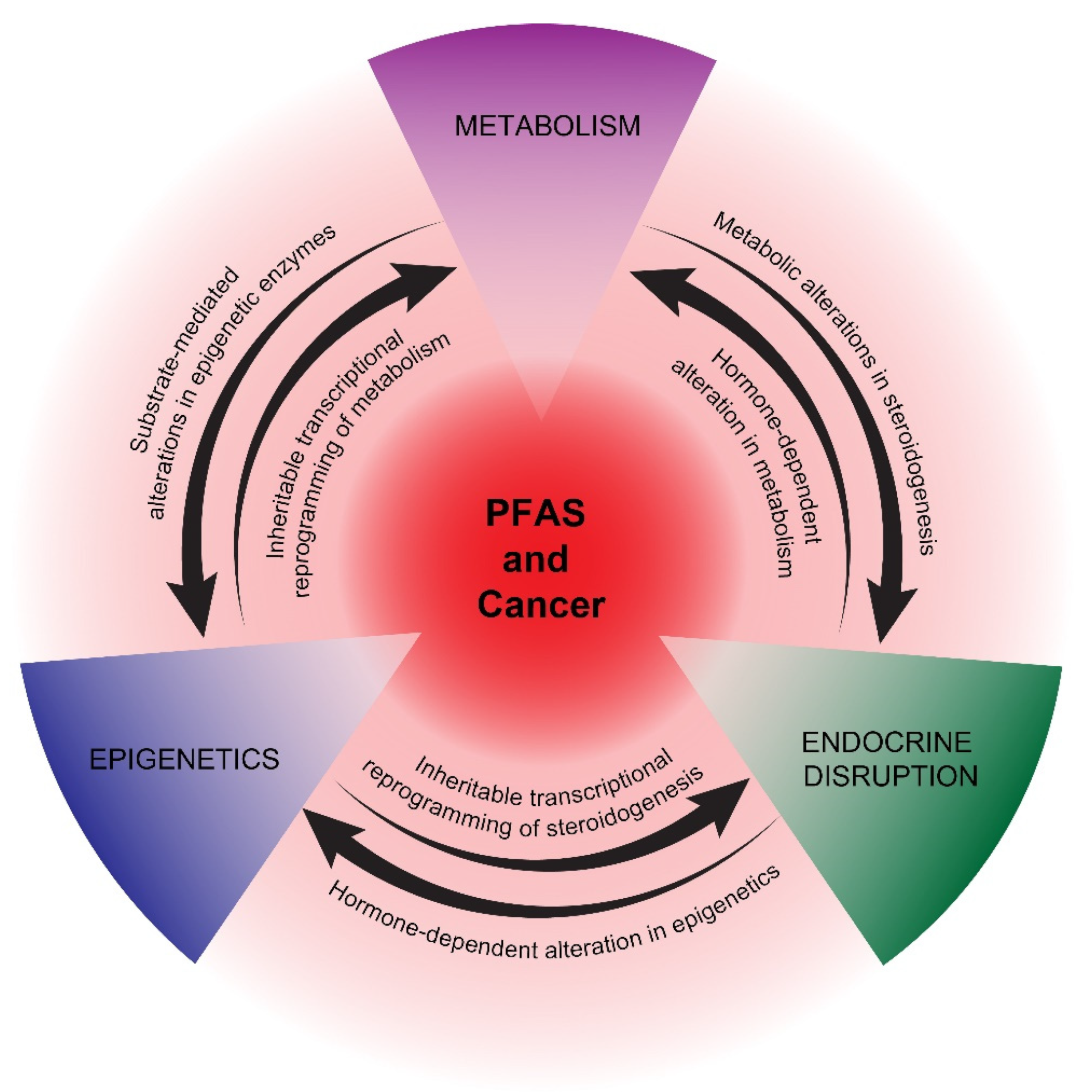
2. Potential Mechanisms of PFAS Carcinogenesis
2.1. Metabolism
2.2. Endocrine Disruption
2.3. Epigenetics
3. The Case for Testicular Cancer
4. The Case for Prostate Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chohan, A.; Petaway, H.; Rivera-Diaz, V.; Day, A.; Colaianni, O.; Keramati, M. Per and polyfluoroalkyl substances scientific literature review: Water exposure, impact on human health, and implications for regulatory reform. Rev. Environ. Health 2021, 36, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Meegoda, J.N.; Kewalramani, J.A.; Li, B.; Marsh, R.W. A review of the applications, environmental release, and remediation technologies of per- and polyfluoroalkyl substances. Int. J. Environ. Res. Public Health 2020, 17, 8117. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Sarkar, B.; Vithanage, M.; Singh, G.; Tsang, D.C.W.; Mukhopadhyay, R.; Ramadass, K.; Vinu, A.; Sun, Y.; Ramanayaka, S.; et al. Distribution, behaviour, bioavailability and remediation of poly- and per-fluoroalkyl substances (PFAS) in solid biowastes and biowaste-treated soil. Environ. Int. 2021, 155, 106600. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, S.; Zhou, J.L.; Zheng, C.; Weifeng, J.; Chen, B.; Zhang, T.; Qiu, W. PFAS and their substitutes in groundwater: Occurrence, transformation and remediation. J. Hazard. Mater. 2021, 412, 125159. [Google Scholar] [CrossRef]
- Laitinen, J.A.; Koponen, J.; Koikkalainen, J.; Kiviranta, H. Firefighters’ exposure to perfluoroalkyl acids and 2-butoxyethanol present in firefighting foams. Toxicol. Lett. 2014, 231, 227–232. [Google Scholar] [CrossRef]
- Calafat, A.M.; Wong, L.Y.; Kuklenyik, Z.; Reidy, J.A.; Needham, L.L. Polyfluoroalkyl chemicals in the U.S. population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007, 115, 1596–1602. [Google Scholar] [CrossRef]
- Fu, J.; Gao, Y.; Cui, L.; Wang, T.; Liang, Y.; Qu, G.; Yuan, B.; Wang, Y.; Zhang, A.; Jiang, G. Occurrence, temporal trends, and half-lives of perfluoroalkyl acids (PFAAs) in occupational workers in China. Sci. Rep. 2016, 6, 38039. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.C.; Andrews, D.Q.; Lindstrom, A.B.; Bruton, T.A.; Schaider, L.A.; Grandjean, P.; Lohmann, R.; Carignan, C.C.; Blum, A.; Balan, S.A.; et al. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [CrossRef]
- Emmett, E.A.; Shofer, F.S.; Zhang, H.; Freeman, D.; Desai, C.; Shaw, L.M. Community exposure to perfluorooctanoate: Relationships between serum concentrations and exposure sources. J. Occup. Environ. Med. 2006, 48, 759–770. [Google Scholar] [CrossRef]
- Ingelido, A.M.; Abballe, A.; Gemma, S.; Dellatte, E.; Iacovella, N.; De Angelis, G.; Zampaglioni, F.; Marra, V.; Miniero, R.; Valentini, S.; et al. Biomonitoring of perfluorinated compounds in adults exposed to contaminated drinking water in the Veneto Region, Italy. Environ. Int. 2018, 110, 149–159. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steenland, K.; Fletcher, T.; Stein, C.R.; Bartell, S.M.; Darrow, L.; Lopez-Espinosa, M.J.; Barry Ryan, P.; Savitz, D.A. Review: Evolution of evidence on PFOA and health following the assessments of the C8 Science Panel. Environ. Int. 2020, 145, 106125. [Google Scholar] [CrossRef]
- Steenland, K.; Winquist, A. PFAS and cancer, a scoping review of the epidemiologic evidence. Environ. Res. 2021, 194, 110690. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, S.; Oh, B.C.; Jung, D.; Ji, K.; Choi, K.; Park, Y.J. Association between perfluoroalkyl substances exposure and thyroid function in adults: A meta-analysis. PLoS ONE 2018, 13, e0197244. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, J.C.; Blossom, S.J.; Schaider, L.A. Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: Epidemiological and toxicological evidence. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 148–156. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. In Some Chemicals Used as Solvents and in Polymer Manufacture; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- E.P.A. Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA); EPA: New York, NY, USA, 2016. [Google Scholar]
- E.P.A. Human Health Toxicity Values for Hexafluoropropylene Oxide (HFPO) Dimer Acid and Its Ammonium Salt (CASRN 13252-13-6 and CASRN 62037-80-3) Also Known as “GenX Chemicals”; Office of Water: Washington, DC, USA, 2018. [Google Scholar]
- Rodgers, K.M.; Udesky, J.O.; Rudel, R.A.; Brody, J.G. Environmental chemicals and breast cancer: An updated review of epidemiological literature informed by biological mechanisms. Environ. Res. 2018, 160, 152–182. [Google Scholar] [CrossRef]
- Stanifer, J.W.; Stapleton, H.M.; Souma, T.; Wittmer, A.; Zhao, X.; Boulware, L.E. perfluorinated chemicals as emerging environmental threats to kidney health: A Scoping Review. Clin. J. Am. Soc. Nephrol. 2018, 13, 1479–1492. [Google Scholar] [CrossRef] [Green Version]
- Gilliland, F.D.; Mandel, J.S. Mortality among employees of a perfluorooctanoic acid production plant. J. Occup. Med. 1993, 35, 950–954. [Google Scholar] [CrossRef]
- Lundin, J.I.; Alexander, B.H.; Olsen, G.W.; Church, T.R. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology 2009, 20, 921–928. [Google Scholar] [CrossRef]
- Barry, V.; Winquist, A.; Steenland, K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ. Health Perspect. 2013, 121, 1313–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, V.M.; Hoffman, K.; Shin, H.M.; Weinberg, J.M.; Webster, T.F.; Fletcher, T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: A geographic analysis. Environ. Health Perspect. 2013, 121, 318–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksen, K.T.; Sørensen, M.; McLaughlin, J.K.; Lipworth, L.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. J. Natl. Cancer Inst. 2009, 101, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Bonefeld-Jorgensen, E.C.; Long, M.; Bossi, R.; Ayotte, P.; Asmund, G.; Krüger, T.; Ghisari, M.; Mulvad, G.; Kern, P.; Nzulumiki, P.; et al. Perfluorinated compounds are related to breast cancer risk in Greenlandic Inuit: A case control study. Environ. Health 2011, 10, 88. [Google Scholar] [CrossRef] [Green Version]
- Bonefeld-Jørgensen, E.C.; Long, M.; Fredslund, S.O.; Bossi, R.; Olsen, J. Breast cancer risk after exposure to perfluorinated compounds in Danish women: A case-control study nested in the Danish National Birth Cohort. Cancer Causes Control. 2014, 25, 1439–1448. [Google Scholar] [CrossRef] [Green Version]
- Chang, E.T.; Adami, H.O.; Boffetta, P.; Cole, P.; Starr, T.B.; Mandel, J.S. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and cancer risk in humans. Crit. Rev. Toxicol. 2014, 44, 1–81. [Google Scholar] [CrossRef] [Green Version]
- Raleigh, K.K.; Alexander, B.H.; Olsen, G.W.; Ramachandran, G.; Morey, S.Z.; Church, T.R.; Logan, P.W.; Scott, L.L.; Allen, E.M. Mortality and cancer incidence in ammonium perfluorooctanoate production workers. Occup. Environ. Med. 2014, 71, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Arrieta-Cortes, R.; Farias, P.; Hoyo-Vadillo, C.; Kleiche-Dray, M. Carcinogenic risk of emerging persistent organic pollutant perfluorooctane sulfonate (PFOS): A proposal of classification. Regul. Toxicol. Pharmacol. 2017, 83, 66–80. [Google Scholar] [CrossRef]
- Bartell, S.M.; Vieira, V.M. Critical review on PFOA, kidney cancer, and testicular cancer. J. Air Waste Manag. Assoc. 2021, 71, 663–679. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Perfluorooctanoic Acid Administered in Feed to Sprague Dawley (Hsd: Sprague Dawley SD) Rats; National Toxicology Program: Triangle Park, NC, USA, 2020. [Google Scholar] [CrossRef]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [Green Version]
- Butenhoff, J.L.; Kennedy, G.L., Jr.; Chang, S.C.; Olsen, G.W. Chronic dietary toxicity and carcinogenicity study with ammonium perfluorooctanoate in Sprague-Dawley rats. Toxicology 2012, 298, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Biegel, L.B.; Hurtt, M.E.; Frame, S.R.; O’Connor, J.C.; Cook, J.C. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol. Sci. 2001, 60, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Temkin, A.M.; Hocevar, B.A.; Andrews, D.Q.; Naidenko, O.V.; Kamendulis, L.M. Application of the key characteristics of carcinogens to per and polyfluoroalkyl substances. Int. J. Environ. Res. Public Health 2020, 17, 1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crebelli, R.; Caiola, S.; Conti, L.; Cordelli, E.; De Luca, G.; Dellatte, E.; Eleuteri, P.; Iacovella, N.; Leopardi, P.; Marcon, F.; et al. Can sustained exposure to PFAS trigger a genotoxic response? A comprehensive genotoxicity assessment in mice after subacute oral administration of PFOA and PFBA. Regul. Toxicol. Pharmacol. 2019, 106, 169–177. [Google Scholar] [CrossRef]
- Emerce, E.; Çetin, Ö. Genotoxicity assessment of perfluoroalkyl substances on human sperm. Toxicol. Ind. Health 2018, 34, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Wielsøe, M.; Long, M.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere 2015, 129, 239–245. [Google Scholar] [CrossRef]
- Panaretakis, T.; Shabalina, I.G.; Grandér, D.; Shoshan, M.C.; DePierre, J.W. Reactive oxygen species and mitochondria mediate the induction of apoptosis in human hepatoma HepG2 cells by the rodent peroxisome proliferator and hepatocarcinogen, perfluorooctanoic acid. Toxicol. Appl. Pharmacol. 2001, 173, 56–64. [Google Scholar] [CrossRef]
- Brase, R.A.; Mullin, E.J.; Spink, D.C. Legacy and emerging per- and polyfluoroalkyl substances: Analytical techniques, environmental fate, and health effects. Int. J. Mol. Sci. 2021, 22, 995. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Hocevar, B.A.; Kamendulis, L.M. Mode of action analysis of perfluorooctanoic acid (PFOA) tumorigenicity and human relevance. Reprod. Toxicol. 2012, 33, 410–418. [Google Scholar] [CrossRef]
- Kim, S.; Thapar, I.; Brooks, B.W. Epigenetic changes by per- and polyfluoroalkyl substances (PFAS). Environ. Pollut. 2021, 279, 116929. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanden Heuvel, J.P.; Thompson, J.T.; Frame, S.R.; Gillies, P.J. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: A comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol. Sci. 2006, 92, 476–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, C.J.; Takacs, M.L.; Schmid, J.E.; Lau, C.; Abbott, B.D. Activation of mouse and human peroxisome proliferator-activated receptor alpha by perfluoroalkyl acids of different functional groups and chain lengths. Toxicol. Sci. 2008, 106, 162–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, G.; Prabhu, A. The pleiotropic peroxisome proliferator activated receptors: Regulation and therapeutics. Exp. Mol. Pathol. 2022, 124, 104723. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Zhang, Y.; Guan, S.; Gong, X.; Wang, X. Maternal exposure to perfluorooctanoic acid (PFOA) causes liver toxicity through PPAR-α pathway and lowered histone acetylation in female offspring mice. Environ. Sci. Pollut. Res. Int. 2019, 26, 18866–18875. [Google Scholar] [CrossRef]
- Sheng, N.; Pan, Y.; Guo, Y.; Sun, Y.; Dai, J. Hepatotoxic effects of hexafluoropropylene oxide trimer acid (HFPO-TA), A novel perfluorooctanoic acid (PFOA) alternative, on mice. Environ. Sci. Technol. 2018, 52, 8005–8015. [Google Scholar] [CrossRef]
- Das, K.P.; Wood, C.R.; Lin, M.T.; Starkov, A.A.; Lau, C.; Wallace, K.B.; Corton, J.C.; Abbott, B.D. Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology 2017, 378, 37–52. [Google Scholar] [CrossRef]
- Chappell, G.A.; Thompson, C.M.; Wolf, J.C.; Cullen, J.M.; Klaunig, J.E.; Haws, L.C. Assessment of the mode of action underlying the effects of GenX in mouse liver and implications for assessing human health risks. Toxicol. Pathol. 2020, 48, 494–508. [Google Scholar] [CrossRef]
- Schlezinger, J.J.; Hyötyläinen, T.; Sinioja, T.; Boston, C.; Puckett, H.; Oliver, J.; Heiger-Bernays, W.; Webster, T.F. Perfluorooctanoic acid induces liver and serum dyslipidemia in humanized PPARα mice fed an American diet. Toxicol. Appl. Pharmacol. 2021, 426, 115644. [Google Scholar] [CrossRef]
- Fragki, S.; Dirven, H.; Fletcher, T.; Grasl-Kraupp, B.; Bjerve Gützkow, K.; Hoogenboom, R.; Kersten, S.; Lindeman, B.; Louisse, J.; Peijnenburg, A.; et al. Systemic PFOS and PFOA exposure and disturbed lipid homeostasis in humans: What do we know and what not? Crit. Rev. Toxicol. 2021, 51, 141–164. [Google Scholar] [CrossRef]
- Frisbee, S.J.; Brooks, A.P., Jr.; Maher, A.; Flensborg, P.; Arnold, S.; Fletcher, T.; Steenland, K.; Shankar, A.; Knox, S.S.; Pollard, C.; et al. The C8 health project: Design, methods, and participants. Environ. Health Perspect. 2009, 117, 1873–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksen, K.T.; Raaschou-Nielsen, O.; McLaughlin, J.K.; Lipworth, L.; Tjønneland, A.; Overvad, K.; Sørensen, M. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS ONE 2013, 8, e56969. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.S.; Wen, L.L.; Chu, P.L.; Lin, C.Y. Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013-2014. Environ. Pollut. 2018, 232, 73–79. [Google Scholar] [CrossRef]
- Hu, W.; Jones, P.D.; DeCoen, W.; King, L.; Fraker, P.; Newsted, J.; Giesy, J.P. Alterations in cell membrane properties caused by perfluorinated compounds. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 135, 77–88. [Google Scholar] [CrossRef]
- Fitzgerald, N.J.M.; Wargenau, A.; Sorenson, C.; Pedersen, J.; Tufenkji, N.; Novak, P.J.; Simcik, M.F. Partitioning and accumulation of perfluoroalkyl substances in model lipid bilayers and bacteria. Environ. Sci. Technol. 2018, 52, 10433–10440. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.C.; Moore, T.; Abbott, B.D.; Rosen, M.B.; Das, K.P.; Zehr, R.D.; Lindstrom, A.B.; Strynar, M.J.; Lau, C. Comparative hepatic effects of perfluorooctanoic acid and WY 14,643 in PPAR-alpha knockout and wild-type mice. Toxicol. Pathol. 2008, 36, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Attané, C.; Milhas, D.; Hoy, A.J.; Muller, C. Metabolic remodeling induced by adipocytes: A new achilles’ heel in invasive breast cancer? Curr. Med. Chem. 2020, 27, 3984–4001. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Y.; Gu, M.; Liu, C.; Chen, Q.; Zhan, M.; Wang, Z. Fatty acid metabolism reprogramming in advanced prostate cancer. Metabolites 2021, 11, 765. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Yu, N.; Wei, S.; Li, M.; Yang, J.; Li, K.; Jin, L.; Xie, Y.; Giesy, J.P.; Zhang, X.; Yu, H. Effects of perfluorooctanoic acid on metabolic profiles in brain and liver of mouse revealed by a high-throughput targeted metabolomics approach. Sci. Rep. 2016, 6, 23963. [Google Scholar] [CrossRef] [Green Version]
- Tan, F.; Jin, Y.; Liu, W.; Quan, X.; Chen, J.; Liang, Z. Global liver proteome analysis using iTRAQ labeling quantitative proteomic technology to reveal biomarkers in mice exposed to perfluorooctane sulfonate (PFOS). Environ. Sci. Technol. 2012, 46, 12170–12177. [Google Scholar] [CrossRef] [PubMed]
- Domazet, S.L.; Grøntved, A.; Timmermann, A.G.; Nielsen, F.; Jensen, T.K. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: The European Youth Heart Study. Diabetes Care 2016, 39, 1745–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alderete, T.L.; Jin, R.; Walker, D.I.; Valvi, D.; Chen, Z.; Jones, D.P.; Peng, C.; Gilliland, F.D.; Berhane, K.; Conti, D.V.; et al. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: A proof-of-concept analysis. Environ. Int. 2019, 126, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, N.; Conti, D.V.; Jin, R.; Margetaki, K.; Valvi, D.; Siskos, A.P.; Maitre, L.; Garcia, E.; Varo, N.; Zhao, Y.; et al. Prenatal exposure to perfluoroalkyl substances associated with increased susceptibility to liver injury in children. Hepatology 2020, 72, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, S.L.; Walker, D.I.; Calafat, A.M.; Chen, A.; Papandonatos, G.D.; Xu, Y.; Jones, D.P.; Lanphear, B.P.; Pennell, K.D.; Braun, J.M. Metabolomics of childhood exposure to perfluoroalkyl substances: A cross-sectional study. Metabolomics 2019, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, L.; Zhang, W.; Zhang, J.; Du, X.; Huang, Q.; Tian, M.; Shen, H. Serum metabolome biomarkers associate low-level environmental perfluorinated compound exposure with oxidative /nitrosative stress in humans. Environ. Pollut. 2017, 229, 168–176. [Google Scholar] [CrossRef]
- Lv, Z.; Li, G.; Li, Y.; Ying, C.; Chen, J.; Chen, T.; Wei, J.; Lin, Y.; Jiang, Y.; Wang, Y.; et al. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ. Toxicol. 2013, 28, 532–542. [Google Scholar] [CrossRef]
- Quist, E.M.; Filgo, A.J.; Cummings, C.A.; Kissling, G.E.; Hoenerhoff, M.J.; Fenton, S.E. Hepatic mitochondrial alteration in CD-1 mice associated with prenatal exposures to low doses of perfluorooctanoic acid (PFOA). Toxicol. Pathol. 2015, 43, 546–557. [Google Scholar] [CrossRef]
- Varsi, K.; Huber, S.; Averina, M.; Brox, J.; Bjørke-Monsen, A.L. Quantitation of linear and branched perfluoroalkane sulfonic acids (PFSAs) in women and infants during pregnancy and lactation. Environ. Int. 2021, 160, 107065. [Google Scholar] [CrossRef]
- Appel, M.; Forsthuber, M.; Ramos, R.; Widhalm, R.; Granitzer, S.; Uhl, M.; Hengstschläger, M.; Stamm, T.; Gundacker, C. The transplacental transfer efficiency of per- and polyfluoroalkyl substances (PFAS): A first meta-analysis. J. Toxicol. Environ. Health B Crit. Rev. 2022, 25, 23–42. [Google Scholar] [CrossRef]
- Ješeta, M.; Navrátilová, J.; Franzová, K.; Fialková, S.; Kempisty, B.; Ventruba, P.; Žáková, J.; Crha, I. Overview of the mechanisms of action of selected bisphenols and perfluoroalkyl chemicals on the male reproductive axes. Front. Genet. 2021, 12, 692897. [Google Scholar] [CrossRef] [PubMed]
- Green, M.P.; Harvey, A.J.; Finger, B.J.; Tarulli, G.A. Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period. Environ. Res. 2021, 194, 110694. [Google Scholar] [CrossRef] [PubMed]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination, endocrine-mediated effects, and disease. Toxicology 2022, 465, 153031. [Google Scholar] [CrossRef] [PubMed]
- Tarapore, P.; Ouyang, B. Perfluoroalkyl Chemicals and Male Reproductive Health: Do PFOA and PFOS Increase Risk for Male Infertility? Int. J. Environ. Res. Public Health 2021, 18, 3794. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.T.; Zhao, Y.G.; Wong, M.H.; Lee, K.F.; Yeung, W.S.; Giesy, J.P.; Wong, C.K. Testicular signaling is the potential target of perfluorooctanesulfonate-mediated subfertility in male mice. Biol. Reprod. 2011, 84, 1016–1023. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Wang, X.; Cao, X.; Xia, Y.; Zhou, R.; Chen, L. Chronic exposure of female mice to an environmental level of perfluorooctane sulfonate suppresses estrogen synthesis through reduced histone H3K14 acetylation of the star promoter leading to deficits in follicular development and ovulation. Toxicol. Sci. 2015, 148, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Li, L.; Liu, J.; Li, H.; Zhang, C.; Han, P.; Zhang, Y.; Yuan, X.; Ge, R.S.; Chu, Y. Exposure to perfluorooctane sulfonate in utero reduces testosterone production in rat fetal Leydig cells. PLoS ONE 2014, 9, e78888. [Google Scholar] [CrossRef] [Green Version]
- Jensen, A.A.; Leffers, H. Emerging endocrine disrupters: Perfluoroalkylated substances. Int. J. Androl. 2008, 31, 161–169. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.; Luo, B.; Yan, S.; Guo, X.; Dai, J. Proteomic analysis of mouse testis reveals perfluorooctanoic acid-induced reproductive dysfunction via direct disturbance of testicular steroidogenic machinery. J. Proteome Res. 2014, 13, 3370–3385. [Google Scholar] [CrossRef]
- López-Doval, S.; Salgado, R.; Pereiro, N.; Moyano, R.; Lafuente, A. Perfluorooctane sulfonate effects on the reproductive axis in adult male rats. Environ. Res. 2014, 134, 158–168. [Google Scholar] [CrossRef]
- Di Nisio, A.; Sabovic, I.; Valente, U.; Tescari, S.; Rocca, M.S.; Guidolin, D.; Dall’Acqua, S.; Acquasaliente, L.; Pozzi, N.; Plebani, M.; et al. Endocrine disruption of androgenic activity by perfluoroalkyl substances: Clinical and experimental evidence. J. Clin. Endocrinol. Metab 2019, 104, 1259–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, S.; Araki, A.; Mitsui, T.; Miyashita, C.; Goudarzi, H.; Sasaki, S.; Cho, K.; Nakazawa, H.; Iwasaki, Y.; Shinohara, N.; et al. Association of perfluoroalkyl substances exposure in utero with reproductive hormone levels in cord blood in the Hokkaido Study on Environment and Children’s Health. Environ. Int. 2016, 94, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, P.; Li, D.; Wang, X.; Zhong, X. Effects of perfluorooctanoic acid exposure during pregnancy on the reproduction and development of male offspring mice. Andrologia 2018, 50, e13059. [Google Scholar] [CrossRef]
- White, S.S.; Stanko, J.P.; Kato, K.; Calafat, A.M.; Hines, E.P.; Fenton, S.E. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environ. Health Perspect. 2011, 119, 1070–1076. [Google Scholar] [CrossRef] [Green Version]
- White, S.S.; Calafat, A.M.; Kuklenyik, Z.; Villanueva, L.; Zehr, R.D.; Helfant, L.; Strynar, M.J.; Lindstrom, A.B.; Thibodeaux, J.R.; Wood, C.; et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol. Sci. 2007, 96, 133–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Bao, J.; Liu, L.; Wang, X. Perfluorooctanoic acid exposure in early pregnancy induces oxidative stress in mice uterus and liver. Environ. Sci. Pollut. Res. Int. 2021, 28, 66355–66365. [Google Scholar] [CrossRef]
- Mallozzi, M.; Leone, C.; Manurita, F.; Bellati, F.; Caserta, D. Endocrine disrupting chemicals and endometrial cancer: An overview of recent laboratory evidence and epidemiological studies. Int. J. Environ. Res. Public Health 2017, 14, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vested, A.; Ramlau-Hansen, C.H.; Olsen, S.F.; Bonde, J.P.; Kristensen, S.L.; Halldorsson, T.I.; Becher, G.; Haug, L.S.; Ernst, E.H.; Toft, G. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ. Health Perspect. 2013, 121, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Espinosa, M.J.; Mondal, D.; Armstrong, B.G.; Eskenazi, B.; Fletcher, T. perfluoroalkyl substances, sex hormones, and insulin-like growth factor-1 at 6-9 years of age: A cross-sectional analysis within the C8 Health Project. Environ. Health Perspect. 2016, 124, 1269–1275. [Google Scholar] [CrossRef]
- Tsai, M.S.; Lin, C.Y.; Lin, C.C.; Chen, M.H.; Hsu, S.H.; Chien, K.L.; Sung, F.C.; Chen, P.C.; Su, T.C. Association between perfluoroalkyl substances and reproductive hormones in adolescents and young adults. Int. J. Hyg. Environ. Health 2015, 218, 437–443. [Google Scholar] [CrossRef]
- Toporova, L.; Balaguer, P. Nuclear receptors are the major targets of endocrine disrupting chemicals. Mol. Cell Endocrinol. 2020, 502, 110665. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.; Sant, K.E. Associations between Exposures to Perfluoroalkyl Substances and Diabetes, Hyperglycemia, or Insulin Resistance: A Scoping Review. J. Xenobiot. 2021, 11, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.L. Minireview: Epigenomic plasticity and vulnerability to EDC exposures. Mol. Endocrinol. 2016, 30, 848–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Dungen, M.W.; Murk, A.J.; Kampman, E.; Steegenga, W.T.; Kok, D.E. Association between DNA methylation profiles in leukocytes and serum levels of persistent organic pollutants in Dutch men. Environ. Epigenet. 2017, 3, dvx001. [Google Scholar] [CrossRef] [Green Version]
- Watkins, D.J.; Wellenius, G.A.; Butler, R.A.; Bartell, S.M.; Fletcher, T.; Kelsey, K.T. Associations between serum perfluoroalkyl acids and LINE-1 DNA methylation. Environ. Int. 2014, 63, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Preston, R.; Goldman, L.R.; Brebi-Mieville, P.; Ili-Gangas, C.; Lebron, C.; Witter, F.R.; Apelberg, B.J.; Hernández-Roystacher, M.; Jaffe, A.; Halden, R.U.; et al. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics 2010, 5, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Kingsley, S.L.; Kelsey, K.T.; Butler, R.; Chen, A.; Eliot, M.N.; Romano, M.E.; Houseman, A.; Koestler, D.C.; Lanphear, B.P.; Yolton, K.; et al. Maternal serum PFOA concentration and DNA methylation in cord blood: A pilot study. Environ. Res. 2017, 158, 174–178. [Google Scholar] [CrossRef]
- Leung, Y.K.; Ouyang, B.; Niu, L.; Xie, C.; Ying, J.; Medvedovic, M.; Chen, A.; Weihe, P.; Valvi, D.; Grandjean, P.; et al. Identification of sex-specific DNA methylation changes driven by specific chemicals in cord blood in a Faroese birth cohort. Epigenetics 2018, 13, 290–300. [Google Scholar] [CrossRef]
- Miura, R.; Araki, A.; Miyashita, C.; Kobayashi, S.; Kobayashi, S.; Wang, S.L.; Chen, C.H.; Miyake, K.; Ishizuka, M.; Iwasaki, Y.; et al. An epigenome-wide study of cord blood DNA methylations in relation to prenatal perfluoroalkyl substance exposure: The Hokkaido study. Environ. Int. 2018, 115, 21–28. [Google Scholar] [CrossRef]
- Kobayashi, S.; Azumi, K.; Goudarzi, H.; Araki, A.; Miyashita, C.; Kobayashi, S.; Itoh, S.; Sasaki, S.; Ishizuka, M.; Nakazawa, H.; et al. Effects of prenatal perfluoroalkyl acid exposure on cord blood IGF2/H19 methylation and ponderal index: The Hokkaido Study. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.Y.; Chen, P.C.; Lien, P.C.; Liao, Y.P. Prenatal perfluorooctyl sulfonate exposure and Alu DNA hypomethylation in cord blood. Int. J. Environ. Res Public Health 2018, 15, 1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.X.; He, Q.Z.; Li, W.; Long, D.X.; Pan, X.Y.; Chen, C.; Zeng, H.C. Brain-derived neurotrophic factor mediated perfluorooctane sulfonate induced-neurotoxicity via epigenetics regulation in SK-N-SH cells. Int. J. Mol. Sci. 2017, 18, 893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonkar, R.; Kay, M.K.; Choudhury, M. PFOS modulates interactive epigenetic regulation in first-trimester human trophoblast cell line HTR-8/SV(neo). Chem. Res. Toxicol. 2019, 32, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Peng, S.; Martin, F.L.; Zhang, J.; Liu, L.; Wang, Z.; Dong, S.; Shen, H. Perfluorooctanoic acid induces gene promoter hypermethylation of glutathione-S-transferase Pi in human liver L02 cells. Toxicology 2012, 296, 48–55. [Google Scholar] [CrossRef]
- van den Dungen, M.W.; Murk, A.J.; Kok, D.E.; Steegenga, W.T. Persistent organic pollutants alter DNA methylation during human adipocyte differentiation. Toxicol. Vitr. 2017, 40, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, J.; Wan, Y.; Peng, Y.; Ding, S.; Li, Y.; Xu, B.; Chen, X.; Xia, W.; Ke, Y.; et al. Low-level perfluorooctanoic acid enhances 3 T3-L1 preadipocyte differentiation via altering peroxisome proliferator activated receptor gamma expression and its promoter DNA methylation. J. Appl. Toxicol. 2018, 38, 398–407. [Google Scholar] [CrossRef]
- Rashid, F.; Ramakrishnan, A.; Fields, C.; Irudayaraj, J. Acute PFOA exposure promotes epigenomic alterations in mouse kidney tissues. Toxicol. Rep. 2020, 7, 125–132. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, J.; Li, J.; Arif, W.; Kalsotra, A.; Irudayaraj, J. Effect of PFOA on DNA methylation and alternative splicing in mouse liver. Toxicol. Lett. 2020, 329, 38–46. [Google Scholar] [CrossRef]
- Wen, Y.; Mirji, N.; Irudayaraj, J. Epigenetic toxicity of PFOA and GenX in HepG2 cells and their role in lipid metabolism. Toxicol. Vitr. 2020, 65, 104797. [Google Scholar] [CrossRef]
- Liu, W.; Irudayaraj, J. Perfluorooctanoic acid (PFOA) exposure inhibits DNA methyltransferase activities and alters constitutive heterochromatin organization. Food Chem. Toxicol. 2020, 141, 111358. [Google Scholar] [CrossRef]
- Ahmad, S.; Wen, Y.; Irudayaraj, J.M.K. PFOA induces alteration in DNA methylation regulators and SARS-CoV-2 targets Ace2 and Tmprss2 in mouse lung tissues. Toxicol. Rep. 2021, 8, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.J.; Li, Y.Y.; Xia, W.; Chen, J.; Lv, Z.Q.; Zeng, H.C.; Zhang, L.; Yang, W.J.; Chen, T.; Lin, Y.; et al. Alterations in tumor biomarker GSTP gene methylation patterns induced by prenatal exposure to PFOS. Toxicology 2010, 274, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Xu, H.; Zhang, Y.; Shi, X.; Wang, W.; Gao, H.; Bi, Y. SAM targeting methylation by the methyl donor, a novel therapeutic strategy for antagonize PFOS transgenerational fertility toxicity. Ecotoxicol. Environ. Saf 2019, 184, 109579. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Ahmad, S.; Irudayaraj, J.M.K. Effect of Perfluorooctanoic acid on the epigenetic and tight junction genes of the mouse intestine. Toxics 2020, 8, 64. [Google Scholar] [CrossRef]
- Jabeen, M.; Fayyaz, M.; Irudayaraj, J. Epigenetic modifications, and alterations in cell cycle and apoptosis pathway in A549 lung carcinoma cell line upon exposure to perfluoroalkyl substances. Toxics 2020, 8, 112. [Google Scholar] [CrossRef]
- Dong, H.; Curran, I.; Williams, A.; Bondy, G.; Yauk, C.L.; Wade, M.G. Hepatic miRNA profiles and thyroid hormone homeostasis in rats exposed to dietary potassium perfluorooctanesulfonate (PFOS). Environ. Toxicol. Pharmacol. 2016, 41, 201–210. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Zhang, W.; Jin, Y.; Dai, J. Association of perfluorooctanoic acid with HDL cholesterol and circulating miR-26b and miR-199-3p in workers of a fluorochemical plant and nearby residents. Environ. Sci. Technol. 2012, 46, 9274–9281. [Google Scholar] [CrossRef]
- Imir, O.B.; Kaminsky, A.Z.; Zuo, Q.Y.; Liu, Y.J.; Singh, R.; Spinella, M.J.; Irudayaraj, J.; Hu, W.Y.; Prins, G.S.; Madak Erdogan, Z. Per- and polyfluoroalkyl substance exposure combined with high-fat diet supports prostate cancer progression. Nutrients 2021, 13, 3902. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, 5473. [Google Scholar] [CrossRef]
- Chandra, V.; Hong, K.M. Effects of deranged metabolism on epigenetic changes in cancer. Arch. Pharm. Res. 2015, 38, 321–337. [Google Scholar] [CrossRef]
- Boukouris, A.E.; Zervopoulos, S.D.; Michelakis, E.D. metabolic enzymes moonlighting in the nucleus: Metabolic regulation of gene transcription. Trends Biochem. Sci. 2016, 41, 712–730. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, A.F.; Wright, R.O.; Baccarelli, A.A. Environmental epigenetics: A role in endocrine disease? J. Mol. Endocrinol. 2012, 49, R61–R67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feroe, A.; Broene, R.; Albuquerque, D.; Ruiz, P. Endocrine disrupting chemicals, transgenerational epigenetics and metabolic diseases. EC Endocrinol. Metab. Res. 2017, 21, 31–51. [Google Scholar] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, J.; Gillis, A.J.M.; Jerónimo, C.; Henrique, R.; Looijenga, L.H.J. Human germ cell tumors are developmental cancers: Impact of epigenetics on pathobiology and clinic. Int. J. Mol. Sci. 2019, 258. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Shih, J.; Hollern, D.P.; Wang, L.; Bowlby, R.; Tickoo, S.K.; Thorsson, V.; Mungall, A.J.; Newton, Y.; Hegde, A.M.; et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep. 2018, 23, 3392–3406. [Google Scholar] [CrossRef]
- Litchfield, K.; Summersgill, B.; Yost, S.; Sultana, R.; Labreche, K.; Dudakia, D.; Renwick, A.; Seal, S.; Al-Saadi, R.; Broderick, P.; et al. Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumours. Nat. Commun. 2015, 6, 5973. [Google Scholar] [CrossRef] [Green Version]
- Bräuner, E.V.; Lim, Y.H.; Koch, T.; Uldbjerg, C.S.; Gregersen, L.S.; Pedersen, M.K.; Frederiksen, H.; Petersen, J.H.; Coull, B.A.; Andersson, A.M.; et al. Endocrine disrupting chemicals and risk of testicular cancer: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2021, 106, e4834–e4860. [Google Scholar] [CrossRef]
- Sharma, A.; Mollier, J.; Brocklesby, R.W.K.; Caves, C.; Jayasena, C.N.; Minhas, S. Endocrine-disrupting chemicals and male reproductive health. Reprod. Med. Biol. 2020, 19, 243–253. [Google Scholar] [CrossRef]
- Singh, R.; Fazal, Z.; Freemantle, S.J.; Spinella, M.J. Between a rock and a hard place: An epigenetic-centric view of testicular germ cell tumors. Cancers 2021, 13, 1506. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Buck Louis, G.M.; Toppari, J.; Andersson, A.M.; Eisenberg, M.L.; Jensen, T.K.; Jørgensen, N.; Swan, S.H.; Sapra, K.J.; et al. Male reproductive disorders and fertility trends: Influences of environment and genetic susceptibility. Physiol. Rev. 2016, 96, 55–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvi, I.; Ozturk, E.; Yikilmaz, T.N.; Sarikaya, S.; Basar, H. Effects of testicular dysgenesis syndrome components on testicular germ cell tumor prognosis and oncological outcomes. Int. Braz. J. Urol. 2020, 46, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Zugna, D.; Richiardi, L.; McGlynn, K.A.; Akre, O. Congenital malformations and testicular germ cell tumors. Int. J. Cancer 2013, 133, 1900–1904. [Google Scholar] [CrossRef] [PubMed]
- Hanson, H.A.; Anderson, R.E.; Aston, K.I.; Carrell, D.T.; Smith, K.R.; Hotaling, J.M. Subfertility increases risk of testicular cancer: Evidence from population-based semen samples. Fertil. Steril. 2016, 105, 322–328.e321. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Fazal, Z.; Corbet, A.K.; Bikorimana, E.; Rodriguez, J.C.; Khan, E.M.; Shahid, K.; Freemantle, S.J.; Spinella, M.J. Epigenetic Remodeling through Downregulation of Polycomb Repressive Complex 2 Mediates Chemotherapy Resistance in Testicular Germ Cell Tumors. Cancers 2019, 11, 796. [Google Scholar] [CrossRef] [Green Version]
- Fazal, Z.; Singh, R.; Fang, F.; Bikorimana, E.; Baldwin, H.; Corbet, A.; Tomlin, M.; Yerby, C.; Adra, N.; Albany, C.; et al. Hypermethylation and global remodelling of DNA methylation is associated with acquired cisplatin resistance in testicular germ cell tumours. Epigenetics 2021, 16, 1071–1084. [Google Scholar] [CrossRef]
- Soteriades, E.S.; Kim, J.; Christophi, C.A.; Kales, S.N. Cancer Incidence and Mortality in Firefighters: A State-of-the-Art Review and Meta-َAnalysis. Asian Pac. J. Cancer Prev. 2019, 20, 3221–3231. [Google Scholar] [CrossRef]
- Joensen, U.N.; Bossi, R.; Leffers, H.; Jensen, A.A.; Skakkebaek, N.E.; Jørgensen, N. Do perfluoroalkyl compounds impair human semen quality? Environ. Health Perspect. 2009, 117, 923–927. [Google Scholar] [CrossRef]
- Petersen, K.U.; Larsen, J.R.; Deen, L.; Flachs, E.M.; Hærvig, K.K.; Hull, S.D.; Bonde, J.P.E.; Tøttenborg, S.S. Per- and polyfluoroalkyl substances and male reproductive health: A systematic review of the epidemiological evidence. J. Toxicol. Environ. Health B Crit. Rev. 2020, 23, 276–291. [Google Scholar] [CrossRef]
- Steves, A.N.; Turry, A.; Gill, B.; Clarkson-Townsend, D.; Bradner, J.M.; Bachli, I.; Caudle, W.M.; Miller, G.W.; Chan, A.W.S.; Easley, C.A.t. Per- and polyfluoroalkyl substances impact human spermatogenesis in a stem-cell-derived model. Syst. Biol. Reprod. Med. 2018, 64, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Iwabuchi, K.; Senzaki, N.; Mazawa, D.; Sato, I.; Hara, M.; Ueda, F.; Liu, W.; Tsuda, S. Tissue toxicokinetics of perfluoro compounds with single and chronic low doses in male rats. J. Toxicol. Sci. 2017, 42, 301–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Zhou, Q.F.; Liao, C.Y.; Fu, J.J.; Jiang, G.B. Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch. Environ. Contam. Toxicol. 2009, 56, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Biegel, L.B.; Liu, R.C.; Hurtt, M.E.; Cook, J.C. Effects of ammonium perfluorooctanoate on Leydig cell function: In vitro, in vivo, and ex vivo studies. Toxicol. Appl. Pharmacol. 1995, 134, 18–25. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, H.; Chen, P.; Chen, X.; Sun, C.; Ge, R.S.; Su, Z.; Ye, L. Effects of gestational perfluorooctane sulfonate exposure on the developments of fetal and adult Leydig cells in F1 males. Environ. Pollut. 2020, 262, 114241. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.P.; Lee, J.C.; Wan, H.T.; Li, J.W.; Wong, A.Y.; Chan, T.F.; Oger, C.; Galano, J.M.; Durand, T.; Leung, K.S.; et al. Effects of in utero PFOS exposure on transcriptome, lipidome, and function of mouse testis. Environ. Sci. Technol. 2017, 51, 8782–8794. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, X.; Chen, X.; Chen, Y.; Liu, J.; Chen, F.; Ge, F.; Ye, L.; Lian, Q.; Ge, R.S. Perfluorooctane sulfonate impairs rat Leydig cell development during puberty. Chemosphere 2018, 190, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Huang, Q.; Wang, H.; Martin, F.L.; Liu, L.; Zhang, J.; Shen, H. Biphasic effects of perfluorooctanoic acid on steroidogenesis in mouse Leydig tumour cells. Reprod. Toxicol. 2019, 83, 54–62. [Google Scholar] [CrossRef]
- York, R.G.; Kennedy, G.L., Jr.; Olsen, G.W.; Butenhoff, J.L. Male reproductive system parameters in a two-generation reproduction study of ammonium perfluorooctanoate in rats and human relevance. Toxicology 2010, 271, 64–72. [Google Scholar] [CrossRef]
- Butenhoff, J.L.; Kennedy, G.L., Jr.; Frame, S.R.; O’Connor, J.C.; York, R.G. The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rat. Toxicology 2004, 196, 95–116. [Google Scholar] [CrossRef]
- Liu, R.C.; Hurtt, M.E.; Cook, J.C.; Biegel, L.B. Effect of the peroxisome proliferator, ammonium perfluorooctanoate (C8), on hepatic aromatase activity in adult male Crl:CD BR (CD) rats. Fundam Appl. Toxicol. 1996, 30, 220–228. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef] [PubMed]
- Hardell, E.; Kärrman, A.; van Bavel, B.; Bao, J.; Carlberg, M.; Hardell, L. Case-control study on perfluorinated alkyl acids (PFAAs) and the risk of prostate cancer. Environ. Int. 2014, 63, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Narita, S.; Nara, T.; Sato, H.; Koizumi, A.; Huang, M.; Inoue, T.; Habuchi, T. Research evidence on high-fat diet-induced prostate cancer development and progression. J. Clin. Med. 2019, 8, 597. [Google Scholar] [CrossRef] [Green Version]
- Freedland, S.J.; Aronson, W.J. Examining the relationship between obesity and prostate cancer. Rev. Urol. 2004, 6, 73–81. [Google Scholar]
- Labbé, D.P.; Zadra, G.; Yang, M.; Reyes, J.M.; Lin, C.Y.; Cacciatore, S.; Ebot, E.M.; Creech, A.L.; Giunchi, F.; Fiorentino, M.; et al. High-fat diet fuels prostate cancer progression by rewiring the metabolome and amplifying the MYC program. Nat. Commun. 2019, 10, 4358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, A.C.; Oyekunle, T.; Howard, L.E.; De Hoedt, A.M.; Kane, C.J.; Terris, M.K.; Cooperberg, M.R.; Amling, C.L.; Klaassen, Z.; Freedland, S.J.; et al. Obesity, race, and long-term prostate cancer outcomes. Cancer 2020, 126, 3733–3741. [Google Scholar] [CrossRef] [PubMed]
- Priolo, C.; Pyne, S.; Rose, J.; Regan, E.R.; Zadra, G.; Photopoulos, C.; Cacciatore, S.; Schultz, D.; Scaglia, N.; McDunn, J.; et al. AKT1 and MYC induce distinctive metabolic fingerprints in human prostate cancer. Cancer Res. 2014, 74, 7198–7204. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.Y.; Lu, R.; Hu, D.P.; Imir, O.B.; Zuo, Q.; Moline, D.; Afradiasbagharani, P.; Liu, L.; Lowe, S.; Birch, L.; et al. Per- and polyfluoroalkyl substances target and alter human prostate stem-progenitor cells. Biochem. Pharmacol. 2021, 197, 114902. [Google Scholar] [CrossRef]
- Madak-Erdogan, Z.; Band, S.; Zhao, Y.C.; Smith, B.P.; Kulkoyluoglu-Cotul, E.; Zuo, Q.; Santaliz Casiano, A.; Wrobel, K.; Rossi, G.; Smith, R.L.; et al. Free fatty acids rewire cancer metabolism in obesity-associated breast cancer via estrogen receptor and mTOR signaling. Cancer Res. 2019, 79, 2494–2510. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Mills, I.G. The interplay between prostate cancer genomics, metabolism, and the epigenome: Perspectives and future prospects. Front. Oncol. 2021, 11, 704353. [Google Scholar] [CrossRef]
- Deblois, G.; Tonekaboni, S.A.M.; Grillo, G.; Martinez, C.; Kao, Y.I.; Tai, F.; Ettayebi, I.; Fortier, A.-M.; Savage, P.; Fedor, A.N.; et al. Epigenetic switch–induced viral mimicry evasion in chemotherapy-resistant breast cancer. Cancer Discov. 2020, 10, 1312–1329. [Google Scholar] [CrossRef] [PubMed]
- McDonald, O.G.; Li, X.; Saunders, T.; Tryggvadottir, R.; Mentch, S.J.; Warmoes, M.O.; Word, A.E.; Carrer, A.; Salz, T.H.; Natsume, S.; et al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat. Genet. 2017, 49, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Makohon-Moore, A.P.; Zhang, M.; Reiter, J.G.; Bozic, I.; Allen, B.; Kundu, D.; Chatterjee, K.; Wong, F.; Jiao, Y.; Kohutek, Z.A.; et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat. Genet. 2017, 49, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutendra, G.; Kinnaird, A.; Dromparis, P.; Paulin, R.; Stenson, T.H.; Haromy, A.; Hashimoto, K.; Zhang, N.; Flaim, E.; Michelakis, E.D. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell 2014, 158, 84–97. [Google Scholar] [CrossRef] [Green Version]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Cross, J.R.; Thompson, C.B. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 2009, 324, 1076–1080. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyd, R.I.; Ahmad, S.; Singh, R.; Fazal, Z.; Prins, G.S.; Madak Erdogan, Z.; Irudayaraj, J.; Spinella, M.J. Toward a Mechanistic Understanding of Poly- and Perfluoroalkylated Substances and Cancer. Cancers 2022, 14, 2919. https://doi.org/10.3390/cancers14122919
Boyd RI, Ahmad S, Singh R, Fazal Z, Prins GS, Madak Erdogan Z, Irudayaraj J, Spinella MJ. Toward a Mechanistic Understanding of Poly- and Perfluoroalkylated Substances and Cancer. Cancers. 2022; 14(12):2919. https://doi.org/10.3390/cancers14122919
Chicago/Turabian StyleBoyd, Raya I., Saeed Ahmad, Ratnakar Singh, Zeeshan Fazal, Gail S. Prins, Zeynep Madak Erdogan, Joseph Irudayaraj, and Michael J. Spinella. 2022. "Toward a Mechanistic Understanding of Poly- and Perfluoroalkylated Substances and Cancer" Cancers 14, no. 12: 2919. https://doi.org/10.3390/cancers14122919
APA StyleBoyd, R. I., Ahmad, S., Singh, R., Fazal, Z., Prins, G. S., Madak Erdogan, Z., Irudayaraj, J., & Spinella, M. J. (2022). Toward a Mechanistic Understanding of Poly- and Perfluoroalkylated Substances and Cancer. Cancers, 14(12), 2919. https://doi.org/10.3390/cancers14122919







