Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment
Abstract
:1. Introduction
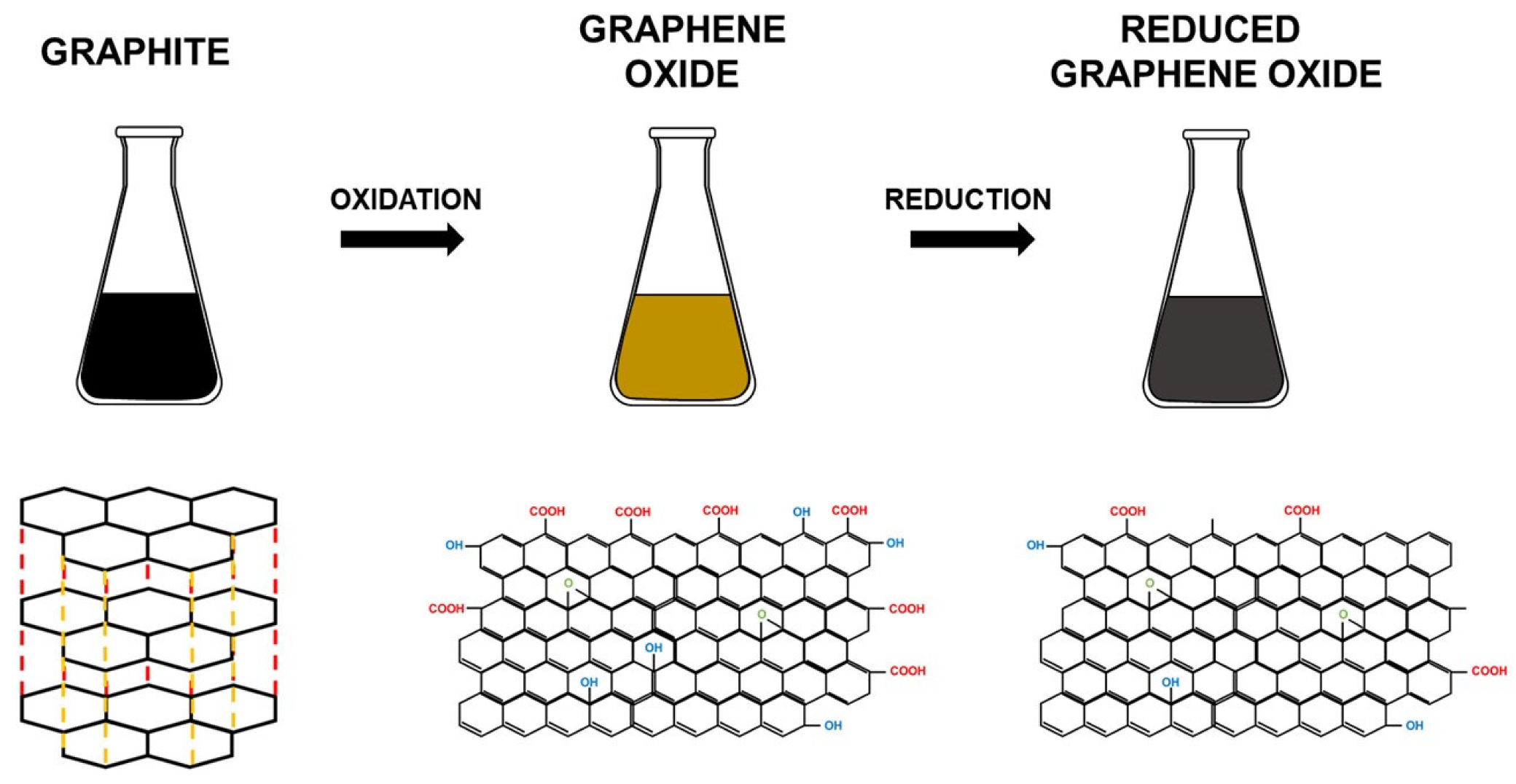
2. Evolution of GO Synthesis Methods
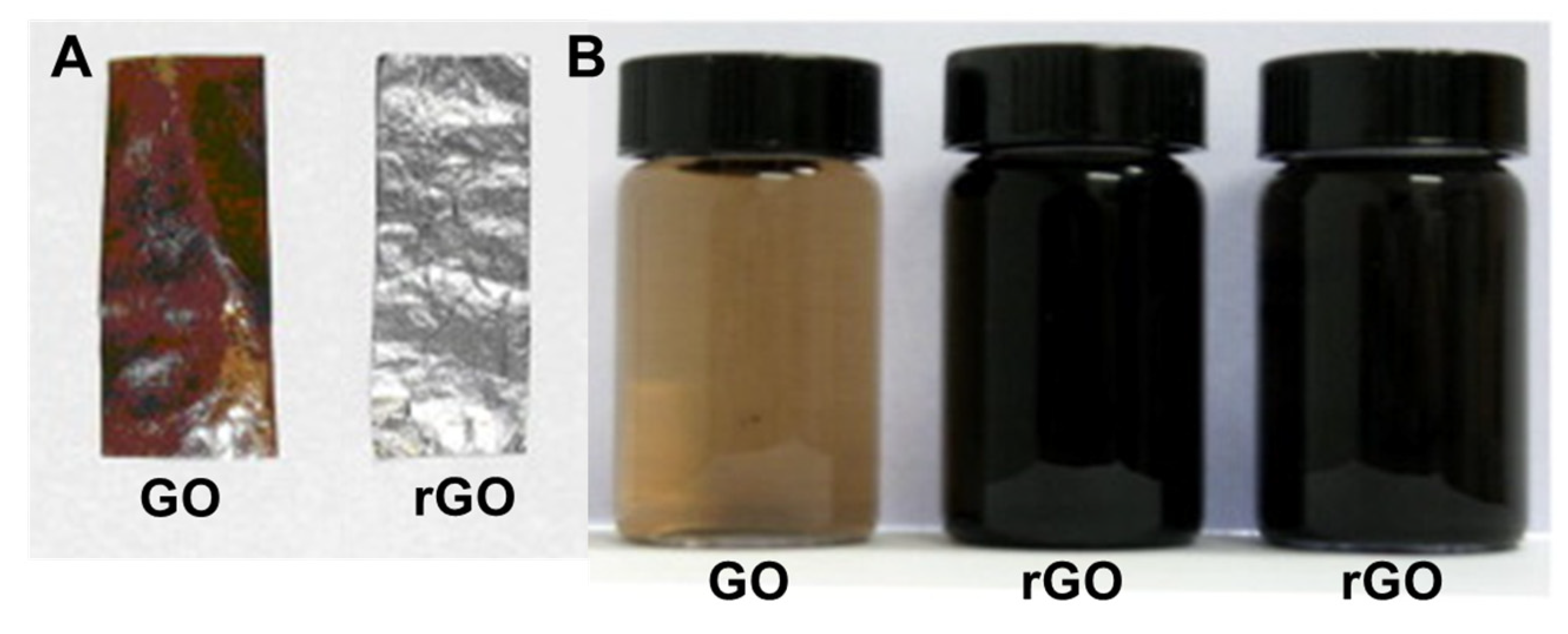
3. Reduction of GO
3.1. Chemical Reduction
3.2. Thermal Reduction
3.3. Solvothermal and Hydrothermal Reduction
4. Factors Affecting the Properties of GO
5. Applications of GO and rGO
5.1. GO as Antimicrobial Agent
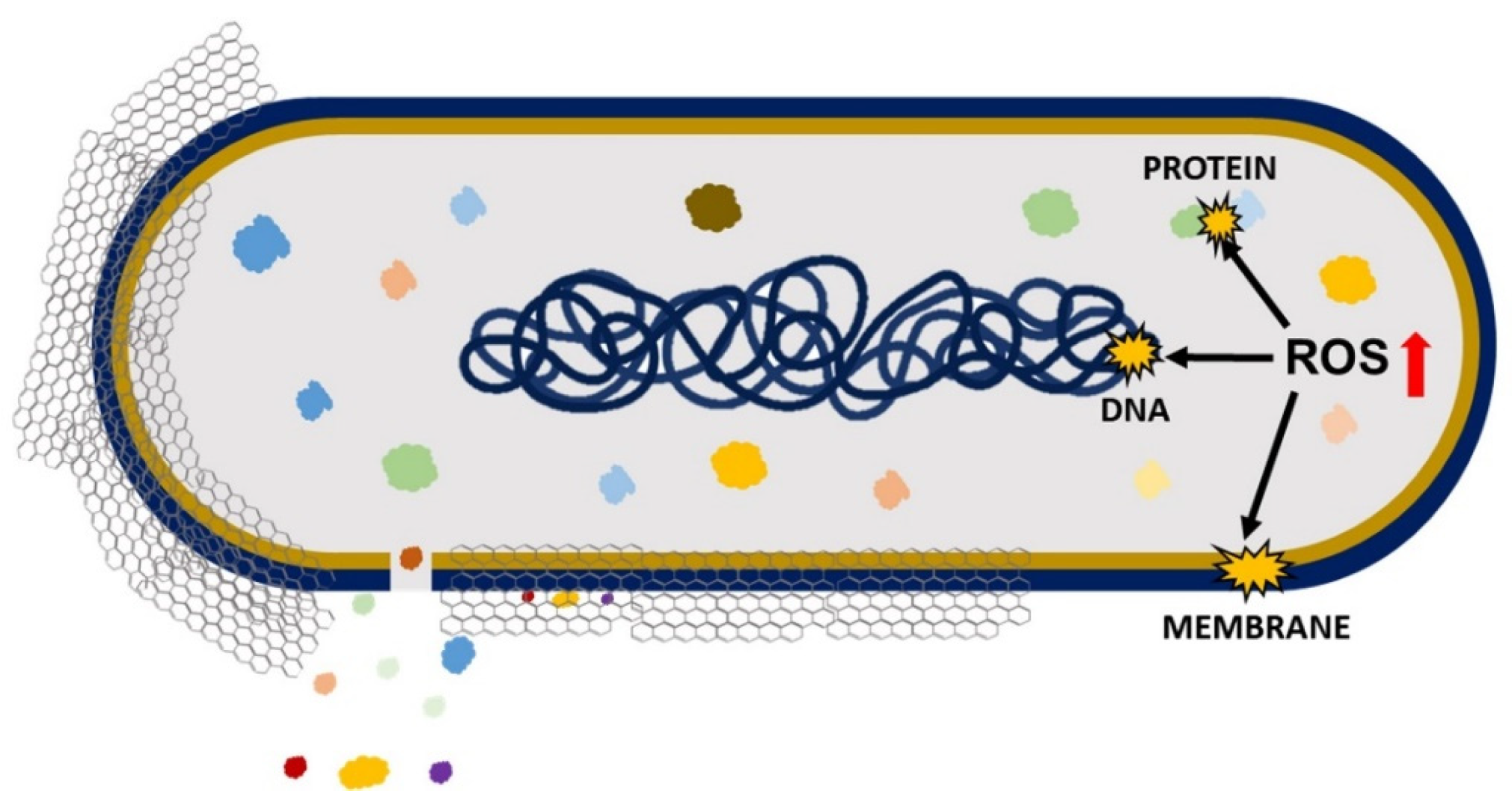
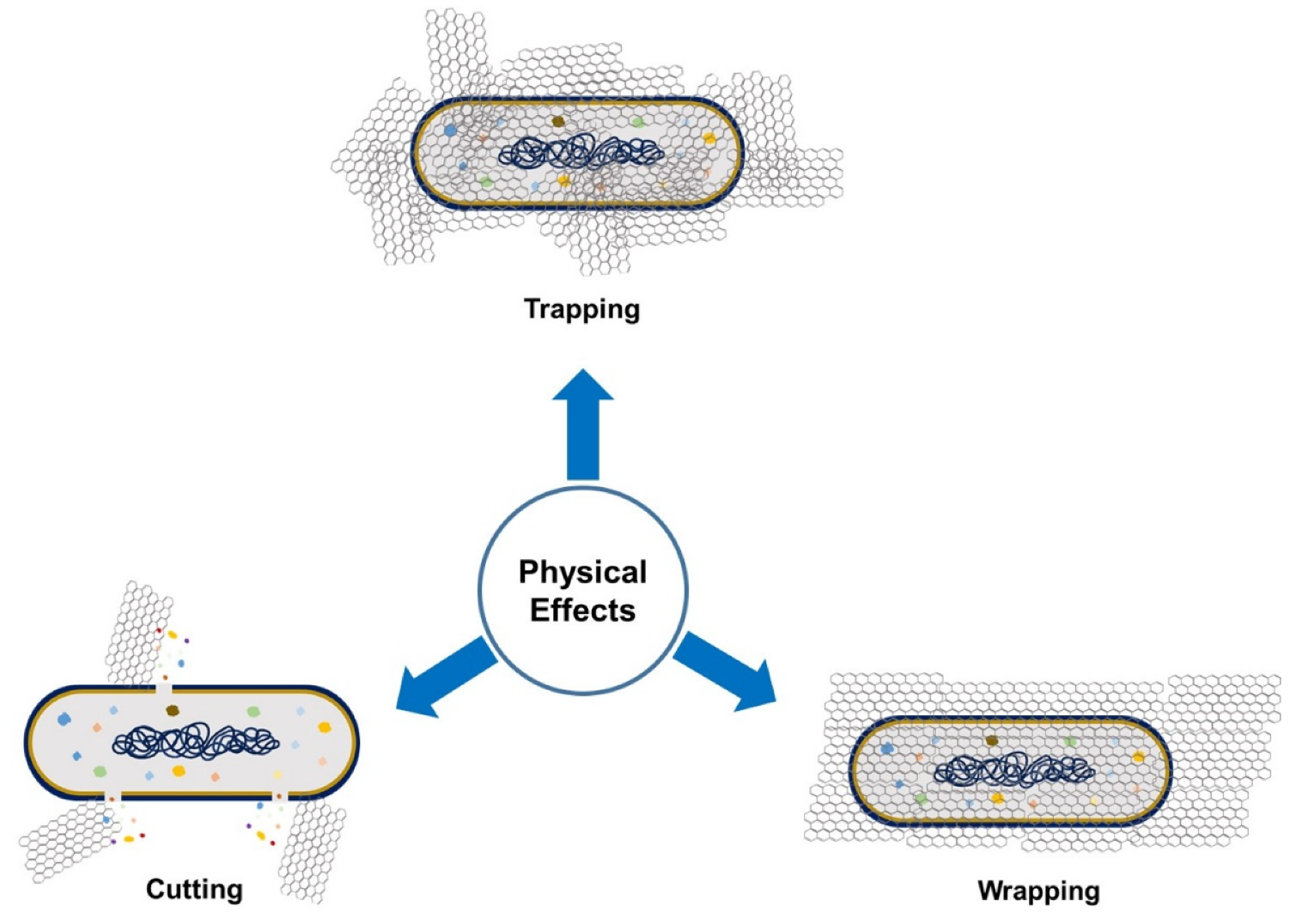
5.1.1. Antimicrobial Mechanism of GO
5.1.2. Factors Affecting the Antimicrobial Nature of GO
Lateral Size
Morphology
Aggregation
Basal Plane
Purity
Composites
5.2. Water Treatment (Purification)
5.3. Water Desalination Membranes
5.4. Removal of Oil Pollution
5.5. Cancer Treatment
5.6. Bone and Teeth Implantation
5.7. Scaffolds for Mammalian Cell Culture
5.8. Biofunctionalization with Proteins and DNA
5.9. Biosensing and Bioimaging
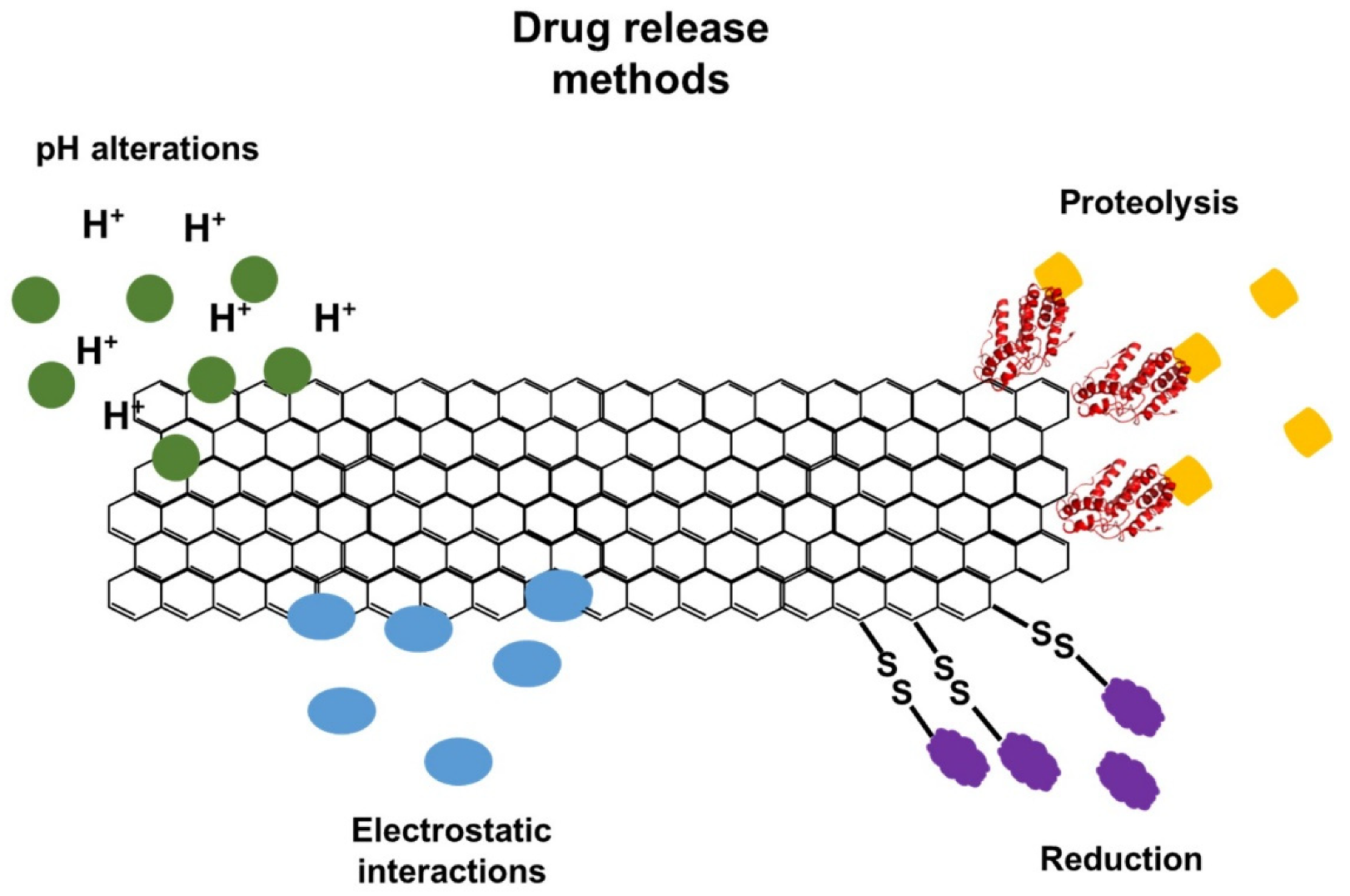
5.10. Gene Delivery and Drug Delivery
6. Environmental Toxicity
7. Cytotoxicity
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNPs | silver nanoparticles |
| ATP | adenosine triphosphate |
| CMC | carboxymethyl cellulose |
| DDT | dichloro-diphenyl-trichloroethane |
| DFT | density functional theory |
| DOX | Doxorubicin |
| EGFR | epidermal growth factor receptor |
| FAM | Carboxyfluorescein |
| FLG | few-layer graphene |
| GFP | green fluorescent protein |
| GO | graphene oxide |
| GOM | GO membrane |
| GPNs | graphene-based nanoparticles |
| GTP | guanosine triphosphate |
| HA | hyaluronic acid |
| hASCs | human adipose-derived stem cells |
| HEK293A | human embryonic kidney cells |
| HepG2 | hepatocellular carcinoma cells |
| hDPSCs | dental pulp stem cells |
| IC50 | half maximal inhibitory concentration |
| l-GO | large graphene oxide |
| LB | Luria-Bertani |
| Lys | lysozyme |
| MCF-7 | human breast cancer cell lines |
| MRI | magnetic resonance imaging |
| MS | melamine sponge |
| MSPE | magnetic solid-phase extraction |
| n-GO | negatively charged GO |
| N-GQDs | nitrogen-doped graphene quantum dots |
| NMP | N-methyl-2-pyrrolidinone |
| NR | natural rubber |
| p-GO | positively charged GO |
| PAHs | polycyclic aromatic hydrocarbons |
| PBS | phosphate-buffered saline |
| PCB 28 | 2,4,4′-trichlorobiphenyl |
| PCBs | polychlorinated biphenyls |
| PEF | porcine skin fibroblasts |
| PEG | polyethylene glycol |
| PEI | polyethylenimine |
| PET | polyethylene terephthalate |
| PHGOM | planar heterogeneous graphene oxide membrane |
| PVK | poly(vinyl-N-carbazole) |
| PSU | polysulfone |
| rGO | reduced graphene oxide |
| ROS | reactive oxygen species |
| s-GO | small graphene oxide |
| siRNA | small interference RNA |
| ssDNA | single-stranded DNA |
| TA | tannic acid |
References
- Anand, A.; Unnikrishnan, B.; Wei, S.; Chou, C.; Zhang, L.; Huang, C. Graphene oxide and carbon dots as broad-spectrum antimicrobial agents- a minireview. J. R. Soc. Chem. 2018, 4, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Kurt, O.K.; Zhang, J.; Pinkerton, K.E. Pulmonary health effects of air pollution. Curr. Opin. Pulm. Med. 2016, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Elmhagen, B.; Destouni, G.; Angerbjörn, A.; Borgström, S.; Boyd, E.; Cousins, S.A.; Lindborg, R. Interacting effects of change in climate, human population, land use, and water use on biodiversity and ecosystem services. Ecol. Soc. 2015, 20, 23. [Google Scholar] [CrossRef] [Green Version]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostructure Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Graphene-Info the Graphene Expert. 29th January 2019. Graphene Oxide: Introduction and Market News, Metalgrass LTD, Viewed 23rd July 2020. Available online: https://www.graphene-info.com/graphene-oxide (accessed on 9 January 2022).
- Bianco, A.; Cheng, H.; Enoki, T.; Gogotsi, Y.; Hurt, R.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.; Tascon, J.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Int. J. Founded Conjunction Am. Carbon Soc. 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Ray, S.C. Chapter 2: Application and Uses of Graphene Oxide. In Applications of Graphene and Graphene-Oxide Based Nanomaterials; University of South Africa Florida Park: Johannesburg, South Africa, 2015; pp. 39–55. [Google Scholar]
- Perrozzi, F.; Prezioso, S.; Ottaviano, L. Graphene oxide: From fundamentals to applications. J. Phys. Condens. Matter 2014, 27, 13002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.; La Chanceab, A.; Zengab, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. J. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizighannad, S.; Mitra, S. Stepwise Reduction of Graphene Oxide (GO) and Its Effects on Chemical and Colloidal Properties. Sci. Rep. 2018, 8, 10083. [Google Scholar] [CrossRef]
- Alam, S.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient Synthesis of Graphene Oxide Based on Improved Hummers Method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcano, D.; Kosynkin, D.; Berlin, J.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.; Lu, W.; Tour, J. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S. Graphene nanosheet: Synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [CrossRef] [PubMed]
- Somanathan, T.; Prasad, K.; Ostrikov, K.K.; Saravanan, A.; Krishna, V.M. Graphene oxide synthesis from agro waste. Nanomaterials 2015, 5, 826–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Pei, S. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar]
- Pei, S.; Zhao, J.; Du, J.; Ren, W.; Cheng, H.M. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon 2010, 48, 4466–4474. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Park, M.R.; Kwon, D.N.; Kim, J.H. Antibacterial activity of dithiothreitol reduced graphene oxide. J. Ind. Eng. Chem. 2013, 19, 1280–1288. [Google Scholar] [CrossRef]
- Khojasteh, H.; Safajou, H.; Mortazavi-Derazkola, S.; Salavati-Niasari, M.; Heydaryan, K.; Yazdani, M. Economic procedure for facile and eco-friendly reduction of graphene oxide by plant extracts; a comparison and property investigation. J. Clean. Prod. 2019, 229, 1139–1147. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Karim, M.; Hayami, S. Chemical, Thermal, and Light-Driven Reduction of Graphene Oxide: Approach to Obtain Graphene and its Functional Hybrids. In Graphene Materials—Advanced Applications; Kyzas, G.Z., Mitropoulos, A.C., Eds.; IntechOpen: London, UK, 2017. [Google Scholar]
- Dideikin, A.; Vul, Y. Graphene Oxide and Derivatives: The Place in Graphene Family. Front. Phys. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Kim, M.C.; Hwang, G.S.; Ruoff, R.S. Epoxide reduction with hydrazine on graphene: A first principles study. J. Chem. Phys. 2009, 131, 64704. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: Strategies for synthesis, reduction and frontier applications. RSC Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Dubin, S.; Gilje, S.; Wang, K.; Tung, V.; Cha, K.; Hall, A.; Farrar, J.; Varshneya, R.; Yang, Y.; Kaner, R. One-Step, Solvothermal Reduction Method for Producing Reduced Graphene Oxide Dispersions in Organic Solvents. ACS Nano 2010, 4, 3845–3852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Bao, Q.; Tang, L.A.L.; Zhong, Y.; Loh, K.P. Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem. Mater. 2009, 21, 2950–2956. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Atapour, A.; Amani, A.M.; Dashtaki, S.A.; Arjmand, O. Green synthesis of silver nanoparticles toward bio and medical applications: Review study. Artif. Cells Nanomed. Biotechnol. 2018, 46, S855–S872. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.; Shin, H. Control of size and physical properties of graphene oxide by changing the oxidation temperature. Carbon Lett. 2011, 13, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Wang, X.; Chang, C. Preparation and Characterization of Graphene Oxide. J. Nanomater. 2014, 2014, 276143. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 465. [Google Scholar] [CrossRef]
- Choudhary, P.; Das, S.K. Bio-reduced graphene oxide as a nanoscale antimicrobial coating for medical devices. ACS Omega 2019, 4, 387–397. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.; Liu, J.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Fallatah, H.; Elhaneid, M.; Ali-Boucetta, H.; Overton, T.; El Kadri, H.; Gkatzionis, K. Antibacterial effect of graphene oxide (GO) nano-particles against Pseudomonas putida biofilm of variable age. Environ. Sci. Pollut. Res. 2019, 26, 25057–25070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alayande, A.B.; Park, H.D.; Vrouwenvelder, J.S.; Kim, I.S. Implications of chemical reduction using hydriodic acid on the antimicrobial properties of graphene oxide and reduced graphene oxide membranes. Small 2019, 15, 1901023. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.N.; Ananda, S.; Rangappa, D. Preparation of reduced graphene oxide and its antibacterial properties. Mater. Today Proc. 2017, 4, 12300–12305. [Google Scholar] [CrossRef]
- Grace, E.; Annamalai, A.; Ponmari, G.; Vani, C.; Rose, A.; Marahatta, A.B.; Gunasekaran, V. Cytotoxicity and antibacterial characteristics of graphene-oxide nanosheets toward human pathogens. J. Nanosci. Nanotechnol. 2016, 16, 2447–2452. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Badiei, E.; Sangpour, P.; Bagheri, M.; Pazouki, M. Graphene oxide antibacterial sheets: Synthesis and characterization. Int. J. Eng. Trans. C 2014, 27, 1803–1808. [Google Scholar] [CrossRef]
- Aunkor, M.T.H.; Raihan, T.; Prodhan, S.H.; Metselaar, H.S.C.; Malik, S.U.F.; Azad, A.K. Antibacterial activity of graphene oxide nanosheet against multidrug resistant superbugs isolated from infected patients. R. Soc. Open Sci. 2020, 7, 200640. [Google Scholar] [CrossRef]
- Wu, X.; Tan, S.; Xing, Y.; Pu, Q.; Wu, M.; Zhao, J.X. Graphene oxide as an efficient antimicrobial nanomaterial for eradicating multi-drug resistant bacteria in vitro and in vivo. Colloids Surf. B Biointerfaces 2017, 157, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, I.; Bhattacharya, P.; Talukdar, M.; Neogi, S.; Pal, S.K.; Chakraborty, S. Bactericidal effect of graphene oxide and reduced graphene oxide: Influence of shape of bacteria. Colloid Interface Sci. Commun. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Yu, C.H.; Chen, G.Y.; Xia, M.Y.; Xie, Y.; Chi, Y.Q.; He, Z.Y.; Peng, Q. Understanding the sheet size-antibacterial activity relationship of graphene oxide and the nano-bio interaction-based physical mechanisms. Colloids Surf. B Biointerfaces 2020, 191, 111009. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Antibacterial activity of graphene-based materials. J. Mater. Chem. B 2016, 4, 6892–6912. [Google Scholar] [CrossRef] [Green Version]
- Mangadlao, J.; Santos, C.; Felipe, M.; Leon, A.; Rodrigues, D.; Advincula, R. On the antibacterial mechanism of graphene oxide (GO) Langmuir–Blodgett films. Chem. Commun. 2015, 51, 2886. [Google Scholar] [CrossRef]
- Palmieri, V.; Lauriola, M.C.; Ciasca, G.; Conti, C.; De Spirito, M.; Papi, M. The graphene oxide contradictory effects against human pathogens. Nanotechnology 2017, 28, 152001. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C.; Fang, G.; Wu, R.; Zhang, H.; Chai, Z.; Chen, C.; Yin, J. Light-Enhanced Antibacterial Activity of Graphene Oxide, Mainly via Accelerated Electron Transfer. Environ. Sci. Technol. 2017, 51, 10154–10161. [Google Scholar] [CrossRef]
- Perreault, F.; Faria, A.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Valentini, F.; Calcaterra, A.; Ruggiero, V.; Pichichero, E.; Martino, A.; Iosi, F.; Bertuccini, L.; Antonaroli, S.; Mardente, S.; Zicari, A.; et al. Functionalized Graphene Derivatives: Antibacterial Properties and Cytotoxicity. J. Nanomater. 2019, 2019, 2752539. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Yang, L.; Kong, J.; Chen, Y. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Zhou, H.; Jeong, D.; Kwon, J.; Eom, S.; Park, T.; Hong, S.; Lee, J. Wrinkled Surface-Mediated Antibacterial Activity of Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 1343–1351. [Google Scholar] [CrossRef]
- Karahan, H.; Wiraja, C.; Xu, C.; Wei, J.; Wang, Y.; Wang, L.; Liu, F.; Chen, Y. Graphene Materials in Antimicrobial Nanomedicine: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, e1701406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, V.; Bugli, F.; Lauriola, M.; Cacaci, M.; Torelli, R.; Ciasca, G.; Conti, C.; Sanguinetti, M.; Papi, M.; Spirito, M. Bacteria Meet Graphene: Modulation of Graphene Oxide Nanosheet Interaction with Human Pathogens for Effective Antimicrobial Therapy. ACS Biomater. Sci. Eng. 2017, 3, 619–627. [Google Scholar] [CrossRef]
- Cobos, M.; La-Pinta, I.; Quindós, G.; Fernández, M.; Fernández, M. Graphene Oxide–Silver Nanoparticle Nanohybrids: Synthesis, Characterization, and Antimicrobial Properties. Nanomaterials 2020, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Barbolina, I.; Woods, C.; Lozano, N.; Kostarelos, K.; Novoselov, K.; Roberts, I. Purity of graphene oxide determines its antibacterial activity. 2D Materials 2016, 3, 25025. [Google Scholar] [CrossRef] [Green Version]
- Dhand, V.; Rhee, K.; Kim, H.; Jung, D. A Comprehensive Review of Graphene Nanocomposites: Research Status and Trends’. J. Nanomater. 2013, 2013, 763953. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, K.; Vaidya, M.; Liauw, C.; Brownson, D.; Ramalingam, P.; Kamieniak, J.; Rowley-Neale, S.; Tetlowa, L.; Wilson-Nieuwenhuisa, J.; Brown, D.; et al. Antimicrobial activity of graphene oxide-metal hybrids. Int. Biodeterior. Biodegrad. 2017, 123, 182–190. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Chen, F.; Zhang, C.; Zhi, X.; Wang, K.; Cui, D. The antifungal activity of graphene oxide–silver nanocomposites’. Biomaterials 2013, 34, 3882–3890. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, A.; Subhan, F.; Jan, A.; Raza, M.; Khan, A.; Rahman, A.; Khan, U.; Tariq, M.; Yuan, Q. Tobramycin mediated silver nanospheres/graphene oxide composite for synergistic therapy of bacterial infection. J. Photochem. Photobiol. B Biol. 2018, 183, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Grande, C.; Mangadlao, J.; Fan, J.; Leon, A.; Ospina, J.; Rojas, J.; Rodrigues, D.; Advincula, R. Chitosan Cross-Linked Graphene Oxide Nanocomposite Films with Antimicrobial Activity for Application in Food Industry. Adv. Polym. Sci. MACROMEX 2014, 374, 1600114. [Google Scholar] [CrossRef]
- Panda, S.; Rout, T.; Prusty, A.; Ajayan, P.; Nayak, S. Electron Transfer Directed Antibacterial Properties of Graphene Oxide on Metals. Adv. Mater. 2018, 30, 1702149. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Marangoni, V.; de Faria, C.; Leite, I.; Silva, C.; Maroneze, C.; da-Silva, M.; Bagnato, V.; Inada, N. Graphene Oxide Mediated Broad-Spectrum Antibacterial Based on Bimodal Action of Photodynamic and Photothermal Effects. Front. Microbiol. 2015, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wu, J.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Chenga, C.; Chen, C. Impact of graphene oxide on the antibacterial activity of antibiotics against bacteria. Environ. Sci. Nano 2017, 4, 1016–1024. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Krishnan, R.; Thangavel, S.; Venugopal, G.; Kim, S. Removal of heavy metal ions from pharma-effluents usinggraphene-oxide nanosorbents and study of their adsorption kinetics. J. Ind. Eng. Chem. 2015, 30, 14–19. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, C.; Chu, W.; Vipin, A.K.; Sun, L. Environmental Remediation Applications of Carbon Nanotubes and Graphene Oxide: Adsorption and Catalysis. Nanomaterials 2019, 9, 439. [Google Scholar] [CrossRef] [Green Version]
- Das, P.; Goswami, S.; Maity, S. Removal of naphthalene present in synthetic wastewater using novel G/GO nano sheet synthesized from rice straw: Comparative analysis, isotherm and kinetics. Front. Nanosci Nanotech. 2016, 2, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhang, G.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Poly (amidoamine) modified graphene oxide as an efficient adsorbent for heavy metal ions. Polym. Chem. 2013, 4, 2164–2167. [Google Scholar] [CrossRef]
- Bhunia, P.; Kim, G.; Baik, C.; Lee, H. A strategically designed porous iron–iron oxide matrix on graphene for heavy metal adsorption. Chem. Commun. 2012, 48, 9888–9890. [Google Scholar] [CrossRef]
- Ali, I.; Basheer, A.; Mbianda, X.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019, 127, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, A.; Zambianchi, M.; Bettini, C.; Liscio, A.; Gazzano, M.; Corticelli, F.; Treossi, E.; Navacchia, M.; Palermo, V.; Melucci, M. Graphene oxide–polysulfone filters for tap water purification, obtained by fast microwave oven treatment. Nanoscale 2019, 11, 22780–22787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Cui, A.; Xu, Y.; Fu, X. Graphene oxide–TiO2 composite filtration membranes and their potential application for water purification. Carbon 2013, 62, 465–471. [Google Scholar] [CrossRef]
- Sweetman, M.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.; Hayball, J. Activated Carbon, Carbon Nanotubes and Graphene: Materials and Composites for Advanced Water Purification. J. Carbon Res. 2017, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 2017, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Qin, J.; Xia, P.; Guo, B.; Yang, C.; Song, C.; Wang, S. Graphene oxide–silver nanoparticle membrane for biofouling control and water purification. Chem. Eng. J. 2015, 281, 53–59. [Google Scholar] [CrossRef]
- Han, B.; Li, Y.; Qian, B.; He, Y.; Peng, L.; Yu, H. Adsorption and determination of polycyclic aromatic hydrocarbons in water through the aggregation of graphene oxide. Open Chem. 2018, 16, 716–725. [Google Scholar] [CrossRef]
- Zeng, S.; Mera, R.; Cao, Y.; Gan, N. Enrichment of polychlorinated biphenyl 28 from aqueous solutions using Fe3O4 grafted graphene oxide. Chem. Eng. J. 2013, 218, 108–115. [Google Scholar] [CrossRef]
- Le, G.; Nguyen, T.; Nguyen, M.; Quan, T.; Nguyen, T.; Sapi, A.; Szenti, I.; Mutyala, S.; Kukovecz, A.; Konya, Z.; et al. Cu–Fe Incorporated Graphene-Oxide Nanocomposite as Highly Efficient Catalyst in the Degradation of Dichlorodiphenyltrichloroethane (DDT) from Aqueous Solution. Top. Catal. 2020, 63, 1314–1324. [Google Scholar] [CrossRef]
- Wen, Q.; Jia, P.; Cao, L.; Li, J.; Quan, D.; Wang, L.; Zhang, Y.; Lu, D.; Jiang, L.; Guo, W. Electric-Field-Induced Ionic Sieving at Planar Graphene Oxide Heterojunctions for Miniaturized Water Desalination. Adv. Mater. 2020, 32, 1903954. [Google Scholar] [CrossRef]
- Shao, C.; Zhao, Y.; Qu, L. Tunable Graphene Systems for Water Desalination. Chemonanomat 2020, 6, 1028–1048. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Peng, G.; Chen, M.; Zhao, M.; He, Y.; Jiang, Y.; Zhang, X.; Qin, Y.; Lin, S. Reduced graphene oxide composites and its real-life application potential for in-situ crude oil removal. Chemosphere 2020, 249, 126141. [Google Scholar] [CrossRef] [PubMed]
- Songsaeng, S.; Thamyongkit, P.; Poompradub, S. Natural rubber/reduced-graphene oxide composite materials: Morphological and oil adsorption properties for treatment of oil spills. J. Adv. Res. 2019, 20, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, N.; Markova, M.; Panusheva, K.; Andreeva, T.; Speranza, G.; Wang, D.; Filipova, M.; Miloshev, G.; Georgieva, M. Aminated Graphene Oxide as a Potential New Therapy for Colorectal Cancer. Oxidative Med. Cell. Longev. 2019, 15, 3738980. [Google Scholar] [CrossRef] [Green Version]
- Campbell, E.; Hasan, M.; Pho, C.; Callaghan, K.; Akkaraju, G.; Naumov, A. Graphene Oxide as a Multifunctional Platform for Intracellular Delivery, Imaging, and Cancer Sensing. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Han, J.W.; Kim, E.S.; Park, J.H.; Kim, J.H. Reduction of graphene oxide by resveratrol: A novel and simple biological method for the synthesis of an effective anticancer nanotherapeutic molecule. Int. J. Nanomed. 2015, 10, 2951. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Han, J.W.; Park, J.H.; Kim, E.; Choi, Y.J.; Kwon, D.N.; Kim, J.H. Reduced graphene oxide–silver nanoparticle nanocomposite: A potential anticancer nanotherapy. Int. J. Nanomed. 2015, 10, 6257. [Google Scholar] [CrossRef] [Green Version]
- Kavinkumar, T.; Varunkumar, K.; Ravikumar, V.; Manivannan, S. Anticancer activity of graphene oxide-reduced graphene oxide-silver nanoparticle composites. J. Colloid Interface Sci. 2017, 505, 1125–1133. [Google Scholar] [CrossRef]
- Ganesan, K.; Jothi, V.K.; Natarajan, A.; Rajaram, A.; Ravichandran, S.; Ramalingam, S. Green synthesis of Copper oxide nanoparticles decorated with graphene oxide for anticancer activity and catalytic applications. Arab. J. Chem. 2020, 13, 6802–6814. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Amani, A.M.; Babapoor, A.; Arjmand, O. Applications of graphene oxide in case of nanomedicines and nanocarriers for biomolecules: Review study’. Drug Metab. Rev. 2019, 51, 12–41. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Tian, B.; Zhang, Y.; Liu, Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 2012, 33, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.J.; Lin, P.Y.; Huang, P.H.; Kuo, C.Y.; Shalumon, K.T.; Chen, M.Y.; Chen, J.P. Magnetic graphene oxide for dual targeted delivery of doxorubicin and photothermal therapy. Nanomaterials 2018, 8, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.; Yuan, Y.; Ren, J.; Zhang, Y.; Wang, Y.; Shan, X.; Liu, Q.; Zhang, Z. In vitro and in vivo comparative study of the phototherapy anticancer activity of hyaluronic acid-modified single-walled carbon nanotubes, graphene oxide, and fullerene. J. Nanoparticle Res. 2017, 19, 286. [Google Scholar] [CrossRef]
- Ren, L.; Pan, S.; Li, H.; Li, Y.; He, L.; Zhang, S.; Che, J.; Niu, Y. Effects of aspirin-loaded graphene oxide coating of a titanium surface on proliferation and osteogenic differentiation of MC3T3-E1 cells. Sci. Rep. 2018, 8, 15143. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Gao, C.; Tang, L.; Wang, C.; Chen, Q.; Zheng, Q.; Yang, S.; Sheng, S.; Zan, X. Lysozyme (Lys), Tannic Acid (TA), and Graphene Oxide (GO) Thin Coating for Antibacterial and Enhanced Osteogenesis. ACS Appl. Bio Mater. 2019, 3, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Raslan, A.; Del Burgo, L.S.; Ciriza, J.; Pedraz, J.L. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int. J. Pharm. 2020, 580, 119226. [Google Scholar] [CrossRef]
- Jasim, D.; Lazano, N.; Barbolina, I.; Busy, C.; Rodrigues, A.; Novoselov, K.; Kostarelos, K. Graphene-Based Papers as Substrates for Cell Growth: Characterisation and Impact on Mammalian Cells. Flat Chem. 2018, 12, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Choi, K.; Kim, Y.; Lim, K.; Seonwoo, H.; Park, Y.; Kim, D.; Choung, P.; Cho, C.; Kim, S.; et al. Bioactive effects of graphene oxide cell culture substratum on structure and function of human adipose-derived stem cells. J. Biomed. Mater. Research Part A 2013, 101, 3520–3530. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef]
- Laaksonen, P.; Kainlauri, M.; Laaksonen, T.; Shchepetov, A.; Jiang, H.; Ahopelto, J.; Linder, M.B. Interfacial engineering by proteins: Exfoliation and functionalization of graphene by hydrophobins. Angew. Chem. Int. Ed. 2010, 49, 4946–4949. [Google Scholar] [CrossRef]
- Chen, J.; Leng, J.; Yang, X.; Liao, L.; Liu, L.; Xiao, A. Enhanced performance of magnetic graphene oxide-immobilized laccase and its application for the decolorization of dyes. Molecules 2017, 22, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, A.; Zhu, C.T.; Xu, Y.; Wang, F.Q.; Wu, F.A.; Wang, J. Moving and unsinkable graphene sheets immobilized enzyme for microfluidic biocatalysis. Sci. Rep. 2017, 7, 4309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.W.; Lee, J.H.; Kang, S.H.; Hwang, E.Y.; Hwang, Y.S.; Lee, M.H.; Park, J.C. Enhanced neural cell adhesion and neurite outgrowth on graphene-based biomimetic substrates. BioMed Res. Int. 2014, 2014, 212149. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Woong Han, J.; Kim, E.; Kwon, D.N.; Park, J.K.; Kim, J.H. Enhanced green fluorescent protein-mediated synthesis of biocompatible graphene. J. Nanobiotechnol. 2014, 12, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- YuqiYang, Y.; Asiri, A.; Tang, Z.; Du, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar]
- Wang, Y.; Li, Z.; Weber, T.J.; Hu, D.; Lin, C.T.; Li, J.; Lin, Y. In situ live cell sensing of multiple nucleotides exploiting DNA/RNA aptamers and graphene oxide nanosheets. Anal. Chem. 2013, 85, 6775–6782. [Google Scholar] [CrossRef]
- Chen, W.; Yi, P.; Zhang, Y.; Zhang, L.; Deng, Z.; Zhang, Z. Composites of aminodextran-coated Fe3O4 nanoparticles and graphene oxide for cellular magnetic resonance imaging. ACS Appl. Mater. Interfaces 2011, 3, 4085–4091. [Google Scholar] [CrossRef]
- Hong, H.; Yang, K.; Zhang, Y.; Engle, J.W.; Feng, L.; Yang, Y.; Cai, W. In vivo targeting and imaging of tumor vasculature with radiolabeled, antibody-conjugated nanographene. ACS Nano 2012, 6, 2361–2370. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, S.K.; Goyal, R.; Gupta, K.C.; Kumar, P. Functionalized graphene oxide mediated nucleic acid delivery. Carbon 2013, 51, 224–235. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Pan, Y.; Sahoo, N.G.; Li, L. The application of graphene oxide in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Molino, N.M.; Wang, S.W. Caged protein nanoparticles for drug delivery. Curr. Opin. Biotechnol. 2014, 28, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trusek, A.; Kijak, E.; Granicka, L. Graphene oxide as a potential drug carrier–Chemical carrier activation, drug attachment and its enzymatic controlled release. Mater. Sci. Eng. C 2020, 116, 111240. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; Hussein, K.H. Graphene oxide as a carrier for drug delivery of methotrexate. Biointerface Res. Appl. Chem. 2021, 11, 14726–14735. [Google Scholar] [CrossRef]
- Rao, Z.; Ge, H.; Liu, L.; Zhu, C.; Min, L.; Liu, M.; Fan, L.; Li, D. Carboxymethyl cellulose modified graphene oxide as pH-sensitive drug delivery system. Int. J. Biol. Macromol. 2018, 107, 1184–1192. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.K. Microbial Extracellular Polymeric Substances: Ecological Functio and Impact on Soil Aggregation. Front. Microbiol. 2018, 19, 1636. [Google Scholar] [CrossRef] [Green Version]
- Malina, T.; Maršálková, E.; Holá, K.; Tuček, J.; Scheibe, M.; Zbořil, R.; Maršálek, B. Toxicity of graphene oxide against algae and cyanobacteria: Nanoblade- morphology- induced mechanical injury and self- protection mechanism. Carbon 2019, 155, 386–396. [Google Scholar] [CrossRef]
- Malhotra, N.; Villaflores, O.B.; Audira, G.; Siregar, P.; Lee, J.S.; Ger, T.R.; Hsiao, C.D. Toxicity studies on graphene-based nanomaterials in aquatic organisms: Current understanding. Molecules 2020, 25, 3618. [Google Scholar] [CrossRef]
- Dasmahapatra, A.; Dasari, T.; Tchounwou, P. Graphene-based nanomaterials toxicity in fish. Rev. Environ. Contam. Toxicol. 2019, 247, 1–58. [Google Scholar]
- Luján, L.; Román, S.; Toledo, C.; Parejo, O.; Mansour, A.; Abad, J.; Amassian, A.; Benito, A.; Maser, W.; Urbina, A. Environmental impact of the production of graphene oxide and reduced graphene oxide. SN Appl. Sci. 2019, 1, 179. [Google Scholar] [CrossRef] [Green Version]
- Bangeppagari, M.; Park, S.H.; Kundapur, R.R.; Lee, S.J. Graphene oxide induces cardiovascular defects in developing zebrafish (Danio rerio) embryo model: In-vivo toxicity assessment. Sci. Total Environ. 2019, 673, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.P.; Venturini, F.P.; Santos, F.; Zucolotto, V. Chronic toxicity in Ceriodaphnia dubia induced by graphene oxide. Chemosphere 2018, 190, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Dong, S.; Petersen, E.J.; Niu, J.; Chang, X.; Wang, P.; Mao, L. Biological uptake, distribution, and depuration of radio-labeled graphene in adult zebrafish: Effects of graphene size and natural organic matter. ACS Nano 2017, 11, 2872–2885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.; Yang, Y.; Tao, Y.; Jiang, Y.; Chen, B.; Zhu, X.; Li, B. A mechanism study on toxicity of graphene oxide to Daphnia magna: Direct link between bioaccumulation and oxidative stress. Environ. Pollut. 2018, 234, 953–959. [Google Scholar] [CrossRef]
- Lu, K.; Dong, S.; Xia, T.; Mao, L. Kupffer cells degrade 14C-labeled few-layer graphene to 14CO2 in liver through erythrophagocytosis. ACS Nano 2020, 15, 396–409. [Google Scholar] [CrossRef]
- Huang, C.; Xia, T.; Niu, J.; Yang, Y.; Lin, S.; Wang, X.; Xing, B. Transformation of 14C-Labeled Graphene to 14CO2 in the Shoots of a Rice Plant. Angew. Chem. 2018, 130, 9907–9911. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.; Jeyaraj, M.; Kim, J. Differential Cytotoxicity of Different Sizes of Graphene Oxide Nanoparticles in Leydig (TM3) and Sertoli (TM4) Cells’. Nanomaterials 2019, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, F.; Aziz, M.; Fatima, M.; Khan, M.; Ahmed, F.; Ahmad, R.; Ahmad, M.; Alkhuraiji, T.; Akram, M.; Raza, R.; et al. In Vitro Cytotoxicity and Morphological Assessments of GO-ZnO against the MCF-7 Cells: Determination of Singlet Oxygen by Chemical Trapping. Nanomaterials 2018, 8, 539. [Google Scholar] [CrossRef] [Green Version]
- Srikanth, K.; Sundar, L.; Pereira, E.; Duarte, A. Graphene oxide induces cytotoxicity and oxidative stress in bluegill sunfish cells. J. Appl. Toxicol. 2018, 8, 504–513. [Google Scholar] [CrossRef]
- Pelin, M.; Fusco, L.; León, V.; Martín, C.; Criado, A.; Sosa, S.; Vázquez, E.; Tubaro, A.; Prato, M. Differential cytotoxic effects of graphene and graphene oxide on skin keratinocytes. Sci. Rep. 2017, 7, 40572. [Google Scholar] [CrossRef] [Green Version]
- Liao, K.; Lin, Y.; Macosko, C.; Haynes, C. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhuc, S.; Jiang, X. Toxicity mechanism of graphene oxide and nitrogen-doped graphene quantum dots in RBCs revealed by surface-enhanced infrared absorption spectroscopy. Toxicol. Res. 2015, 4, 885–894. [Google Scholar] [CrossRef]
- Yang, H.; Pan, Y.; Chen, T.; Li, L.; Zou, W.; Liu, D.; Xue, D.; Wang, X.; Lin, G. Cytotoxicity and Immune Dysfunction of Dendritic Cells Caused by Graphene Oxide. Front. Pharmacol. 2020, 1206. [Google Scholar] [CrossRef] [PubMed]
| Microorganisms | Type | Nanomaterial | Evaluation Method | Dose | Antimicrobial Activity | Ref. |
|---|---|---|---|---|---|---|
| Bacillus subtilis | Gram-positive bacteria | rGO | Optical density | 1 × 1 cm2 graphene-based membrane | No growth detected | [38] |
| rGO | Agar well diffusion | 0.1–0.8 mg/mL | 1–3.5 mm | [39] | ||
| GO | Microdilution | 25–200 μg/μL | 48.86–91.40% | [40] | ||
| Escherichia coli | Gram-negative bacteria | GO | Colony counting | 40 μg/mL | 69.3 ± 6.1% | [41] |
| rGO | Colony counting | 40 μg/mL | 45.9 ± 4.8% | [41] | ||
| GO | Colony counting | 25–150 μg/mL | 18–87% | [20] | ||
| rGO | Colony counting | 25–150 μg/mL | 14–81 | [20] | ||
| GO | Colony counting | 3 mg/mL | 80% | [42] | ||
| rGO | Agar well diffusion | 0.1–0.8 mg/mL | 2–5 mm | [39] | ||
| GO | Agar well diffusion | 1 μg/μL | 39 mm | [43] | ||
| GO | Optical density | 62.5–500 μg/mL | ~40–95% | [44] | ||
| Fusarium graminearum | Fungal | GO | Germination spores | 10–500 μg/mL | 21.66–85.48% | [45] |
| Fusarium oxysporum | Fungal | GO | Germination spores | 10–500 μg/mL | 17.31–81.16% | [45] |
| Klebsiella pneumoniae | Gram-negative bacteria | GO | Agar well diffusion | 1 μg/μL | 41 mm | [43] |
| GO | Microdilution | 25–200 μg/μL | 50.76–92.80% | [40] | ||
| GO | Optical density | 62.5–500 μg/mL | 71.8–96.8% | [44] | ||
| Proteus mirabilis | Gram-negative bacteria | GO | Agar well diffusion | 1 μg/μL | 27 mm | [43] |
| Pseudomonas aeruginosa | Gram-negative bacteria | GO | Optical density | 25–200 µg/mL | 0–100% | [46] |
| rGO | Optical density | 25–200 µg/mL | 0–100% | [46] | ||
| GO | Growth curve | 1–5 mg/mL | Up to 78.7% | [47] | ||
| rGO | Growth curve | 1–3 mg/mL | 90.3–93.3% | [47] | ||
| GO | Agar well diffusion | 1 μg/μL | 38 mm | [43] | ||
| rGO | Optical density | 1 × 1 cm2 graphene-based membrane | No growth detected | [38] | ||
| GO | Optical density | 62.5–500 μg/mL | ~30–Above 95% | [44] | ||
| Pseudomonas syringae | Gram-negative bacteria | GO | Optical density | 10–500 μg/mL | 5–88.8% | [45] |
| Salmonella typhi | Gram-negative bacteria | GO | Microdilution | 25–200 μg/μL | 44.28–90.71% | [40] |
| Serratia marcescens | Gram-negative bacteria | GO | Agar well diffusion | 1 μg/μL | 39 mm | [43] |
| Staphylococcus aureus | Gram-positive bacteria | GO | Growth curve | 1–3 mg/mL | Up to 93.7% | [47] |
| rGO | Growth curve | 1–3 mg/mL | Up to 48.6% | [47] | ||
| GO | Agar well diffusion | 1 μg/μL | 38 mm | [43] | ||
| GO | Microdilution | 25–200 μg/μL | 51.36–92.12% | [40] | ||
| Streptococcus mutans | Gram-positive bacteria | GO | Colony counting | 12.5–50 μg/mL | Up to 80% | [48] |
| Xanthomonas campestris pv. Undulosa | Gram-negative bacteria | GO | Optical density | 10–500 μg/mL | 6.96–86.8% | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghulam, A.N.; dos Santos, O.A.L.; Hazeem, L.; Pizzorno Backx, B.; Bououdina, M.; Bellucci, S. Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. J. Funct. Biomater. 2022, 13, 77. https://doi.org/10.3390/jfb13020077
Ghulam AN, dos Santos OAL, Hazeem L, Pizzorno Backx B, Bououdina M, Bellucci S. Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. Journal of Functional Biomaterials. 2022; 13(2):77. https://doi.org/10.3390/jfb13020077
Chicago/Turabian StyleGhulam, Aminah N., Otávio A. L. dos Santos, Layla Hazeem, Bianca Pizzorno Backx, Mohamed Bououdina, and Stefano Bellucci. 2022. "Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment" Journal of Functional Biomaterials 13, no. 2: 77. https://doi.org/10.3390/jfb13020077
APA StyleGhulam, A. N., dos Santos, O. A. L., Hazeem, L., Pizzorno Backx, B., Bououdina, M., & Bellucci, S. (2022). Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. Journal of Functional Biomaterials, 13(2), 77. https://doi.org/10.3390/jfb13020077








