Tea (Camellia sinensis): A Review of Nutritional Composition, Potential Applications, and Omics Research
Abstract
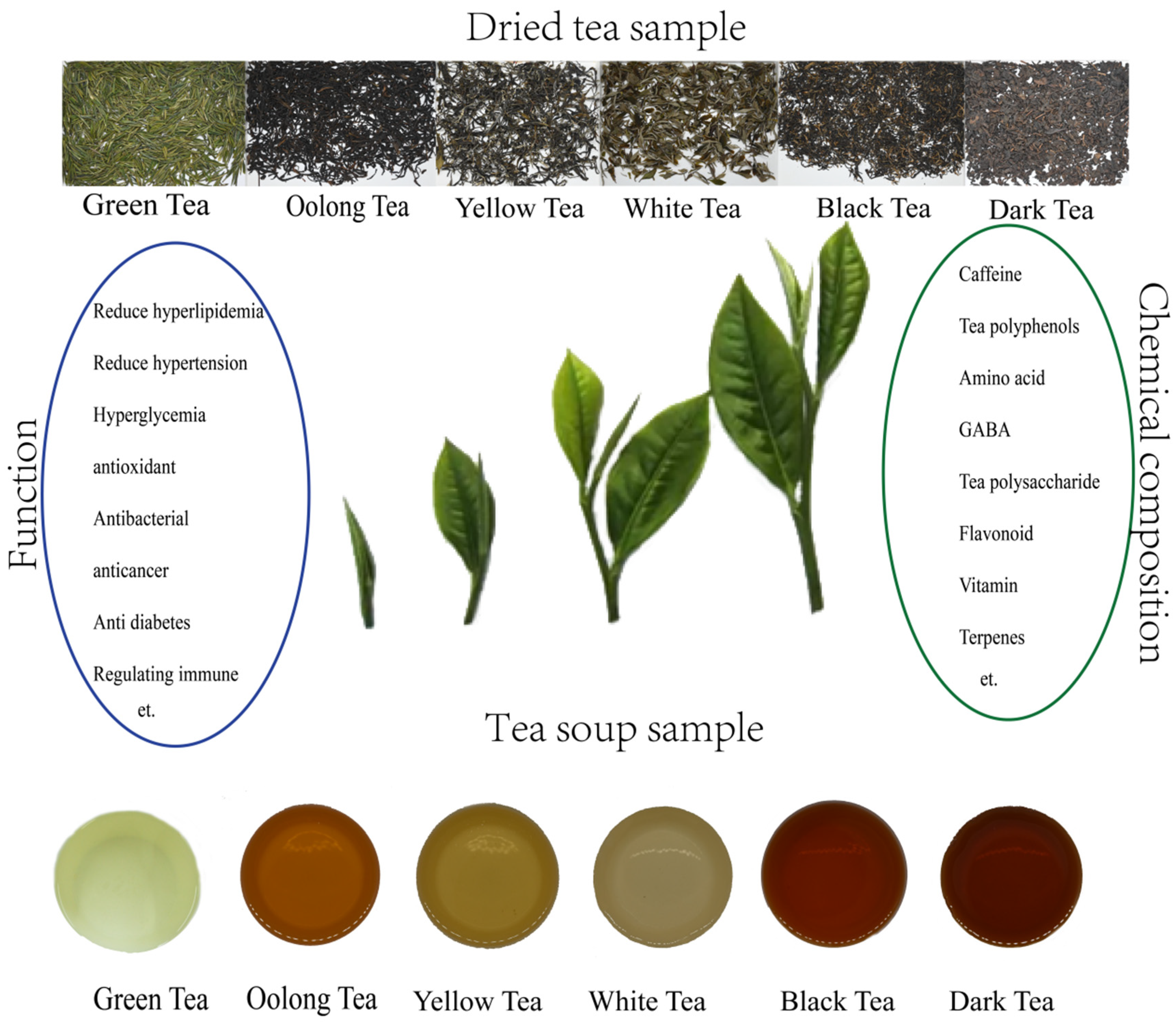
1. Introduction
2. Uses and Potential Applications of Tea
2.1. Food Products
2.2. Cosmetic Products
2.3. Folk Medicines
3. Bioactive Compounds
3.1. Polyphenol Compounds
3.2. Amino Acids and Peptides
3.3. Alkaloids
3.4. Aroma Compounds
3.5. Saponins
3.6. Polysaccharides
4. Pharmacological Properties
4.1. Antioxidant Properties
4.2. Anti-Cardiovascular and Anti-Cancer Properties
4.3. Anti-Obesity and Antidiabetic Properties
4.4. Neuroprotective Effects of Tea
4.5. Antimicrobial Properties
5. Omics Research
5.1. Genomics Research
5.2. Transcriptomics Research (RNA-Seq)
5.3. Metabolomics
6. Function Genes
6.1. House-Keeping Genes
6.2. Stress-Related Genes
6.3. Metabolism-Related Genes
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cherotich, L.; Kamunya, S.M.; Alakonya, A.; Msomba, S.W.; Uwimana, M.A.; Wanyoko, J.K.; Owuor, P.O. Variation in Catechin Composition of Popularly Cultivated Tea Clones in East Africa (Kenya). Am. J. Plant Sci. 2013, 04, 628–640. [Google Scholar] [CrossRef]
- Katoh, Y.; Katoh, M.; Omori, M. Identification of Teas Cultivated in Eastern, Southeastern and Southern Asia Based on Nucleotide Sequence Comparison of Ribulose 1,5-bisphosphate Carboxylase Large-subunit of Chloroplast DNA and 18S Ribosomal RNA of Nuclear DNA. Food Sci. Technol. Res. 2015, 21, 381–389. [Google Scholar] [CrossRef]
- Bora, D.K. Scaling relations of moment magnitude, local magnitude, and duration magnitude for earthquakes originated in northeast India. Earthq. Sci. 2016, 29, 153–164. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sharma, M.; Latkar, S.; Maurya, P. A Study on Aflatoxin Content in Black Tea Available in Domestic Market in India. J. Chem. Chem. Sci. 2018, 8, 562–568. [Google Scholar] [CrossRef]
- Berry, D. My tea party. Dairy Foods 2006, 107, 56. [Google Scholar] [CrossRef]
- Mondal, T.K. Micropropagation of tea (Camellia sinensis L.). In Micropropagation of Woody Trees and Fruits; Springer: Berlin/Heidelberg, Germany, 2003; pp. 671–719. [Google Scholar] [CrossRef]
- Camargo, L.E.A.; Pedroso, L.S.; Vendrame, S.C.; Mainardes, R.M.; Khalil, N.M. Antioxidant and antifungal activities of Camellia sinensis (L.) Kuntze leaves obtained by different forms of production. Braz. J. Biol. 2016, 76, 428–434. [Google Scholar] [CrossRef]
- Liu, J.Y.; He, D.; Xing, Y.F.; Zeng, W.; Xing, X.H. Effects of bioactive components of Pu-erh tea on gut microbiomes and health: A review. Food Chem. 2021, 353, 129439. [Google Scholar] [CrossRef]
- Sun, Q.; Yan, X. History of pu’er tea and comparative study for the effect of its various extracts on lipid-lowering diet. Pak. J. Pharm. Sci. 2014, 27, 1015–1022. [Google Scholar]
- Taylor, A.E.; Smith, G.D.; Munafò, M.R. Associations of coffee genetic risk scores with consumption of coffee, tea and other beverages in the UK Biobank. Addiction 2018, 113, 148–157. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.W.; Cao, J.; Jiang, W. Tea polyphenols (TP): A promising natural additive for the manufacture of multifunctional active food packaging films. Crit. Rev. Food Sci. Nutr. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Dubey, K.K.; Janve, M.; Ray, A.; Singhal, R.S. Ready-to-Drink Tea. In Trends in Non-Alcoholic Beverages; Academic Press: Cambridge, MA, USA, 2020; pp. 101–140. [Google Scholar] [CrossRef]
- Zhu, F.; Sakulnak, R.; Wang, S. Effect of black tea on antioxidant, textural, and sensory properties of Chinese steamed bread. Food Chem. 2016, 194, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Hou, G.G.; Ding, J.; Du, X. Comparative study on textural and rheological properties between dry white salted noodle and yellow alkaline noodle as influenced by different tea extracts. J. Food Process. Preserv. 2020, 44, 14981. [Google Scholar] [CrossRef]
- Lavelli, V.; Vantaggi, C.; Corey, M.; Kerr, W. Formulation of a Dry Green Tea-Apple Product: Study on Antioxidant and Color Stability. J. Food Sci. 2010, 75, C184–C190. [Google Scholar] [CrossRef] [PubMed]
- Goh, R.; Gao, J.; Ananingsih, V.K.; Ranawana, V.; Henry, J.; Zhou, W. Green tea catechins reduced the glycaemic potential of bread: An in vitro digestibility study. Food Chem. 2015, 180, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Z.; Kerry, J.P.; Sheehan, D.; Buckley, D.J.; Morrissey, P.A. Antioxidative effect of dietary tea catechins on lipid oxidation of long-term frozen stored chicken meat. Meat Sci. 2001, 57, 331–336. [Google Scholar] [CrossRef]
- Sullivan, C.; Lynch, A.M.; Lynch, P.B.; Buckley, D.J.; Kerry, J.P. Use of Antioxidants in Chicken Nuggets Manufactured with and Without the Use of Salt and/or Sodium Tripolyphosphate: Effects on Product Quality and Shelf-life Stability. Int. J. Poult. Sci. 2004, 3, 345–353. [Google Scholar] [CrossRef]
- Mitsumoto, M.; O’Grady, M.N.; Kerry, J.P.; Buckley, D.J. Addition of tea catechins and vitamin C on sensory evaluation, colour and lipid stability during chilled storage in cooked or raw beef and chicken patties. Meat Sci. 2005, 69, 773–779. [Google Scholar] [CrossRef]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of Tea (Camellia sinensis) and Its Active Constituents in Cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Mukhtar, H. Green tea polyphenol (–)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J. Leukoc. Biol. 2001, 69, 719–726. [Google Scholar] [CrossRef]
- Hong, Y.H.; Jung, E.Y.; Noh, D.O.; Suh, H.J. Physiological effects of formulation containing tannase-converted green tea extract on skin care: Physical stability, collagenase, elastase, and tyrosinase activities. Integr. Med. Res. 2014, 3, 25–33. [Google Scholar] [CrossRef]
- Bi, A.; Hang, Q.; Huang, Y.; Zheng, S.; Bi, X.; Zhang, Z.; Yin, Z.; Luo, L. l-Theanine attenuates neointimal hyperplasia via suppression of vascular smooth muscle cell phenotypic modulation. J. Nutr. Biochem. 2020, 82, 108398. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Pandit, A.H.; Nadeem, M.; Pandit, A.H.; Rattan, S. γ-Radiation induced L-glutamic acid grafted highly porous, pH-responsive chitosan hydrogel beads: A smart and biocompatible vehicle for controlled anti-cancer drug delivery. Int. J. Biol. Macromol. 2021, 182, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Makoto, B.; Nobuhiro, K.; Kaori, O.; Keiko, N.; Noboru, M. The Effects of Glycine on Subjective Daytime Performance in Partially Sleep-Restricted Healthy Volunteers. Front. Neurol. 2012, 3, 61. [Google Scholar] [CrossRef]
- Shah, N.A.; Ren, Y.; Lan, R.T.; Lv, J.C.; Gul, R.M.; Tan, P.F.; Huang, S.S.; Tan, L.; Xu, J.Z.; Li, Z.M. Ultrahigh molecular weight polyethylene with improved crosslink density, oxidation stability, and microbial inhibition by chemical crosslinking and tea polyphenols for total joint replacements. J. Appl. Polym. Sci. 2021, 138, 51261. [Google Scholar] [CrossRef]
- Ahmad, R.S.; Butt, M.S.; Sultan, M.T.; Mushtaq, Z.; Ahmad, S.; Dewanjee, S.; De Feo, V.; Zia-Ul-Haq, M. Preventive role of green tea catechins from obesity and related disorders especially hypercholesterolemia and hyperglycemia. J. Transl. Med. 2015, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Guijie, C.; Yuan, Q.; Saeeduddin, M.; Ou, S.; Zeng, X.; Hong, Y. Recent advances in tea polysaccharides: Extraction, purification, physicochemical characterization and bioactivities. Carbohydr. Polym. 2016, 153, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, N.; Morikawa, T.; Motai, C.; Ninomiya, K.; Okugawa, S.; Nishida, A.; Yoshikawa, M.; Muraoka, O. The Antiproliferative Effect of Chakasaponins I and II, Floratheasaponin A, and Epigallocatechin 3-O-Gallate Isolated from Camellia sinensis on Human Digestive Tract Carcinoma Cell Lines. Int. J. Mol. Sci. 2016, 17, 1979. [Google Scholar] [CrossRef]
- Belous, O.; Platonova, N. Content of Vitamin C and Ruthin in Krasnodar Tea. Green Rep. 2020, 1, 1–4. [Google Scholar] [CrossRef]
- Jin, L.; Puligundla, P.; Ko, S.; Wan, X.C. A review on selenium-enriched green tea: Fortification methods, biological activities and application prospect. Sains Malays. 2014, 43, 1685–1692. [Google Scholar]
- Zafar, K.; Naeem, A.; Mirza, F. A Comparative Study of Caffeinated Beverages; Tea and Energy Drink Consumption on Attention Span of Healthy Male and Female Subjects. Int. J. Endorsing Health Sci. Res. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Ning, J.; Li, D.; Luo, X.; Ding, D.; Song, Y.; Zhang, Z.; Wan, X. Stepwise Identification of Six Tea (Camellia sinensis (L.)) Categories Based on Catechins, Caffeine, and Theanine Contents Combined with Fisher Discriminant Analysis. Food Anal. Methods 2016, 9, 3242–3250. [Google Scholar] [CrossRef]
- Sun, L.; Xu, H.; Ye, J.; Gaikwad, N.W. Comparative effect of black, green, oolong, and white tea intake on weight gain and bile acid metabolism. Nutrition 2019, 65, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Engelhardt, U.H.; Lin, Z.; Kaiser, N.; Maiwald, B. Flavonoids, phenolic acids, alkaloids and theanine in different types of authentic Chinese white tea samples. J. Food Compos. Anal. 2017, 57, 8–15. [Google Scholar] [CrossRef]
- Yılmaz, C.; Özdemir, F.; Gökmen, V. Investigation of free amino acids, bioactive and neuroactive compounds in different types of tea and effect of black tea processing. LWT 2020, 117, 108655. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, S.; Xu, P.; Chen, H.; Lin-Shiau, S.Y.; Deng, Y.T.; Lin, J.K. Fermentation process enhanced production and bioactivities of oolong tea polysaccharides. Food Res. Int. 2012, 46, 158–166. [Google Scholar] [CrossRef]
- Jiang, H.; Engelhardt, U.H.; Thräne, C.; Maiwald, B.; Stark, J. Determination of flavonol glycosides in green tea, oolong tea and black tea by UHPLC compared to HPLC. Food Chem. 2015, 183, 30–35. [Google Scholar] [CrossRef]
- Su, H.; Wu, W.; Wan, X.; Ning, J. Discriminating geographical origins of green tea based on amino acid, polyphenol, and caffeine content through high-performance liquid chromatography: Taking Lu’an guapian tea as an example. Food Sci. Nutr. 2019, 7, 2167–2175. [Google Scholar] [CrossRef]
- Rahman, M.; Jahan, I.A.; Ahmed, S.; Ahmed, K.S.; Ahmad, I. Bioactive compounds and antioxidant activity of black and green tea available in Bangladesh. Food Res. 2021, 5, 107–111. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, F.; Qin, L.; Zhang, N.; Cao, Q.; Schwab, W.; Li, D.; Song, C. Dynamic change in amino acids, catechins, alkaloids, and gallic acid in six types of tea processed from the same batch of fresh tea (Camellia sinensis L.) leaves. J. Food Compos. Anal. 2019, 77, 28–38. [Google Scholar] [CrossRef]
- Lee, C.Y.; Oh, J.H.; Chung, J.O.; Rha, C.S.; Park, M.Y.; Hong, Y.D.; Kim, W.K.; Shim, S.M. Effect of whole green tea products including catechins, polysaccharides, and flavonols on the metabolism of added sugars. Food Biosci. 2021, 41, 100936. [Google Scholar] [CrossRef]
- Liu, Z.H.; Cao, Y.H.; Xu, Y.; Li, C.W.; Si, W.Y. Assessment of the content and stability of tea polyphenols, free amino acids and vitamin C in four kinds of tea beverages. Sci. Technol. Food Ind. 2019, 40, 38–44. [Google Scholar] [CrossRef]
- Han, M.; Zhao, G.; Wang, Y.; Wang, D.; Sun, F.; Ning, J.; Wan, X.; Zhang, J. Erratum: Corrigendum: Safety and anti-hyperglycemic efficacy of various tea types in mice. Sci. Rep. 2016, 6, 35699. [Google Scholar] [CrossRef] [PubMed]
- Horanni, R.; Engelhardt, U.H. Determination of amino acids in white, green, black, oolong, pu-erh teas and tea products. J. Food Compos. Anal. 2013, 31, 94–100. [Google Scholar] [CrossRef]
- Wu, T.; Guo, Y.; Liu, R.; Wang, K.; Zhang, M. Black tea polyphenols and polysaccharides improve body composition, increase fecal fatty acid, and regulate fat metabolism in high-fat diet-induced obese rats. Food Funct. 2016, 7, 2469–2478. [Google Scholar] [CrossRef] [PubMed]
- Luximon-Ramma, A.; Bahorun, T.; Crozier, A.; Zbarsky, V.; Datla, K.P.; Dexter, D.T.; Aruoma, O.I. Characterization of the antioxidant functions of flavonoids and proanthocyanidins in Mauritian black teas. Food Res. Int. 2005, 38, 357–367. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Li, W.; Zhao, L.; Meng, F.; Wang, Y.; Tan, H.; Yang, H.; Wei, C.; Wan, X.; et al. Tissue-Specific, Development-Dependent Phenolic Compounds Accumulation Profile and Gene Expression Pattern in Tea Plant (Camellia sinensis). PLoS ONE 2013, 8, e62315. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Zhang, J.; Xu, L.; Zhou, H.; Wei, K.; Peng, L.; Zhang, J.; Liu, Z.; Wei, X. Integration of non-targeted metabolomics and E-tongue evaluation reveals the chemical variation and taste characteristics of five typical dark teas. LWT 2021, 150, 111875. [Google Scholar] [CrossRef]
- Gong, Z.P.; Ouyang, J.; Wu, X.L.; Zhou, F.; Lu, D.M.; Zhao, C.J.; Liu, C.F.; Zhu, W.; Zhang, J.C.; Li, N.X.; et al. Dark tea extracts: Chemical constituents and modulatory effect on gastrointestinal function. Biomed. Pharmacother. 2020, 130, 110514. [Google Scholar] [CrossRef]
- Beecher, G.R.; Warden, B.A.; Merken, H. Analysis of Tea Polyphenols. Proc. Soc. Exp. Boil. Med. 1999, 220, 267–270. [Google Scholar] [CrossRef]
- Lin, Y.L.; Juan, I.M.; Chen, Y.L.; Liang, Y.C.; Lin, J.K. Composition of Polyphenols in Fresh Tea Leaves and Associations of Their Oxygen-Radical-Absorbing Capacity with Antiproliferative Actions in Fibroblast Cells. J. Agric. Food Chem. 1996, 44, 1387–1394. [Google Scholar] [CrossRef]
- Anesini, C.; Ferraro, G.E.; Filip, R. Total Polyphenol Content and Antioxidant Capacity of Commercially Available Tea (Camellia sinensis) in Argentina. J. Agric. Food Chem. 2008, 56, 9225–9229. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Z.Y.; Liu, Z.Y.; Feng, Z.H.; Zhang, L.; Wan, X.C.; Yang, X.G. Identification of d-amino acids in tea leaves. Food Chem. 2020, 317, 126428. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Yang, W.J.; Ahammed, G.J.; Shen, C.; Yan, P.; Li, X.; Han, W.Y. Developmental changes in carbon and nitrogen metabolism affect tea quality in different leaf position. Plant Physiol. Biochem. 2016, 106, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.; Zhu, Y.; Guo, L.; Zhang, Y.; et al. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [CrossRef]
- Feng, L.; Yang, T.; Zhang, Z.; Li, F.; Chen, Q.; Sun, J.; Shi, C.; Deng, W.W.; Tao, M.; Tai, Y.; et al. Identification and characterization of cationic amino acid transporters (CATs) in tea plant (Camellia sinensis). Plant Growth Regul. 2017, 84, 57–69. [Google Scholar] [CrossRef]
- Carrera, C.S.; Reynoso, C.M.; Funes, G.J.; Martínez, M.J.; Dardanelli, J.; Resnik, S.L. Amino acid composition of soybean seeds as affected by climatic variables. Pesqui. Agropecuária Bras. 2011, 46, 1579–1587. [Google Scholar] [CrossRef]
- Korbee, N.; Figueroa, F.D.L.; Aguilera, J. Effect of light quality on the accumulation of photosynthetic pigments, proteins and mycosporine-like amino acids in the red alga Porphyra leucosticta (Bangiales, Rhodophyta). J. Photochem. Photobiol. B Biol. 2005, 80, 71–78. [Google Scholar] [CrossRef]
- Miyauchi, S.; Yonetani, T.; Yuki, T.; Tomio, A.; Bamba, T.; Fukusaki, E. Quality evaluation of green tea leaf cultured under artificial light condition using gas chromatography/mass spectrometry. J. Biosci. Bioeng. 2017, 123, 197–202. [Google Scholar] [CrossRef]
- Ye, Y.; Yan, J.; Cui, J.; Mao, S.; Li, M.; Liao, X.; Tong, H. Dynamic changes in amino acids, catechins, caffeine and gallic acid in green tea during withering. J. Food Compos. Anal. 2018, 66, 98–108. [Google Scholar] [CrossRef]
- Bai, L.; Takagi, S.; Ando, T.; Yoneyama, H.; Ito, K.; Mizugai, H.; Isogai, E. Antimicrobial activity of tea catechin against canine oral bacteria and the functional mechanisms. J. Veter. Med. Sci. 2016, 78, 1439–1445. [Google Scholar] [CrossRef]
- Hečimović, I.; Belščak-Cvitanović, A.; Horžić, D.; Komes, D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011, 129, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liang, L.; Li, Y.; Yang, T.; Wang, Y. Studies of quality development and major chemical composition of green tea processed from tea with different shoot maturity. LWT 2021, 142, 111055. [Google Scholar] [CrossRef]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Baljak, A. Determination of Caffeine Content in Tea and Maté Tea by using Different Methods. Czech J. Food Sci. 2009, 27, S213–S216. [Google Scholar] [CrossRef]
- Zareef, M.; Chen, Q.; Ouyang, Q.; Kutsanedzie, F.Y.; Hassan, M.M.; Viswadevarayalu, A.; Wang, A. Prediction of amino acids, caffeine, theaflavins and water extract in black tea using FT-NIR spectroscopy coupled chemometrics algorithms. Anal. Methods 2018, 10, 3023–3031. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef]
- Shao, C.; Zhang, C.; Lv, Z.; Shen, C. Pre- and post-harvest exposure to stress influence quality-related metabolites in fresh tea leaves (Camellia sinensis). Sci. Hortic. 2021, 281, 109984. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, H.; Kang, J.; Warusawitharana, H.K.; Chen, S.; Wang, K.; Yu, F.; Wu, Y.; He, P.; Tu, Y.; et al. Comparative Transcriptome and Phytochemical Analysis Provides Insight into Triterpene Saponin Biosynthesis in Seeds and Flowers of the Tea Plant (Camellia sinensis). Metabolites 2022, 12, 204. [Google Scholar] [CrossRef]
- Wu, X.; Jia, L.; Wu, J.; Liu, Y.; Kang, H.; Liu, X.; Li, P.; He, P.; Tu, Y.; Li, B. Simultaneous determination and quantification of triterpene saponins from Camellia sinensis seeds using UPLC-PDA-QTOF-MS/MS. Molecules 2019, 24, 3794. [Google Scholar] [CrossRef]
- Wei, X.L.; Chen, M.A.; Xiao, J.B.; Liu, Y.; Yu, L.; Zhang, H.; Wang, Y.F. Composition and bioactivity of tea flower polysaccharides obtained by different methods. Carbohydr. Polym. 2010, 79, 418–422. [Google Scholar] [CrossRef]
- Yang, C.S.; Hong, J. Prevention of Chronic Diseases by Tea: Possible Mechanisms and Human Relevance. Annu. Rev. Nutr. 2013, 33, 161–181. [Google Scholar] [CrossRef]
- Dufresne, C.J.; Farnworth, E.R. A review of latest research findings on the health promotion properties of tea. J. Nutr. Biochem. 2001, 12, 404–421. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; Higdon, J.V. Antioxidant Activity of Tea Polyphenols In Vivo: Evidence from Animal Studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.; Taskeen, M.; Mohammad, I.; Huo, C.; Chan, T.H.; Dou, Q.P. Recent advances on tea polyphenols. Front. Biosci. 2012, 4, 111. [Google Scholar] [CrossRef]
- Mukhtar, H.; Ahmad, N. Tea polyphenols: Prevention of cancer and optimizing health. Am. J. Clin. Nutr. 2000, 71, 1698S–1702S. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Lambert, J.D.; Sang, S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch. Toxicol. 2009, 83, 11–21. [Google Scholar] [CrossRef]
- Kimura, K.; Ozeki, M.; Juneja, L.R.; Ohira, H. l-Theanine reduces psychological and physiological stress responses. Biol. Psychol. 2007, 74, 39–45. [Google Scholar] [CrossRef]
- Juneja, L.; Chu, D.C.; Okubo, T.; Nagato, Y.; Yokogoshi, H. L-theanine—A unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci. Technol. 1999, 10, 199–204. [Google Scholar] [CrossRef]
- Mu, W.; Zhang, T.; Jiang, B. An overview of biological production of L-theanine. Biotechnol. Adv. 2015, 33, 335–342. [Google Scholar] [CrossRef]
- Yokogoshi, H.; Kato, Y.; Sagesaka, Y.M.; Takihara-Matsuura, T.; Kakuda, T.; Takeuchi, N. Reduction Effect of Theanine on Blood Pressure and Brain 5-Hydroxyindoles in Spontaneously Hypertensive Rats. Biosci. Biotechnol. Biochem. 1995, 59, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Sayama, K.; Okubo, T.; Juneja, L.R.; Oguni, I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. Vivo 2004, 18, 55–62. [Google Scholar] [CrossRef]
- Papamichael, C.M.; Aznaouridis, K.A.; Karatzis, E.N.; Karatzi, K.N.; Stamatelopoulos, K.S.; Vamvakou, G.; Lekakis, J.P.; Mavrikakis, M.E. Effect of coffee on endothelial function in healthy subjects: The role of caffeine. Clin. Sci. 2005, 109, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.M.; Griffiths, R.R. Caffeine withdrawal: A parametric analysis of caffeine dosing conditions. J. Pharmacol. Exp. Ther. 1999, 289, 285–294. [Google Scholar] [PubMed]
- Lopes, J.M.; Aubier, M.; Jardim, J.; Aranda, J.V.; Macklem, P.T. Effect of caffeine on skeletal muscle function before and after fatigue. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 54, 1303–1305. [Google Scholar] [CrossRef]
- Rees, K.; Allen, D.; Lader, M. The influences of age and caffeine on psychomotor and cognitive function. Psychopharmacology 1999, 145, 181–188. [Google Scholar] [CrossRef]
- Ruxton, C.H.S. The impact of caffeine on mood, cognitive function, performance and hydration: A review of benefits and risks. Nutr. Bull. 2008, 33, 15–25. [Google Scholar] [CrossRef]
- Chen, X.; Ye, Y.; Cheng, H.; Jiang, Y.; Wu, Y. Thermal Effects on the Stability and Antioxidant Activity of an Acid Polysaccharide Conjugate Derived from Green Tea. J. Agric. Food Chem. 2009, 57, 5795–5798. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Liu, Y.; Chen, X.; Wei, X. Extraction, characterization and antioxidant activities of Se-enriched tea polysaccharides. Int. J. Biol. Macromol. 2015, 77, 76–84. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- He, N.; Shi, X.; Zhao, Y.; Tian, L.; Wang, N.; Yang, X. Inhibitory Effects and Molecular Mechanisms of Selenium-Containing Tea Polysaccharides on Human Breast Cancer MCF-7 Cells. J. Agric. Food Chem. 2013, 61, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Z.; Jin, G.M.; Wang, L.K.; Yang, J.F. Effect of tea polysaccharides on immune functions and antioxdative activity in broilers. J. Tea Sci. 2005, 25, 61–64. [Google Scholar]
- Wang, H.; Shi, S.; Bao, B.; Li, X.; Wang, S. Structure characterization of an arabinogalactan from green tea and its anti-diabetic effect. Carbohydr. Polym. 2015, 124, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.M.; Zhong, Y.Z.; Duan, Y.H.; Chen, Q.H.; Li, F.N. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Kalender, Y.; Kaya, S.; Durak, D.; Uzun, F.G.; Demir, F. Protective effects of catechin and quercetin on antioxidant status, lipid peroxidation and testis-histoarchitecture induced by chlorpyrifos in male rats. Environ. Toxicol. Pharmacol. 2012, 33, 141–148. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, S.Q.; Wang, J.; Chen, Q.R.; Zeng, J.; Liu, A.; Chen, Z.X.; Lu, X.M. Oral administration of green tea polyphenols (TP) improves ileal injury and intestinal flora disorder in mice with Salmonella typhimurium infection via resisting inflammation, enhancing antioxidant action and preserving tight junction. J. Funct. Foods 2019, 64, 103654. [Google Scholar] [CrossRef]
- Cao, G.; Chen, M.; Song, Q.; Liu, Y.; Xie, L.; Han, Y.; Liu, Z.; Ji, Y. EGCG protects against UVB-induced apoptosis via oxidative stress and the JNK1/c-Jun pathway in ARPE19 cells. Mol. Med. Rep. 2011, 5, 54–59. [Google Scholar] [CrossRef]
- Chen, C.; Yu, R.; Owuor, E.D.; Tony Kong, A.N. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch. Pharmacal Res. 2000, 23, 605–612. [Google Scholar] [CrossRef]
- Liu, S.; Huang, H. Assessments of antioxidant effect of black tea extract and its rationals by erythrocyte haemolysis assay, plasma oxidation assay and cellular antioxidant activity (CAA) assay. J. Funct. Foods 2015, 18, 1095–1105. [Google Scholar] [CrossRef]
- Su, J.; Wang, X.; Song, W.; Bai, X.; Li, C. Reducing oxidative stress and hepatoprotective effect of water extracts from Pu-erh tea on rats with high-fat diet. Food Sci. Hum. Wellness 2016, 5, 199–206. [Google Scholar] [CrossRef]
- Fang, J.; Sureda, A.; Silva, A.S.; Khan, F.; Xu, S.; Nabavi, S.M. Trends of tea in cardiovascular health and disease: A critical review. Trends Food Sci. Technol. 2019, 88, 385–396. [Google Scholar] [CrossRef]
- Cai, S.; Khoo, J.; Mussa, S.; Alp, N.J.; Channon, K.M. Endothelial nitric oxide synthase dysfunction in diabetic mice: Importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia 2005, 48, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Actis-Goretta, L.; Ottaviani, J.I.; Fraga, C.G. Inhibition of Angiotensin Converting Enzyme Activity by Flavanol-Rich Foods. J. Agric. Food Chem. 2005, 54, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Annaba, F.; Kumar, P.; Dudeja, A.K.; Saksena, S.; Gill, R.K.; Alrefai, W.A. Green tea catechin EGCG inhibits ileal apical sodium bile acid transporter ASBT. Am. J. Physiol. Liver Physiol. 2010, 298, G467–G473. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.; Elmets, C. Green tea polyphenolic antioxidants and skin photoprotection (Review). Int. J. Oncol. 2001, 18, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Park, J.-Y.; Lambert, J.D. Differential prooxidative effects of the green tea polyphenol, (-)-epigallocatechin-3-gallate, in normal and oral cancer cells are related to differences in sirtuin 3 signaling. Mol. Nutr. Food Res. 2014, 59, 203–211. [Google Scholar] [CrossRef]
- Lubet, R.A.; Yang, C.S.; Lee, M.J.; Hara, Y.; Kapetanovic, I.M.; Crowell, J.A.; Steele, V.E.; Juliana, M.M.; Grubbs, C.J. Preventive effects of Polyphenon E on urinary bladder and mammary cancers in rats and correlations with serum and urine levels of tea polyphenols. Mol. Cancer Ther. 2007, 6, 2022–2028. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Li, D.; Chen, Y.; Dou, Q.P. Perspectives on the recent developments with green tea polyphenols in drug discovery. Expert Opin. Drug Discov. 2018, 13, 643–660. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, S.; Chen, R.; Peng, Z.; Wan, J.; Wu, B. Taurine and tea polyphenols combination ameliorate nonalcoholic steatohepatitis in rats. BMC Complement. Altern. Med. 2017, 17, 455. [Google Scholar] [CrossRef]
- Calgarotto, A.K.; Maso, V.; Junior, G.C.F.; Nowill, A.E.; Filho, P.L.; Vassallo, J.; Saad, S.T.O. Antitumor activities of Quercetin and Green Tea in xenografts of human leukemia HL60 cells. Sci. Rep. 2018, 8, 3459. [Google Scholar] [CrossRef]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) Is the Most Effective Cancer Chemopreventive Polyphenol in Green Tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Shomali, T.; Mosleh, N.; Nazifi, S. Two weeks of dietary supplementation with green tea powder does not affect performance, d-xylose absorption, and selected serum parameters in broiler chickens. Comp. Clin. Pathol. 2011, 21, 1023–1027. [Google Scholar] [CrossRef]
- Xiong, J.; Xu, W.; Huang, H.; Jin, B.; Zhao, H. Hypertension and risk of cholangiocarcinoma: A systematic review and meta-analysis. Transl. Cancer Res. 2018, 7, 543–551. [Google Scholar] [CrossRef]
- Yang, H.H.; Zhou, H.; Zhu, W.Z.; Chen, C.L.; Qin, L.Q. Green Tea Consumption May Be Associated with Cardiovascular Disease Risk and Nonalcoholic Fatty Liver Disease in Type 2 Diabetics: A Cross-Sectional Study in Southeast China. J. Med. Food 2020, 23, 1120–1127. [Google Scholar] [CrossRef]
- Lang, T.; Bureau, J.F.; Degoulet, P.; Salah, H.; Benattar, C. Blood pressure, coffee, tea and tobacco consumption: An epidemiological study in algiers. Eur. Heart J. 1983, 4, 602–607. [Google Scholar] [CrossRef]
- Jang, H.J.; Ridgeway, S.D.; Kim, J.A. Effects of the green tea polyphenol epigallocatechin-3-gallate on high-fat diet-induced insulin resistance and endothelial dysfunction. Am. J. Physiol-Endoc. M. 2013, 305, E1444–E1451. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Yu, C.L.; Liu, C.Y.; Wang, S.F.; Pan, P.C.; Wu, M.T.; Ho, C.K.; Lo, Y.S.; Li, Y.; Christiani, D.C.; et al. A population-based, case–control study of green tea consumption and leukemia risk in southwestern Taiwan. Cancer Causes Control 2008, 20, 57–65. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape Seed and Tea Extracts and Catechin 3-Gallates Are Potent Inhibitors of α-Amylase and α-Glucosidase Activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef]
- Mandel, S.; Youdim, M.B. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef]
- Chen, S.Q.; Wang, Z.S.; Ma, Y.X.; Zhang, W.; Lu, J.L.; Liang, Y.R.; Zheng, X.Q. Neuroprotective Effects and Mechanisms of Tea Bioactive Components in Neurodegenerative Diseases. Molecules 2018, 23, 512. [Google Scholar] [CrossRef]
- Weinreb, O.; Amit, T.; Youdim, M.B. A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant–iron chelator green tea polyphenol (-)-epigallocatechin-3-gallate. Free Radic. Biol. Med. 2007, 43, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Shumin, Y.; Wang, W.; Bai, F.L.; Zhu, J.L.; Li, J.R.; Li, X.P.; Xu, Y.X.; Sun, T.; He, Y.T. Antimicrobial effect and membrane-active mechanism of tea polyphenols against Serratia marcescens. World J. Microbiol. Biotechnol. 2013, 30, 451–460. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Guo, N. The antimicrobial activities and action-mechanism of tea tree oil against food-borne bacteria in fresh cucumber juice. Microb. Pathog. 2018, 125, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Shigemune, N.; Tsugukuni, T.; Jun, H.; Matsushita, T.; Mekada, Y.; Kurahachi, M.; Miyamoto, T. Mechanism of the combined anti-bacterial effect of green tea extract and NaCl against Staphylococcus aureus and Escherichia coli O157:H7. Food Control 2011, 25, 225–232. [Google Scholar] [CrossRef]
- Xia, K.Q.; Zhu, Z.Y.; Fu, J.M.; Li, Y.M.; Chi, Y.; Zhang, H.Z.; Du, C.L.; Xu, Z.V. A triboelectric nanogenerator based on waste tea leaves and packaging bags for powering electronic office supplies and behavior monitoring. Nano Energy 2019, 60, 61–71. [Google Scholar] [CrossRef]
- Xia, E.H.; Zhang, H.B.; Sheng, J.; Li, K.; Zhang, Q.J.; Kim, C.; Zhang, Y.; Liu, Y.; Zhu, T.; Li, W.; et al. The Tea Tree Genome Provides Insights into Tea Flavor and Independent Evolution of Caffeine Biosynthesis. Mol. Plant 2017, 10, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.; Tong, W.; Hou, Y.; An, Y.L.; Chen, L.B.; Wu, Q.; Liu, Y.L.; Yu, J.; Li, F.D.; Li, L.P.; et al. The Reference Genome of Tea Plant and Resequencing of 81 Diverse Accessions Provide Insights into Its Genome Evolution and Adaptation. Mol. Plant 2020, 13, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Zhang, Y.J.; Qiu, H.J.; Guo, Y.F.; Wan, H.L.; Zhang, X.L.; Scossa, F.; Alseekh, S.; Zhang, Q.; Wang, P.; et al. Genome assembly of wild tea tree DASZ reveals pedigree and selection history of tea varieties. Nat. Commun. 2020, 11, 3719. [Google Scholar] [CrossRef]
- Niu, S.; Song, Q.; Koiwa, H.; Qiao, D.; Zhao, D.; Chen, Z.; Liu, X.; Wen, X. Genetic diversity, linkage disequilibrium, and population structure analysis of the tea plant (Camellia sinensis) from an origin center, Guizhou plateau, using genome-wide SNPs developed by genotyping-by-sequencing. BMC Plant Biol. 2019, 19, 328. [Google Scholar] [CrossRef]
- Lee, E.S.; Shin, K.S.; Kim, M.S.; Park, H.; Cho, S.; Kim, C.B. The mitochondrial genome of the smaller tea tortrix Adoxophyes honmai (Lepidoptera: Tortricidae). Gene 2006, 373, 52–57. [Google Scholar] [CrossRef]
- Hao, X.; Yang, Y.; Yue, C.; Wang, L.; Horvath, D.P.; Wang, X. Comprehensive Transcriptome Analyses Reveal Differential Gene Expression Profiles of Camellia sinensis Axillary Buds at Para-, Endo-, Ecodormancy, and Bud Flush Stages. Front. Plant Sci. 2017, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, F.; Lin, H.; Ye, Y.; Zheng, Y.; Li, J.; Hao, Z.; Ye, N.; Yue, C. Transcriptome and metabolite analyses provide insights into zigzag-shaped stem formation in tea plants (Camellia sinensis). BMC Plant Biol. 2020, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Chen, C.; Zeng, Z.; Zou, Z.; Li, H.; Zhou, Q.; Chen, X.; Sun, K.; Li, X. Transcriptomic analysis between self- and cross-pollinated pistils of tea plants (Camellia sinensis). BMC Genom. 2018, 19, 289. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lv, X.; Wang, L.; Qiu, Z.; Song, X.; Lin, J.; Chen, W. Transcriptome analysis reveals the accumulation mechanism of anthocyanins in ‘Zijuan’ tea (Camellia sinensis var. asssamica (Masters) kitamura) leaves. Plant Growth Regul. 2016, 81, 51–61. [Google Scholar] [CrossRef]
- Wang, S.; Chen, J.; Ma, J.; Jin, J.; Chen, L.; Yao, M. Novel insight into theacrine metabolism revealed by transcriptome analysis in bitter tea (Kucha, Camellia sinensis). Sci. Rep. 2020, 10, 6286. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhao, Q.Y.; Ma, C.L.; Zhang, Z.H.; Cao, H.L.; Kong, Y.M.; Yue, C.; Hao, X.Y.; Chen, L.; Ma, J.Q.; et al. Global transcriptome profiles of Camellia sinensis during cold acclimation. BMC Genom. 2013, 14, 415. Available online: http://www.biomedcentral.com/1471-2164/14/415 (accessed on 22 June 2013). [CrossRef]
- Wang, W.; Xin, H.; Wang, M.; Ma, Q.; Wang, L.; Kaleri, N.A.; Wang, Y.; Li, X. Transcriptomic Analysis Reveals the Molecular Mechanisms of Drought-Stress-Induced Decreases in Camellia sinensis Leaf Quality. Front. Plant Sci. 2016, 7, 385. [Google Scholar] [CrossRef]
- Liu, Z.W.; Li, H.; Wang, W.L.; Wu, Z.J.; Cui, X.; Zhuang, J. CsGOGAT Is Important in Dynamic Changes of Theanine Content in Postharvest Tea Plant Leaves under Different Temperature and Shading Spreadings. J. Agric. Food Chem. 2017, 65, 9693–9702. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, D.; Zhou, L.; Zhang, X.; Liao, J.; Duan, Y.; Wen, B.; Ma, Y.; Wang, Y.; Fang, W.; et al. Transcriptomic and metabolomic profiling of Camellia sinensis L. cv. ‘Suchazao’ exposed to temperature stresses reveals modification in protein synthesis and photosynthetic and anthocyanin biosynthetic pathways. Tree Physiol. 2019, 39, 1583–1599. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, X.; Zhou, F.; Gao, X.; Shen, C. Comprehensive transcriptome profiling of tea leaves (Camellia sinensis) in response to simulated acid rain. Sci. Hortic. 2020, 272, 109491. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Yang, T.; Chen, T.; Dong, C.; Gu, Q.; Cheng, X. Transcriptome analysis provides insights into the molecular bases in response to different nitrogen forms-induced oxidative stress in tea plant roots (Camellia sinensis). Funct. Plant Biol. 2020, 47, 1073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; He, W.; Yuan, Q.; Wei, K.; Ruan, L.; Wang, L.; Cheng, H. Transcriptome analysis identifies CsNRT genes involved in nitrogen uptake in tea plants, with a major role of CsNRT2.4. Plant Physiol. Biochem. 2021, 167, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Y.; Ma, L.; Jin, X.; Guo, G.; Tan, R.; Liu, Z.; Zheng, L.; Ye, F.; Liu, W. Transcriptome analysis of differentially expressed genes involved in selenium accumulation in tea plant (Camellia sinensis). PLoS ONE 2018, 13, e0197506. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, P.; Liu, S.; Du, Y.; Ni, D.; Song, X.; Chen, Y. An RNA-Seq transcriptome analysis revealing novel insights into fluorine absorption and transportation in the tea plant. Botany 2020, 98, 249–259. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Song, X.; Zhang, Z.; Jiang, Y.; Zhu, Y.; Zhao, H.; Ni, D. An RNA-Seq transcriptome analysis revealing novel insights into aluminum tolerance and accumulation in tea plant. Planta 2017, 246, 91–103. [Google Scholar] [CrossRef]
- Jayaswall, K.; Mahajan, P.; Singh, G.; Parmar, R.; Seth, R.; Raina, A.; Swarnkar, M.K.; Singh, A.K.; Shankar, R.; Sharma, R.K. Transcriptome Analysis Reveals Candidate Genes involved in Blister Blight defense in Tea (Camellia sinensis (L.) Kuntze). Sci. Rep. 2016, 6, 30412. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Cao, H.; Hao, X.; Zeng, J.; Yang, Y.; Wang, X. Transcriptome Analysis of an Anthracnose-Resistant Tea Plant Cultivar Reveals Genes Associated with Resistance to Colletotrichum camelliae. PLoS ONE 2016, 11, e0148535. [Google Scholar] [CrossRef]
- Bandyopadhyay, T.; Gohain, B.; Bharalee, R.; Gupta, S.; Bhorali, P.; Das, S.K.; Kalita, M.C.; Das, S. Molecular Landscape of Helopeltis theivora Induced Transcriptome and Defense Gene Expression in Tea. Plant Mol. Biol. Rep. 2014, 33, 1042–1057. [Google Scholar] [CrossRef]
- Li, C.F.; Zhu, Y.; Yu, Y.; Zhao, Q.Y.; Wang, S.J.; Wang, X.C.; Yao, M.Z.; Luo, D.; Li, X.; Chen, L.; et al. Global transcriptome and gene regulation network for secondary metabolite biosynthesis of tea plant (Camellia sinensis). BMC Genom. 2015, 16, 560. [Google Scholar] [CrossRef]
- Ku, K.M.; Choi, J.N.; Kim, J.; Kim, J.K.; Yoo, L.G.; Lee, S.J.; Hong, Y.-S.; Lee, C.H. Metabolomics Analysis Reveals the Compositional Differences of Shade Grown Tea (Camellia sinensis L.). J. Agric. Food Chem. 2009, 58, 418–426. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, Y.; Jiang, J.; Xie, H.; Ding, Z.; Ding, S.; Wang, H. The Quality Evaluation of Tea (Camellia sinensis) Varieties Based on the Metabolomics. HortScience 2019, 54, 409–415. [Google Scholar] [CrossRef]
- Wu, W.; Lu, M.; Peng, J.; Lv, H.; Shi, J.; Zhang, S.; Liu, Z.; Duan, J.; Chen, D.; Dai, W.; et al. Nontargeted and targeted metabolomics analysis provides novel insight into nonvolatile metabolites in Jianghua Kucha tea germplasm (Camellia sinensis var. Assamica cv. Jianghua). Food Chem. X 2022, 13, 100270. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hua, J.; Yu, Q.; Li, J.; Wang, J.; Deng, Y.; Yuan, H.; Jiang, Y. Widely targeted metabolomic analysis reveals dynamic changes in non-volatile and volatile metabolites during green tea processing. Food Chem. 2021, 363, 130131. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, X.; Liu, S.; Liu, J.; Guo, Y.; Sun, Y.; Lin, J.; Guo, Y.; Wei, S. Understanding the formation mechanism of oolong tea characteristic non-volatile chemical constitutes during manufacturing processes by using integrated widely-targeted metabolome and DIA proteome analysis. Food Chem. 2019, 310, 125941. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, J.; Liu, H.; Gong, Z.; Wang, X.; Li, M.; Aharoni, A.; Yang, Z.; Yu, X. Insights into tissue-specific specialized metabolism in Tieguanyin tea cultivar by untargeted metabolomics. Molecules 2018, 23, 1817. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Guo, X.; Liu, G.; Song, Y.; Ho, C.T.; Hou, R.; Zhang, L.; Wan, X. A comparative analysis for the volatile compounds of various Chinese dark teas using combinatory metabolomics and fungal solid-state fermentation. J. Food Drug Anal. 2018, 26, 112–123. [Google Scholar] [CrossRef]
- Tan, J.; Dai, W.; Lu, M.; Lv, H.; Guo, L.; Zhang, Y.; Zhu, Y.; Peng, Q.; Lin, Z. Study of the dynamic changes in the non-volatile chemical constituents of black tea during fermentation processing by a non-targeted metabolomics approach. Food Res. Int. 2015, 79, 106–113. [Google Scholar] [CrossRef]
- Long, P.; Wen, M.; Granato, D.; Zhou, J.; Wu, Y.; Hou, Y.; Zhang, L. Untargeted and targeted metabolomics reveal the chemical characteristic of pu-erh tea (Camellia assamica) during pile-fermentation. Food Chem. 2019, 311, 125895. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Y.; Long, P.; Ho, C.T.; Wang, Y.; Kan, Z.; Cao, L.; Zhang, L.; Wan, X.; Zhou, J.; et al. LC-MS-Based Metabolomics Reveals the Chemical Changes of Polyphenols during High-Temperature Roasting of Large-Leaf Yellow Tea. J. Agric. Food Chem. 2018, 67, 5405–5412. [Google Scholar] [CrossRef]
- Ma, Y.; Ling, T.J.; Su, X.Q.; Jiang, B.; Nian, B.; Chen, L.J.; Liu, M.L.; Zhang, Z.Y.; Wang, D.P.; Mu, Y.Y.; et al. Integrated proteomics and metabolomics analysis of tea leaves fermented by Aspergillus niger, Aspergillus tamarii and Aspergillus fumigatus. Food Chem. 2020, 334, 127560. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, D.; Liu, M.; Ruan, J. Integrated analyses of the transcriptome and metabolome of the leaves of albino tea cultivars reveal coordinated regulation of the carbon and nitrogen metabolism. Sci. Hortic. 2018, 231, 272–281. [Google Scholar] [CrossRef]
- Liang, L.L.; Song, Y.K.; Qian, W.J.; Ruan, J.Y.; Ding, Z.T.; Zhang, Q.F.; Hu, J.H. Metabolomics analysis reveals the responses of tea plants to excessive calcium. J. Sci. Food Agric. 2021, 101, 5678–5687. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hu, K.; Qu, F.; Ni, D.; Zhang, H.; Liu, S.; Chen, Y. Metabolomics Analysis Reveals Major Differential Metabolites and Metabolic Alterations in Tea Plant Leaves (Camellia sinensis L.) Under Different Fluorine Conditions. J. Plant Growth Regul. 2020, 40, 798–810. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Tao, M.; Li, M.; Wu, Y.; Qi, Q.; Yang, H.; Wan, X. Comparative Metabolic Responses and Adaptive Strategies of Tea Leaves (Camellia sinensis) to N2 and CO2 Anaerobic Treatment by a Nontargeted Metabolomics Approach. J. Agric. Food Chem. 2018, 66, 9565–9572. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Y.; Chen, C.; Ding, Z.; Hu, J.; Zheng, C.; Li, Y. Metabolite profiling of tea (Camellia sinensis L.) leaves in winter. Sci. Hortic. 2015, 192, 1–9. [Google Scholar] [CrossRef]
- Nyarukowa, C.; Koech, R.; Loots, T.; Apostolides, Z. SWAPDT: A method for short-time withering assessment of probability for drought tolerance in Camellia sinensis validated by targeted metabolomics. J. Plant Physiol. 2016, 198, 39–48. [Google Scholar] [CrossRef][Green Version]
- Zhang, Q.; Liu, M.; Ruan, J. Metabolomics analysis reveals the metabolic and functional roles of flavonoids in light-sensitive tea leaves. BMC Plant Biol. 2017, 17, 64. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Gu, M.; Chen, X.; Chen, X.; Yang, J.; Zhao, F.; Ye, N. Exploration of the Effects of Different Blue LED Light Intensities on Flavonoid and Lipid Metabolism in Tea Plants via Transcriptomics and Metabolomics. Int. J. Mol. Sci. 2020, 21, 4606. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, M.; Mumm, R.; Vos, R.C.H.; Ruan, J. Metabolomics reveals the within-plant spatial effects of shading on tea plants. Tree Physiol. 2020, 41, 317–330. [Google Scholar] [CrossRef]
- Huang, A.; Jiang, Z.; Tao, M.; Wen, M.; Xiao, Z.; Zhang, L.; Zha, M.; Chen, J.; Liu, Z. Targeted and nontargeted metabolomics analysis for determining the effect of storage time on the metabolites and taste quality of keemun black tea. Food Chem. 2021, 359, 129950. [Google Scholar] [CrossRef]
- Zeng, C.; Lin, H.; Liu, Z.; Liu, Z. Metabolomics analysis of Camellia sinensis with respect to harvesting time. Food Res. Int. 2020, 128, 108814. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Shang, X.; Liu, G.; Zou, Z.; Zhu, X.; Ma, Y.; Li, F.; Fang, W. The effects of tea plants-soybean intercropping on the secondary metabolites of tea plants by metabolomics analysis. BMC Plant Biol. 2021, 21, 482. [Google Scholar] [CrossRef]
- Lin, F.; Jiang, L.; Liu, Y.; Lv, Y.; Dai, H.; Zhao, H. Genome-wide identification of housekeeping genes in maize. Plant Mol. Biol. 2014, 86, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.W.; Deng, H.L.; Wu, Q.Y.; Liu, B.B.; Yue, C.; Deng, T.T.; Lai, Z.X.; Sun, Y. Validation of reference genes for gene expression studies in post-harvest leaves of tea plant (Camellia sinensis). PeerJ 2019, 7, e6385. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dong, Y.A.; Yu, Y.C.; Xing, Y.X.; Li, X.W.; Zhang, X.; Hou, X.J.; Sun, X.L. Identification and evaluation of reliable reference genes for quantitative real-time PCR analysis in tea plants under differential biotic stresses. Sci. Rep. 2020, 10, 2429. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Dhanapal, S.; Yadav, B.S. The dynamic responses of plant physiology and metabolism during environmental stress progression. Mol. Biol. Rep. 2019, 47, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S. Physiological role of signal molecules in improving plant tolerance under abiotic stress. Int. J. ChemTech Res. 2016, 9, 46–60. [Google Scholar]
- Li, X.W.; Feng, Z.G.; Yang, H.M.; Zhu, X.P.; Liu, J.; Yuan, H.Y. A novel cold-regulated gene from Camellia sinensis, CsCOR1, enhances salt- and dehydration-tolerance in tobacco. Biochem. Biophys. Res. Commun. 2010, 394, 354–359. [Google Scholar] [CrossRef]
- Wang, M.L.; Zou, Z.W.; Li, Q.H.; Sun, K.; Chen, X.; Li, X.H. The CsHSP17.2 molecular chaperone is essential for thermotolerance in Camellia sinensis. Sci. Rep. 2017, 7, 1237. [Google Scholar] [CrossRef]
- Li, N.N.; Qian, W.J.; Wang, L.; Cao, H.L.; Hao, X.Y.; Yang, Y.J.; Wang, X.C. Isolation and expression features of hexose kinase genes under various abiotic stresses in the tea plant (Camellia sinensis). J. Plant Physiol. 2017, 209, 95–104. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Wang, S.; Shi, C.; Zhang, R.; Rao, J.; Wang, X.; Gu, X.; Wang, Y.; Li, D.; et al. Molecular Cloning and Characterization of Galactinol Synthases in Camellia sinensis with Different Responses to Biotic and Abiotic Stressors. J. Agric. Food Chem. 2017, 65, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, K.; Liu, Y.; Jiao, T.; Ma, G.; Qian, Y.; Wang, P.; Dai, X.; Gao, L.; Xia, T. Functional Analysis of Two Flavanone-3-Hydroxylase Genes from Camellia sinensis: A Critical Role in Flavonoid Accumulation. Genes 2017, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, N.; Gao, T.; Jin, J.; Jing, T.; Wang, J.; Wu, Y.; Wan, X.; Schwab, W.; Song, C. Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New Phytol. 2019, 226, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wei, K.; Wang, L.; Ruan, L.; Li, H.; Zhou, X.; Lin, Z.; Shan, R.; Cheng, H. Expression of Key Structural Genes of the Phenylpropanoid Pathway Associated with Catechin Epimerization in Tea Cultivars. Front. Plant Sci. 2017, 8, 702. [Google Scholar] [CrossRef]
- Yao, L.; Hao, X.; Cao, H.; Ding, C.; Yang, Y.; Wang, L.; Wang, X. ABA-dependent bZIP transcription factor, CsbZIP18, from Camellia sinensis negatively regulates freezing tolerance in Arabidopsis. Plant Cell Rep. 2020, 39, 553–565. [Google Scholar] [CrossRef]
- Deng, W.W.; Zhang, M.; Wu, J.; Jiang, Z.Z.; Tang, L.; Li, Y.Y.; Wei, C.L.; Jiang, C.J.; Wan, X.C. Molecular cloning, functional analysis of three cinnamyl alcohol dehydrogenase (CAD) genes in the leaves of tea plant, Camellia sinensis. J. Plant Physiol. 2013, 170, 272–282. [Google Scholar] [CrossRef]
- Deng, W.W.; Wu, Y.L.; Li, Y.Y.; Tan, Z.; Wei, C.L. Molecular Cloning and Characterization of Hydroperoxide Lyase Gene in the Leaves of Tea Plant (Camellia sinensis). J. Agric. Food Chem. 2016, 64, 1770–1776. [Google Scholar] [CrossRef]
- Kariola, T.; Palomäki, T.A.; Brader, G.; Palva, E.T. Erwinia carotovora subsp. carotovora and Erwinia-derived Elicitors HrpN and PehA Trigger Distinct but Interacting Defense Responses and Cell Death in Arabidopsis. Mol. Plant Microbe Interact. 2003, 16, 179–187. [Google Scholar] [CrossRef]
- Acharya, K.; Pal, A.K.; Gulati, A.; Kumar, S.; Singh, A.K.; Ahuja, P.S. Overexpression of Camellia sinensis Thaumatin-Like Protein, CsTLP in Potato Confers Enhanced Resistance to Macrophomina phaseolina and Phytophthora infestans Infection. Mol. Biotechnol. 2012, 54, 609–622. [Google Scholar] [CrossRef]
- Velazhahan, R.; Muthukrishnan, S. Transgenic Tobacco Plants Constitutively Overexpressing a Rice Thaumatin-like Protein (PR-5) Show Enhanced Resistance to Alternaria alternata. Biol. Plant. 2003, 46, 347–354. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Guan, C.; Wu, D.; Zhou, T.; Yu, Y. Transcription factor WRKY14 mediates resistance of tea plants (Camellia sinensis (L.) O. Kuntze) to blister blight. Physiol. Mol. Plant Pathol. 2021, 115, 101667. [Google Scholar] [CrossRef]
- Su, X.J.; Wang, W.W.Z.; Xia, T.; Gao, L.P.; Shen, G.A.; Pang, Y.Z. Characterization of a heat responsive UDP: Flavonoid glucosyltransferase gene in tea plant (Camellia sinensis). PLoS ONE 2018, 13, e0207212. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Wang, L.; Zhang, Y.; Ruan, L.; Li, H.; Wu, L.; Xu, L.; Zhang, C.; Zhou, X.; Cheng, H.; et al. A coupled role for CsMYB75 and CsGSTF1 in anthocyanin hyperaccumulation in purple tea. Plant J. 2018, 97, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Y.; Guo, L.; Gong, N.; Pang, Y.; Jiang, W.; Liu, Y.; Jiang, X.; Zhao, L.; Wang, Y.; et al. Functional Characterization of Tea (Camellia sinensis) MYB4a Transcription Factor Using an Integrative Approach. Front. Plant Sci. 2017, 8, 943. [Google Scholar] [CrossRef]
- He, X.; Zhao, X.; Gao, L.; Shi, X.; Dai, X.; Liu, Y.; Xia, T.; Wang, Y. Isolation and Characterization of Key Genes that Promote Flavonoid Accumulation in Purple-leaf Tea (Camellia sinensis L.). Sci. Rep. 2018, 8, 130. [Google Scholar] [CrossRef]
- Wang, P.Q.; Zhang, L.J.; Jiang, X.L.; Dai, X.L.; Xu, L.J.; Li, T.; Xing, D.W.; Li, Y.Z.; Li, M.Z.; Gao, L.P.; et al. Evolutionary and functional characterization of leucoanthocyanidin reductases from Camellia sinensis. Planta 2017, 247, 139–154. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, H.; Jiang, X.; Wang, P.; Dai, X.; Chen, W.; Gao, L.; Xia, T. A WD40 Repeat Protein from Camellia sinensis Regulates Anthocyanin and Proanthocyanidin Accumulation through the Formation of MYB–bHLH–WD40 Ternary Complexes. Int. J. Mol. Ences 2018, 19, 1686. [Google Scholar] [CrossRef]
- Ma, W.; Kang, X.; Liu, P.; Zhang, Y.; Lin, X.; Li, B.; Chen, Z. The analysis of transcription factor CsHB1 effects on caffeine accumulation in tea callus through CRISPR/Cas9 mediated gene editing. Process Biochem. 2021, 101, 304–311. [Google Scholar] [CrossRef]
- Yen, H.T.T.; Thanh, D.T.; Hang, P.T.; Dung, D.T.; Hue, H.T.T. Isolation and characterization of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase from the green Trung Du tea in Thai Nguyen (Camellia sinensis). Vietnam J. Biotechnol. 2018, 16, 473–480. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Wang, H.; Li, Y.; Wang, B.; Zhang, L.; Wan, X.; Li, M. Transcription factor CsDOF regulates glutamine metabolism in tea plants (Camellia sinensis). Plant Sci. 2020, 302, 110720. [Google Scholar] [CrossRef]
- Mizutani, M.; Nakanishi, H.; Ema, J.; Ma, S.J.; Noguchi, E.; Ochiai, M. Cloning of beta-primeverosidase from tea leaves, a key enzyme in tea aroma formation. Plant Physiol. 2002, 130, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.W.; Wang, R.; Yang, T.; Jiang, L.; Zhang, Z.Z. Functional Characterization of Salicylic Acid Carboxyl Methyltransferase from Camellia sinensis, Providing the Aroma Compound of Methyl Salicylate during the Withering Process of White Tea. J. Agric. Food Chem. 2017, 65, 11036–11045. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, H.; Sano, H.; Crozier, A. Caffeine and related purine alkaloids: Biosynthesis, catabolism, function and genetic engineering. Phytochemistry 2008, 69, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Feldheim, W.; Yongvanit, P.; Cummings, P.H. Investigation of the presence and significance of theanine in the tea plant. J. Sci. Food Agric. 1986, 37, 527–534. [Google Scholar] [CrossRef]
- Wei, C.; Yang, H.; Wang, S.; Zhao, J.; Liu, C.; Gao, L.; Xia, E.; Lu, Y.; Tai, Y.; She, G.; et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar] [CrossRef]
- Sasaoka, K.; Kito, M. Synthesis of Theanine by Tea Seedling Homogenate. Agric. Biol. Chem. 1964, 28, 313–317. [Google Scholar] [CrossRef]
| Compounds | Contents (mg/g Dry Weight, DW) | |||||
|---|---|---|---|---|---|---|
| White Tea | Oolong Tea | Green Tea | Yellow Tea | Black Tea | Dark Tea | |
| EGC | 5.9–10.7 a | 19.4–29.2 a | 21.91–37.46 g | 12.3–24.1 a | 3.5–8.3 a | 4.1–10.89 p,r |
| C | 0.9–3.3 a | 0.5–10.0 a | 0.47–1.46 g | 0.5–1.3 a | 0.1–0.5 a | 1.69–2.2 p,r |
| EC | 2.7–4.7 a | 5.9–7.9 a | 3.78–6.7 g | 3.5–8.1 a | 1.9–4.8 a | 5.7–6.63 p,r |
| EGCG | 32.4–58.4 a | 37.6–61.8 a | 58.02–119.68 g | 41.7–67.5 a | 3.9–13.1 a | 0.8–19.25 p,r |
| ECG | 12.9–26.3 a | 9.2–14.4 a | 12.06–25.49 g | 16.9–27.9 a | 4.2–9.2 a | 0.5–6.54 p,r |
| TC | 101.4–153.9 | 72.6–114.3 | 96.24–190.79 | 74.9–128.9 | 13.6–35.9 | 12.79–45.51 |
| CAFF | 34.3–43.1 a | 17.1–28.5 a | 21.3–47.47 g,h | 29.8–38.0 a | 20.58–24.22 m | 19.7–36.3 a |
| TPS | 1.30 b | 1.799–1.95 e | 0.9904–1.3 i | 3.6 l | 1.645–2.092 n | 7.225 q |
| Flavonols | 2.3–18.9 c | 3.885–5.021 f | 5.85–11.93 j | - | 15–26 o | 11.173 q |
| Asp | 0.451–2.234 d | 0.571–1.29 d | 1.04–2.15 g | 0.212–0.41 d | 0.39–1.25 m | 0.102 r |
| Glu | 0.59–1.407 d | 0.403–1.017 d | 1.36–2.04 g | 0.16–0.396 d | 0.45–1.73 m | 0.042 r |
| Asn | 0.874–5.335 d | 0.046–0.451 d | - | - | 0.11–0.91 m | - |
| Gln | 0.259–0.595 d | 0.053–0.598 d | - | 0.107–0.555 d | 0.18–3.11 m | - |
| Ser | 0.359–0.903 d | 0.135–0.293 d | 0.34–1.07 g | 0.194–0.31 d | 0.17–1.11 m | 0.122 r |
| Arg | 0.208–0.713 d | 0.015–0.217 d | 0.58–1.87 g | 0.113–0.245 d | 0.12–0.80 m | 0.019 r |
| Gly | 0.029 d | 0.001–0.054 d | 0.03–0.04 g | 0.255–0.283 d | 0.03–0.09 m | 0.074 r |
| Thr | 0.155–0.42 d | 0.072–0.16 d | 0.13–0.33 g | 0.117–0.189 d | 0.16–0.41 m | 0.012 r |
| Ala | 0.204–0.938 d | 0.153–0.655 d | 0.16–0.25 g | 0.303–0.373 d | 0.07–0.79 m | 0.065 r |
| Thea | 3.571–8.002 d | 0.726–5.248 d | 3.07–21.18 g | 8.26–10.72 d | 0.89–17.27 m | 2.318 r |
| Pro | 0.215–0.83 d | 0.015–0.103 d | 0.41–0.62 g | 0.212–0.286 d | 0.12–0.78 m | 0.018 r |
| GABA | - | - | - | 0.135–0.189 d | 0.07–0.55 m | 0.03 r |
| Val | 0.296–1.124 d | 0.04–0.198 d | 0.08–0.29 g | 0.361–0.369 d | 0.12–0.58 m | 0.032 r |
| Ile | 0.311–1.172 d | 0.042–0.154 d | 0.07–0.26 g | 0.171–0.191 d | 0.14–0.84 m | - |
| Leu | 0.309–1.06 d | 0.034–0.165 d | 0.11–0.34 g | 0.392–0.422 d | 0.12–0.42 m | - |
| Phe | 0.444–1.161 d | 0.06–0.296 d | 0.13–0.49 g | 0.277–0.353 d | 0.08–0.81 m | 0.013 r |
| Trp | 0.143–0.39 d | 0.063–0.149 d | - | - | 0.10–0.36 m | - |
| His | 0.066–0.248 d | 0.007–0.057 d | 0.29–0.52 g | 0.001 d | 0.047–0.81 m | 0.027 r |
| Lys | 0.132–0.507 d | 0.015–0.098 d | 0.14–0.33 g | 0.175–0.227 d | 0.15–0.48 m | 0.105 r |
| TFAA | 8.484–27.07 d | 2.451–11.203 d | 7.94–31.52 g | 11.443–15.51 d | 7.93–29.48 m | 2.961 r |
| VC | - | - | 0.0174–0.043 k | - | 0.094–0.122 k | - |
| Polyphenols | Free Amino Acids | Caffeine | Polysaccharides | |
|---|---|---|---|---|
| Function | Antitumorigenesis A | Sleep peacefully, relieve tension G | Anti-fatigue L | Scavenging free radicals Q |
| Antioxidant activity B | Decrease blood pressure H | Cardiotonic agent M | Antioxidant R | |
| Anti-cancer C,D | Promote relaxation, concentration and learning ability I | Vasodilation N | Immunostimulatory activity S | |
| Anticardiovascular disease E | Reduce hypertension J | Improve attention O | Anticancer T,U | |
| AntioxidantsxxxxxPrevent oxidative stress, modulate carcinogen metabolism F | Anti-obesity K | Improve physical endurance and cognitive function P | Antidiabetes V | |
| - | - | - | Antiobesity W |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Han, J.; Pu, Y.; Wang, X. Tea (Camellia sinensis): A Review of Nutritional Composition, Potential Applications, and Omics Research. Appl. Sci. 2022, 12, 5874. https://doi.org/10.3390/app12125874
Wang C, Han J, Pu Y, Wang X. Tea (Camellia sinensis): A Review of Nutritional Composition, Potential Applications, and Omics Research. Applied Sciences. 2022; 12(12):5874. https://doi.org/10.3390/app12125874
Chicago/Turabian StyleWang, Cheng, Jingxue Han, Yuting Pu, and Xiaojing Wang. 2022. "Tea (Camellia sinensis): A Review of Nutritional Composition, Potential Applications, and Omics Research" Applied Sciences 12, no. 12: 5874. https://doi.org/10.3390/app12125874
APA StyleWang, C., Han, J., Pu, Y., & Wang, X. (2022). Tea (Camellia sinensis): A Review of Nutritional Composition, Potential Applications, and Omics Research. Applied Sciences, 12(12), 5874. https://doi.org/10.3390/app12125874





