The Expression and Bioinformatics Analysis of Circular RNAs in Endometritis Mouse Uterus Tissues
Abstract
:1. Introduction
2. Results
2.1. Establishment of a Mouse Model of Endometritis
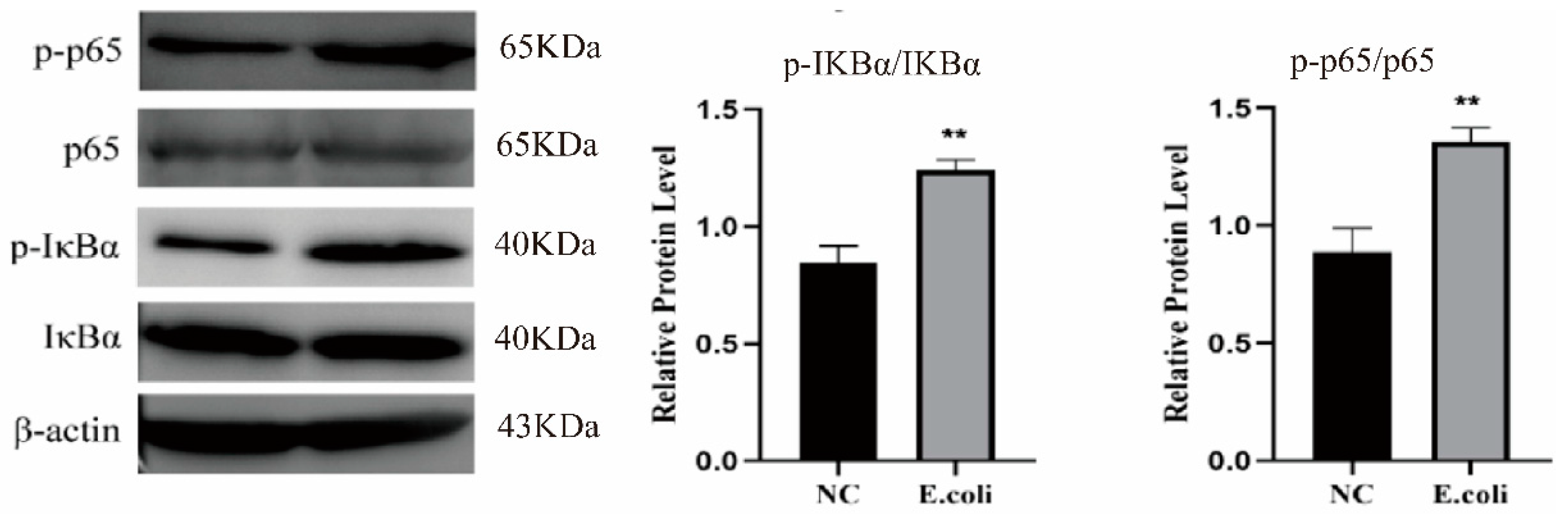
2.2. Effect of Escherichia coli on NF-κB Signaling Pathway
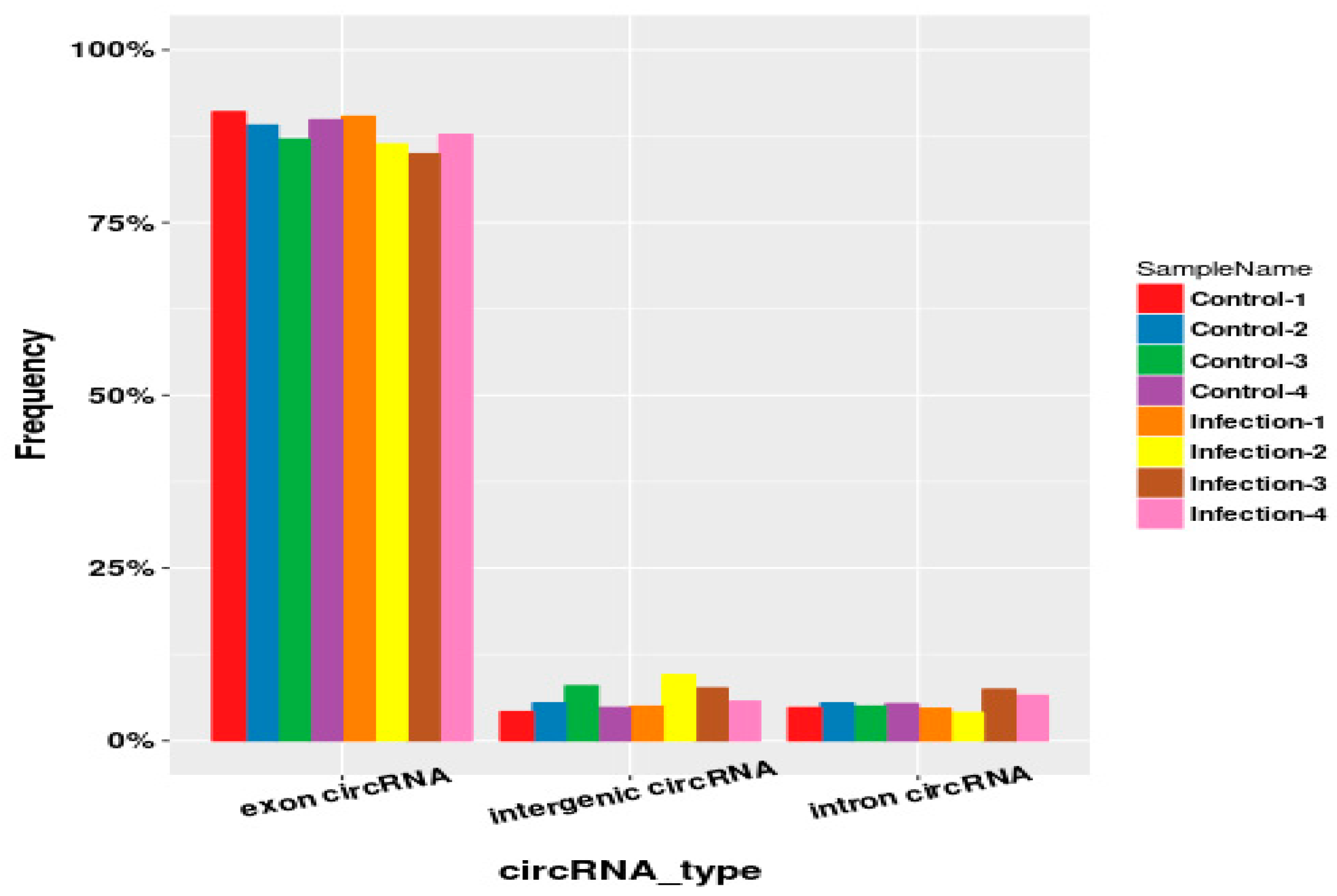
2.3. Identification and Type Statistics of Circular RNAs
2.4. Screening and Cluster Analysis of Circular RNAs
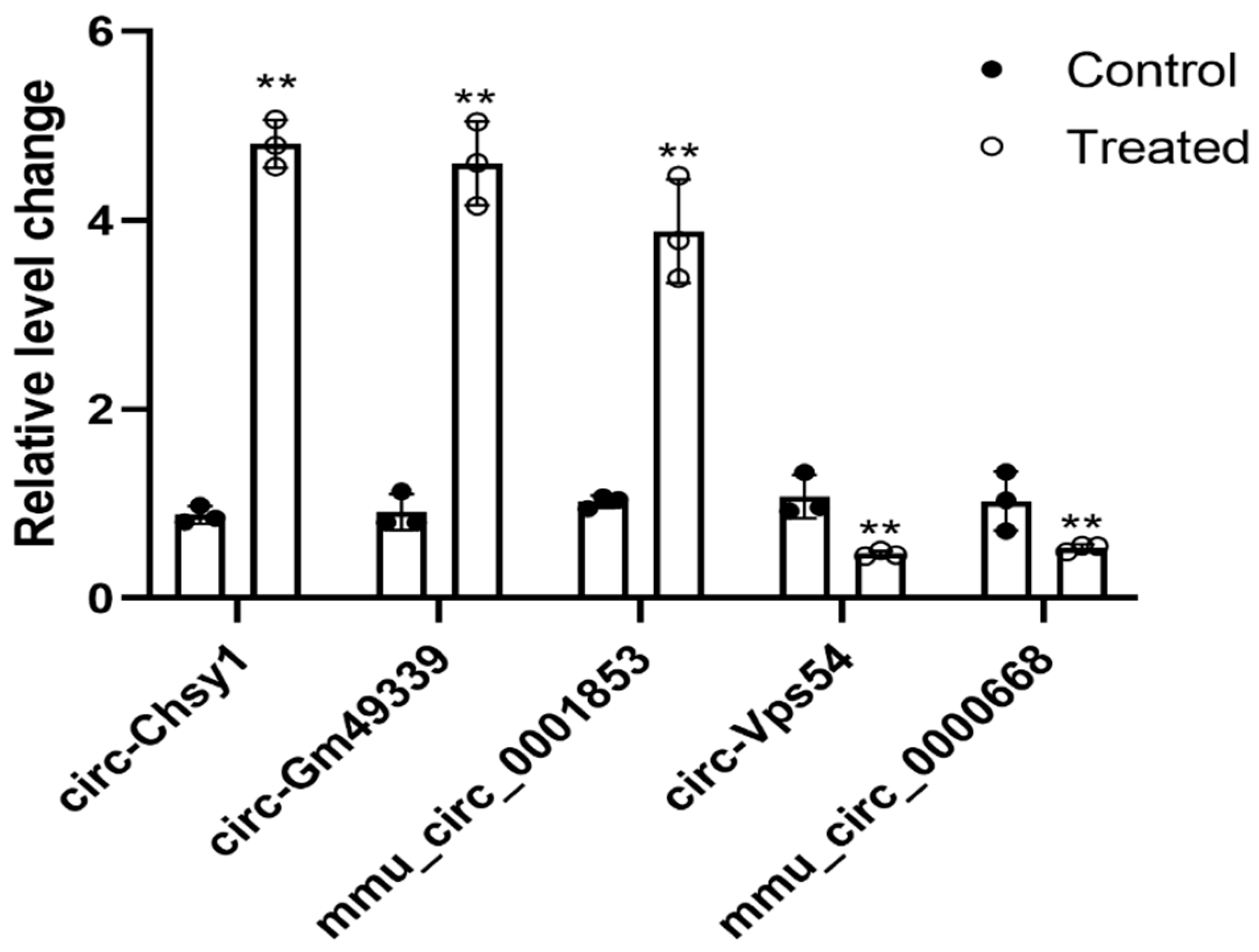
2.5. Validation of Selected Differentially Expressed Circular RNAs
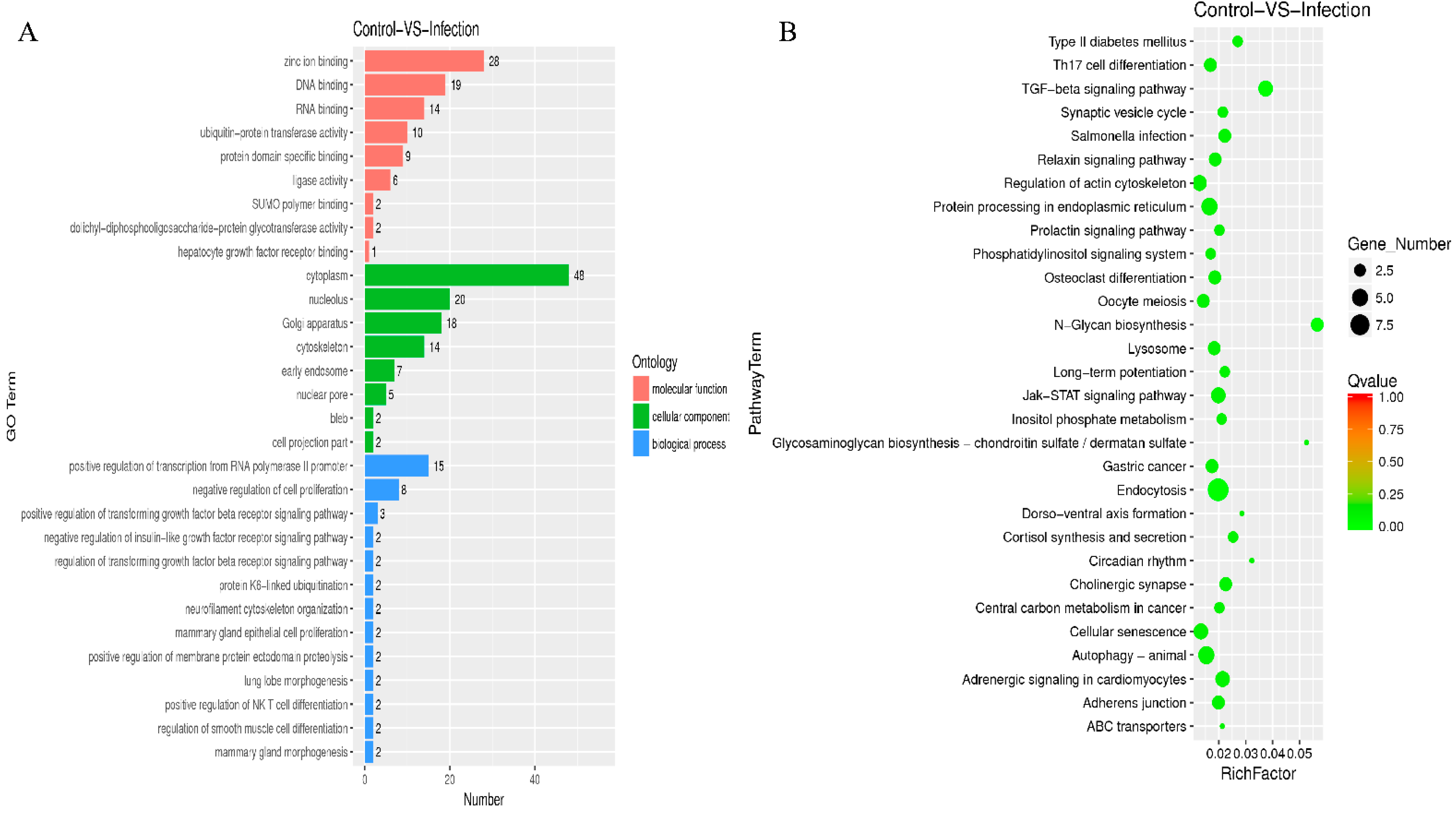
2.6. GO and KEGG Analysis for the Circular RNAs
2.7. The Role of TGF-β Signaling Pathway in Endometritis
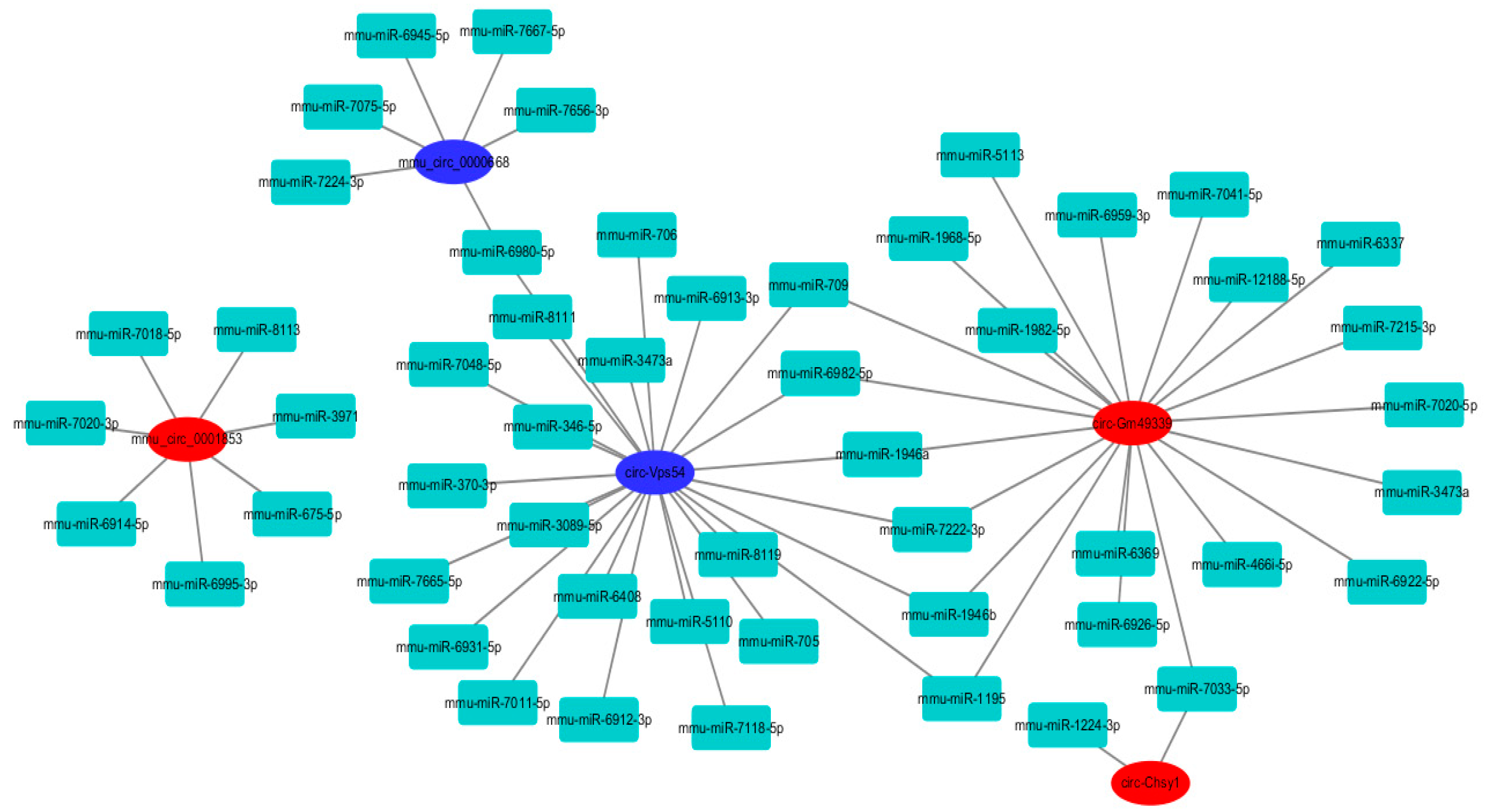
2.8. Circular RNAs–miRNAs Network
3. Discussion
4. Materials and Methods
4.1. Animal Treatment
4.2. Hematoxylin and Eosin
4.3. qRT-PCR
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Western Blot
4.6. Circular RNA Sequencing and Identification
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Piersanti, R.L.; Zimpel, R.; Molinari, P.C.C.; Dickson, M.J.; Ma, Z.; Jeong, K.C.; Santos, J.E.P.; Sheldon, I.M.; Bromfield, J.J. A model of clinical endometritis in Holstein heifers using pathogenic Escherichia coli and Trueperella pyogenes. J. Dairy. Sci. 2019, 102, 2686–2697. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fu, K.; Lv, X.; Wang, Y.; Wang, J.; Li, H.; Tian, W.; Cao, R. Lactoferrin suppresses lipopolysaccharide-induced endometritis in mice via down-regulation of the NF-κB pathway. Int. Immunopharmacol. 2015, 28, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Patop, I.L.; Wüst, S.; Kadener, S. Past, present, and future of circRNAs. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.B.; Wiklund, E.D.; Bramsen, J.B.; Villadsen, S.B.; Statham, A.L.; Clark, S.J.; Kjems, J. MiRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011, 30, 4414–4422. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Xie, H.; Ren, X.; Xin, S.; Lan, X.; Lu, G.; Lin, Y.; Yang, S.; Zeng, Z.; Liao, W.; Ding, Y.-Q.; et al. Emerging roles of circRNA_001569 targeting miR-145 in the proliferation and invasion of colorectal cancer. Oncotarget 2016, 7, 26680–26691. [Google Scholar] [CrossRef]
- Zhong, Z.; Lv, M.; Chen, J. Screening differential circular RNA expression profiles reveals the regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in bladder carcinoma. Sci. Rep. 2016, 6, 30919. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yu, Y.; Huang, Z.; Kong, Y.; Hu, X.; Xiao, W.; Quan, J.; Fan, X. CircRNA-5692 inhibits the progression of hepatocellular carcinoma by sponging miR-328-5p to enhance DAB2IP expression. Cell Death Dis. 2019, 10, 900. [Google Scholar] [CrossRef]
- Wang, J.; Wu, A.; Yang, B.; Zhu, X.; Teng, Y.; Ai, Z. Profiling and bioinformatics analyses reveal differential circular RNA expression in ovarian cancer. Gene 2020, 724, 144150. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, F.; Bao, S.; Sun, J. Systematic Characterization of Circular RNA-Associated CeRNA Network Identified Novel circRNA Biomarkers in Alzheimer’s Disease. Front. Bioeng. Biotechnol 2019, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yu, J.-W. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-β1. Biochem. Biophys. Res. Commun. 2017, 487, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Burd, C.E.; Jeck, W.R.; Liu, Y.; Sanoff, H.K.; Wang, Z.; Sharpless, N.E. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010, 6, e1001233. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cheng, M.; Gu, B.; Wang, J.; Yan, S.; Xu, D. CircRNA_09505 aggravates inflammation and joint damage in collagen-induced arthritis mice via miR-6089/AKT1/NF-κB axis. Cell Death Dis. 2020, 11, 833. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Suo, L.; Yang, S.; Zhang, W. CircRNA 001372 Reduces Inflammation in Propofol-Induced Neuroinflammation and Neural Apoptosis through PIK3CA/Akt/NF-κB by miRNA-148b-3p. J. Invest Surg. 2021, 34, 1167–1177. [Google Scholar] [CrossRef]
- Xu, X.; Jia, S.-Z.; Dai, Y.; Zhang, J.-J.; Li, X.; Shi, J.; Leng, J.; Lang, J. The Relationship of Circular RNAs With Ovarian Endometriosis. Reprod. Sci. 2018, 25, 1292–1300. [Google Scholar] [CrossRef]
- Liang, Y.; Ming, Q.; Shen, T.; Jin, Y.; Zhao, X.; Luo, R.; Wang, J.; Lu, J. CircRNA circFADS2 is Downregulated in Endometritis and its Overexpression Promotes miR-643 Maturation in Human Endometrial Epithelial Cells to Suppress Cell Apoptosis. Reprod. Sci. 2021, 28, 3508–3514. [Google Scholar] [CrossRef]
- Neurath, M.F.; Fuss, I.; Kelsall, B.L.; Presky, D.H.; Waegell, W.; Strober, W. Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J. Exp. Med. 1996, 183, 2605–2616. [Google Scholar] [CrossRef] [Green Version]
- Wira, C.R.; Grant-Tschudy, K.S.; Crane-Godreau, M.A. Epithelial cells in the female reproductive tract: A central role as sentinels of immune protection. Am. J. Reprod. Immunol. 2005, 53, 65–76. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Liu, Y.; Cui, J.; Che, S.; An, X.; Song, Y.; Cao, B. Circ-8073 regulates CEP55 by sponging miR-449a to promote caprine endometrial epithelial cells proliferation via the PI3K/AKT/mTOR pathway. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1130–1147. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, Y.; He, J.; Zhang, J.; Liu, X.; Chen, X.; Su, Y.; Wang, Y.; Gao, R. Altered expression patterns of circular RNAs between implantation sites and interimplantation sites in early pregnant mice. J. Cell. Physiol. 2019, 234, 9862–9872. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Pallone, F.; MacDonald, T.T. Smad7 in TGF-beta-mediated negative regulation of gut inflammation. Trends. Immunol. 2004, 25, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Y. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Qiu, H.; Xue, M.; Zhang, S.; Zhang, X.; Xu, J.; Chen, J.; Yang, Y.; Xie, J. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem. Cell Res. Ther. 2019, 10, 345. [Google Scholar] [CrossRef]
- Chen, B.; Huang, S.; Su, Y.; Wu, Y.-J.; Hanna, A.; Brickshawana, A.; Graff, J.; Frangogiannis, N.G. Macrophage Smad3 Protects the Infarcted Heart, Stimulating Phagocytosis and Regulating Inflammation. Circ. Res. 2019, 125, 55–70. [Google Scholar] [CrossRef]
- Li, X.; Nair, A.; Wang, S.; Wang, L. Quality control of RNA-seq experiments. Methods Mol. Biol. 2015, 1269, 137–146. [Google Scholar]
- Loboda, A.; Sobczak, M.; Jozkowicz, A.; Dulak, J. TGF-β1/Smads and miR-21 in Renal Fibrosis and Inflammation. Mediat. Inflamm. 2016, 2016, 8319283. [Google Scholar] [CrossRef] [Green Version]
- Cock, P.J.A.; Fields, C.J.; Goto, N.; Heuer, M.L.; Rice, P.M. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2010, 38, 1767–1771. [Google Scholar] [CrossRef] [Green Version]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]



| Sample | Clran Reads | Mapped Reads | Mapped Percent (%) | circRNAs Count |
|---|---|---|---|---|
| Control-1 | 105,483,211 | 105,260,546 | 99.79 | 692 |
| Control-2 | 103,908,448 | 103,776,493 | 99.87 | 660 |
| Control-3 | 103,188,307 | 103,012,390 | 99.83 | 642 |
| Control-4 | 103,201,392 | 103,053,994 | 99.86 | 730 |
| Infection-1 | 98,259,493 | 98,061,134 | 99.80 | 664 |
| Infection-2 | 101,642,157 | 101,435,481 | 99.80 | 492 |
| Infection-3 | 101,230,476 | 101,053,010 | 99.82 | 591 |
| Infection-4 | 97,820,008 | 97,690,406 | 99.87 | 702 |
| circRNA ID | GeneName | log2 Fold Change | p-Value | Regulation |
|---|---|---|---|---|
| circ-Chsy1 | Chsy1 | 8.400876 | 0.00015 | Ups |
| circ-Gm49339 | Gm49339 | 8.480887 | 0.00016 | Ups |
| mmu_circ_0001853 | Tgfbr2 | 7.324954 | 0.00244 | Ups |
| circ-Vps54 | Vps54 | −7.012614 | 0.00429 | Down |
| mmu_circ_0000668 | Mapk1 | −5.975774 | 0.02260 | Down |
| Genes | Primer Sequence (5′–3′) | Tm (°C) |
|---|---|---|
| IL-6 | F: CCACTTCACAAGTCGGAGGCTTA R: CCAGTTTGGTAGCATCCATCATTTC | 60 |
| IL-1β | F: TCCAGGATGAGGACATGAGCAC R: GAACGTCACACACCAGCAGGTTA | 60 |
| TNF-α | F: TATGGCCCAGACCCTCACA R: GGAGTAGACAAGGTACAACCCATC | 60 |
| β-actin | F: CTTCCTGGGCATGGAATCCT R: TTGATCTTCATTGTGCTGGGTG | 60 |
| Mus-circ-Gm49339 | F: TGGAGTCCTGGTGTCATTCC R: CCCAACTGTTCAGCTCTGCA | 62 |
| mmu_circ_0001853 | F: ACTCTGGAACATGCCGCTTC R: ATTGTCGCTGGCCATGACAT | 62 |
| Mus-circ-Chsy1 | F: AGATGTGTCCGGAGATTCGC R: CCGGGAATTGTCTTGGACCA | 62 |
| Mus-circ-Ghr | F: CAGTCACCAGCAGCACATTT R: TTGCCATCACCTCCTTTCCC | 62 |
| mmu_circ_0000668 | F: GGCACCAACCATTGAGCAAA R: TCAAGCCGATCAACACGTCA | 62 |
| Tgfb1 | F: GTGGAAATCAACGGGATCAG R: ACTTCCAACCCAGGTCCTTC | 58 |
| Smad3 | F: CCAGCACACAATAACTTGGA R: AGACACACTGGAACAGCGGA | 61 |
| Smad7 | F: CAAACCAACTGCAGGCTGTC R: CCCCCAGGGGCCAGATAATTC | 58 |
| Antibodies | Source and Cat No. | Dilution |
|---|---|---|
| Phosopho-NFκB p65 | CST (3033S) | 1:1000 |
| NFκB p65 | CST (8242S) | 1:1000 |
| Phosopho-IκBα | CST (2859S) | 1:1000 |
| IκBα | CST (4812S) | 1:1000 |
| Smad3 | CST (9523S) | 1:1000 |
| Phosopho-Smad3 | CST (9520S) | 1:1000 |
| Smad7 | SANTA CRUZ (sc-365846) | 1:1000 |
| ACTB | ABclonal (AC038) | 1:10,000 |
| Peroxidase-conjugated Affinipure Goat Anti-Rabbit IgG(H + L) | Proteintech SA00001-2 | 1:10,000 |
| Peroxidase-conjugated Affinipure Goat Anti-Mouse IgG(H + L) | Proteintech SA00001-1 | 1:10,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Shi, L.; Li, Q.; Zhao, J.; Lu, W.; Wang, J. The Expression and Bioinformatics Analysis of Circular RNAs in Endometritis Mouse Uterus Tissues. Molecules 2022, 27, 3682. https://doi.org/10.3390/molecules27123682
Li Z, Shi L, Li Q, Zhao J, Lu W, Wang J. The Expression and Bioinformatics Analysis of Circular RNAs in Endometritis Mouse Uterus Tissues. Molecules. 2022; 27(12):3682. https://doi.org/10.3390/molecules27123682
Chicago/Turabian StyleLi, Zhiqiang, Liying Shi, Qianqing Li, Jing Zhao, Wenfa Lu, and Jun Wang. 2022. "The Expression and Bioinformatics Analysis of Circular RNAs in Endometritis Mouse Uterus Tissues" Molecules 27, no. 12: 3682. https://doi.org/10.3390/molecules27123682
APA StyleLi, Z., Shi, L., Li, Q., Zhao, J., Lu, W., & Wang, J. (2022). The Expression and Bioinformatics Analysis of Circular RNAs in Endometritis Mouse Uterus Tissues. Molecules, 27(12), 3682. https://doi.org/10.3390/molecules27123682





