Diagnosing Pulmonary Embolism
Abstract
Résumé
Introduction
Why are diagnostic strategies necessary?
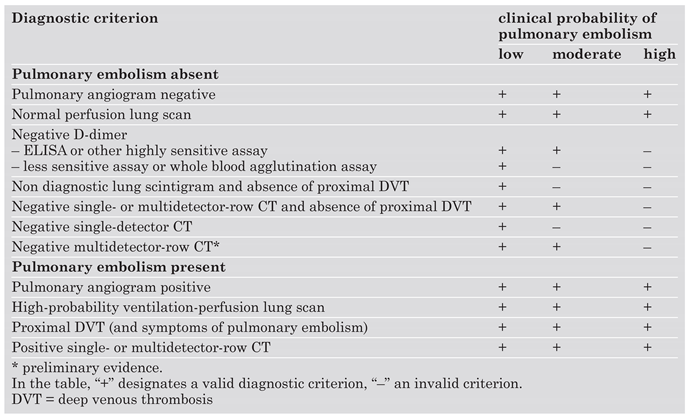
What is a validated diagnostic strategy for pulmonary embolism?
The role of ventilation-perfusion scintigraphy
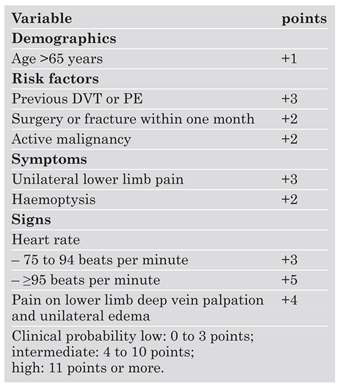
The role of clinical probability assessment
The role of D-dimer
The role of lower limb venous compression ultrasonography
The role of echocardiography
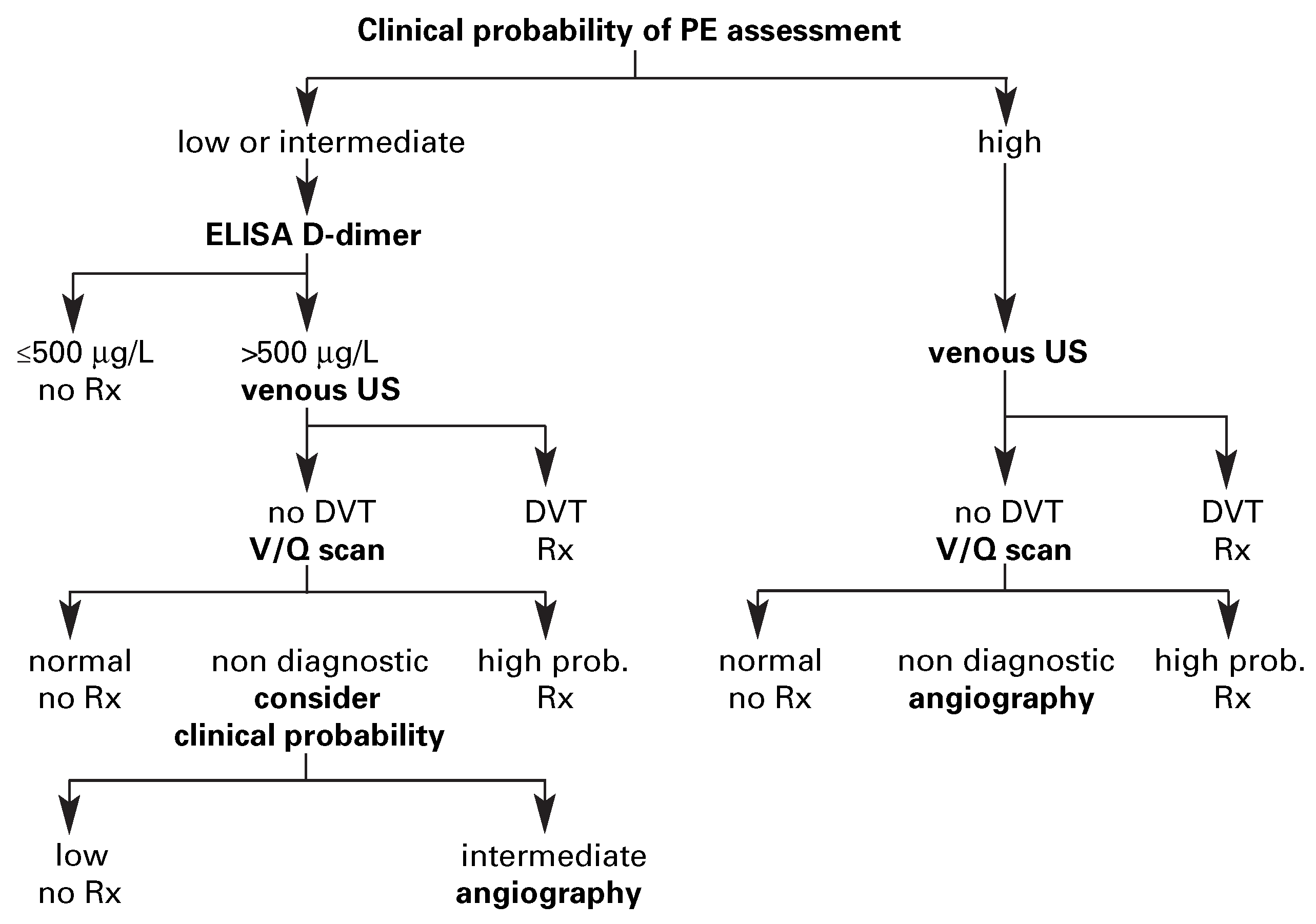
Diagnostic strategy based on ventilation-perfusion lung scintigraphy
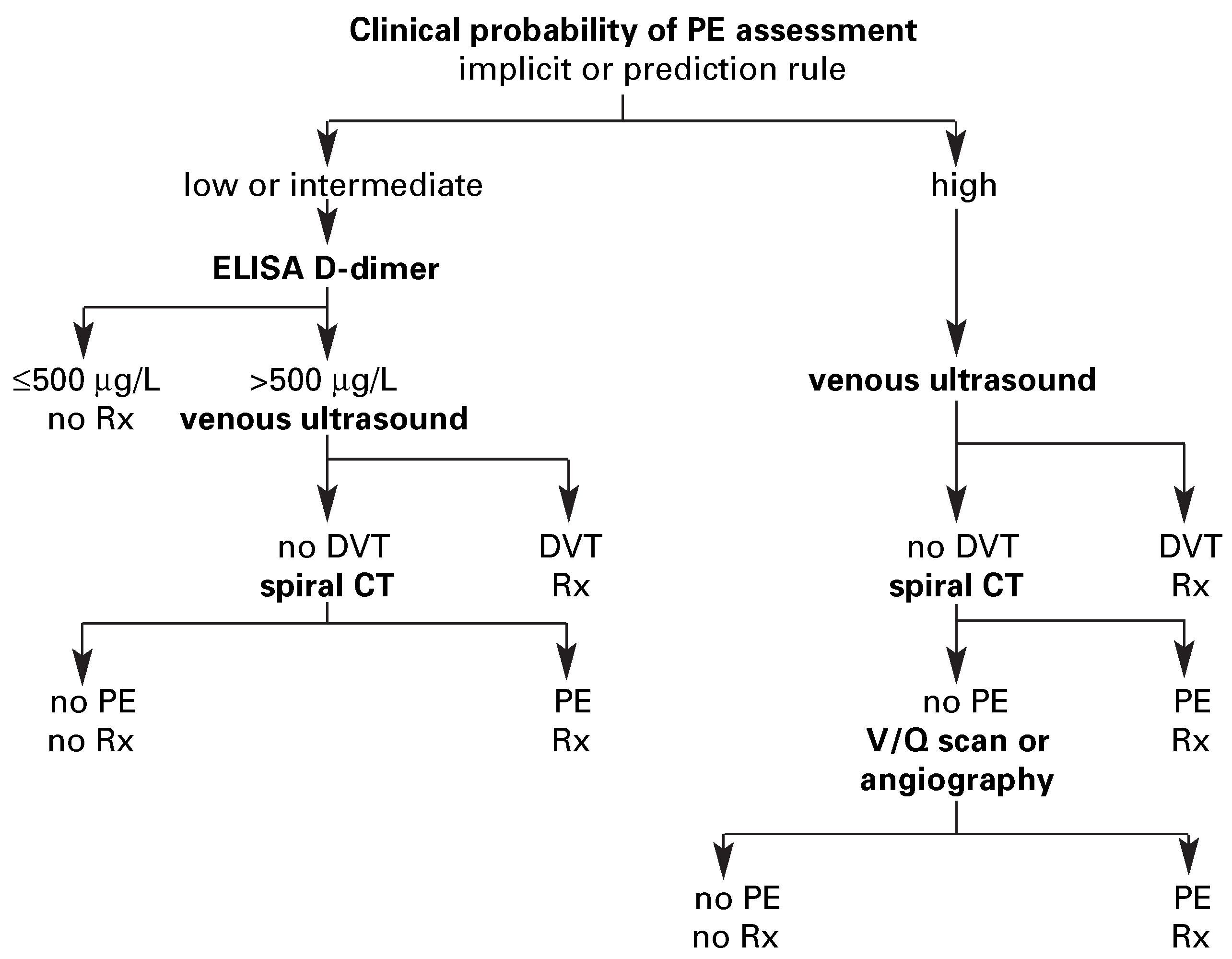
Diagnostic strategy based on spiral computed tomography
Diagnostic strategy for the haemodynamically unstable patient
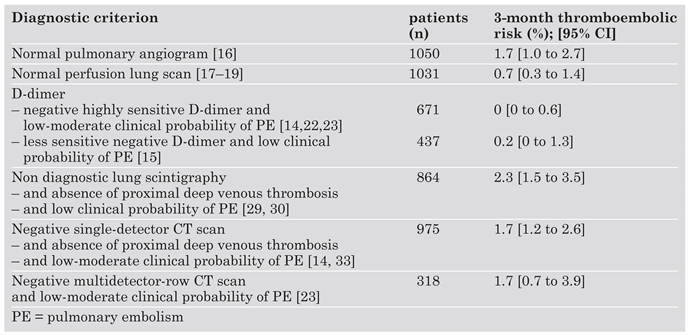
Conclusions
References
- The PIOPED Investigators. Value of the ventilation-perfusion scan in acute pulmonary embolism. JAMA 1990, 263, 2753–2759. [Google Scholar] [CrossRef]
- Stein, P.D.; Hull, R.D.; Patel, K.C.; Olson, R.E.; Ghali, W.A.; Brant, R. et al. D-Dimer for the exclusion of acute venous thrombosis and pulmonary embolism: A systematic review. Ann Intern Med 2004, 140, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Kruip, M.J.; Leclercq, M.G.; van der Heul, C.; Prins, M.H.; Buller, H.R. Diagnostic strategies for excluding pulmonary embolism in clinical outcome studies. A systematic review. Ann Intern Med 2003, 138, 941–951. [Google Scholar] [CrossRef]
- Bounameaux, H.; Perrier, A. D-dimer in the diagnosis of venous thromboembolism. In Venous Thromboembolism; Dalen, J.E., Ed.; Marcel Dekker Inc: New York, NY, USA, 2003; pp. 133–148. [Google Scholar]
- Kearon, C.; Ginsberg, J.S.; Hirsh, J. The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism. Ann Intern Med 1998, 129, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, S.W.; Raskob, G.E.; Whitsett, T.L. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: A systematic review. Ann Intern Med 2000, 132, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Mullins, M.D.; Becker, D.M.; Hagspiel, K.D.; Philbrick, J.T. The role of spiral volumetric computed tomography in the diagnosis of pulmonary embolism. Arch Intern Med 2000, 160, 293–298. [Google Scholar] [CrossRef]
- Fedullo, P.F.; Tapson, V.F. Clinical practice. The evaluation of suspected pulmonary embolism. N Engl J Med 2003, 349, 1247–1256. [Google Scholar] [CrossRef]
- Goldhaber, SZ. Pulmonary embolism. Lancet 2004, 364, 244. [Google Scholar] [CrossRef]
- British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax 2003, 58, 470–483. [CrossRef]
- Stein, P.D.; Athanasoulis, C.; Alavi, A.; Greenspan, R.H.; Hales, C.A.; Saltzman, H.A. et al. Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation 1992, 85, 462–468. [Google Scholar] [CrossRef]
- Perrier, A.; Nendaz, M.R.; Sarasin, F.P.; Howarth, N.; Bounameaux, H. Cost-effectiveness of diagnostic strategies for suspected pulmonary embolism including helical computed tomography. Am J Respir Crit Care Med 2003, 167, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Schoepf, U.J.; Costello, P. CT Angiography for diagnosis of pulmonary embolism: State of the art. Radiology 2004, 230, 329–337. [Google Scholar] [CrossRef]
- Perrier, A.; Roy, P.M.; Aujesky, D.; Chagnon, I.; Howarth, N.; Gourdier, A.L. et al. Diagnosing pulmonary embolism in outpatients with clinical assessment, D-dimer measurement, venous ultrasound, and helical computed tomography: A multicenter management study. Am J Med 2004, 116, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.S.; Anderson, D.R.; Rodger, M.; Stiell, I.; Dreyer, J.F.; Barnes, D. et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: Management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001, 135, 98–107. [Google Scholar] [CrossRef]
- Van Beek, E.J.; Brouwerst, E.M.; Song, B.; Stein, P.D.; Oudkerk, M. Clinical validity of a normal pulmonary angiogram in patients with suspected pulmonary embolism – a critical review. Clin Radiol 2001, 56, 838–842. [Google Scholar] [CrossRef]
- Hull, R.D.; Raskob, G.E.; Coates, G.; Panju, A.A. Clinical validity of a normal perfusion lung scan in patients with suspected pulmonary embolism. Chest 1990, 97, 23–26. [Google Scholar] [CrossRef]
- Kipper, M.S.; Moser, K.M.; Kortman, K.E.; Ashburn, W.L. Longterm follow-up of patients with suspected pulmonary embolism and a normal lung scan. Chest 1982, 82, 411–415. [Google Scholar] [CrossRef]
- Van Beek, E.J.R.; Kuyer, P.M.M.; Schenk, B.S.; Brandjes, D.P.M.; ten Cate, J.W.; Büller, H.R. A normal perfusion lung scan in patients with clinically suspected pulmonary embolism. Frequency and clinical validity. Chest 1995, 108, 170–173. [Google Scholar] [CrossRef]
- Wicki, J.; Perneger, T.V.; Junod, A.; Bounameaux, H.; Perrier, A. Assessing clinical probability of pulmonary embolism in the emergency ward: A simple score. Arch Intern Med 2001, 161, 92–97. [Google Scholar] [CrossRef]
- Le Gal, G.; Righini, M.; Roy, P.M.; Sanchez, O.; Aujesky, D.; Bounameaux, H. et al. Prediction of pulmonary embolism in emergency patients: The revised Geneva score. Ann Intern Med 2005, in press. [Google Scholar]
- Perrier, A.; Desmarais, S.; Miron, M.J.; de Moerloose, P.; Lepage, R.; Slosman, D. et al. Non-invasive diagnosis of venous thromboembolism in outpatients. Lancet 1999, 353, 190–195. [Google Scholar] [CrossRef]
- Perrier, A.; Roy, P.M.; Sanchez, O.; Le Gal, G.; Meyer, G.; Gourdier, A.L. et al. Multi-detector row computed tomography in suspected pulmonary embolism. N Engl J Med 2005, 352, 1760–1768. [Google Scholar] [CrossRef]
- Perrier, A. D-dimer for suspected pulmonary embolism: Whom should we test? Chest 2004, 125, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, S.Z. Echocardiography in the management of pulmonary embolism. Ann Intern Med 2002, 136, 691–700. [Google Scholar] [CrossRef]
- Roy, P.M.; Colombet, I.; Durieux, P.; Chatellier, G.; Sors, H.; Meyer, G. Systematic review and meta-analysis of strategies for the diagnosis of suspected pulmonary embolism. BMJ 2005, 331, 259. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.; Geibel, A.; Heusel, G.; Heinrich, F.; Kasper, W. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002, 347, 1143–1150. [Google Scholar] [CrossRef]
- Righini, M.; Aujesky, D.; Roy, P.M.; Cornuz, J.; de Moerloose, P.; Bounameaux, H. et al. Clinical usefulness of D-dimer depending on clinical probability and cutoff value in outpatients with suspected pulmonary embolism. Arch Intern Med 2004, 164, 2483–2487. [Google Scholar] [CrossRef] [PubMed]
- Perrier, A.; Miron, M.J.; Desmarais, S.; de Moerloose, P.; Slosman, D.; Didier, D. et al. Using clinical evaluation and lung scan to rule out suspected pulmonary embolism: Is it a valid option in patients with normal results of lower-limb venous compression ultrasonography? Arch Intern Med 2000, 160, 512–516. [Google Scholar] [CrossRef][Green Version]
- Wells, P.S.; Ginsberg, J.S.; Anderson, D.R.; Kearon, C.; Gent, M.; Turpie, A.G. et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med 1998, 129, 997–1005. [Google Scholar] [CrossRef]
- Perrier, A.; Howarth, N.; Didier, D.; Loubeyre, P.; Unger, P.F.; de Moerloose, P. et al. Performances of helical computed tomography in unselected outpatients with suspected pulmonary embolism. Ann Intern Med 2001, 135, 88–97. [Google Scholar] [CrossRef]
- Van Strijen, M.J.; De Monye, W.; Kieft, G.J.; Pattynama, P.M.; Prins, M.H.; Huisman, M.V. Accuracy of single-detector spiral CT in the diagnosis of pulmonary embolism: A prospective multicenter cohort study of consecutive patients with abnormal perfusion scintigraphy. J Thromb Haemost 2005, 3, 17–25. [Google Scholar] [CrossRef]
- Musset, D.; Parent, F.; Meyer, G.; Maitre, S.; Girard, P.; Leroyer, C. et al. Diagnostic strategy for patients with suspected pulmonary embolism: A prospective multicentre outcome study. Lancet 2002, 360, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Van Strijen, M.J.; De Monye, W.; Schiereck, J.; Kieft, G.J.; Prins, M.H.; Huisman, M.V. et al. Single-detector helical computed tomography as the primary diagnostic test in suspected pulmonary embolism: A multicenter clinical management study of 510 patients. Ann Intern Med 2003, 138, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, A.; Buller, H.R.; Huisman, M.V.; Huisman, P.M.; Kaasjager, K.; Kamphuisen, P.W. et al. Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006, 295, 172–179. [Google Scholar] [PubMed]
- Wan, S.; Quinlan, D.J.; Agnelli, G.; Eikelboom, J.W. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: A meta-analysis of the randomize controlled trials. Circulation 2004, 110, 744–749. [Google Scholar] [CrossRef]
 |
 |
 |
© 2006 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Perrier, A. Diagnosing Pulmonary Embolism. Cardiovasc. Med. 2006, 9, 136. https://doi.org/10.4414/cvm.2006.01167
Perrier A. Diagnosing Pulmonary Embolism. Cardiovascular Medicine. 2006; 9(4):136. https://doi.org/10.4414/cvm.2006.01167
Chicago/Turabian StylePerrier, Arnaud. 2006. "Diagnosing Pulmonary Embolism" Cardiovascular Medicine 9, no. 4: 136. https://doi.org/10.4414/cvm.2006.01167
APA StylePerrier, A. (2006). Diagnosing Pulmonary Embolism. Cardiovascular Medicine, 9(4), 136. https://doi.org/10.4414/cvm.2006.01167




