Classification
There are two main classes of valvular prostheses: the mechanical and the tissue valve prostheses. The mechanical prostheses are extremely robust and designed to function mechanically way over a human live. Because of the nature of the foreign material, they require, however, an anticoagulation to reduce their thrombo-embolic complication rate. Tissue valve prostheses are made out of human or animal tissue. Thanks to their biocompatibility, the risk of spontaneous thrombo-embolic complication is low and does not justify additional anticoagulation beyond the operative period. The main disadvantage of tissue prostheses is their absence of repair process leading to inevitable degeneration. Many factors converge to induce prosthesis degeneration; the most important ones are the haemodynamic stress on the leaflets and the patient’s immunological reaction to the foreign tissues.
Another important consideration in classification of prostheses (for the semilunar valves) is the breaking down in valvular and in root prostheses (the latter being sometimes also called “composite graft” for the aortic root and “valved conduit” for the pulmonary root) (
Figure 1). The aim of valvular prostheses is to replace the valve cusps only, the aim of a composite graft or a valved conduit is to replace the cusps as well as the sinuses and, as needed, the ascending aorta or the main pulmonary artery.
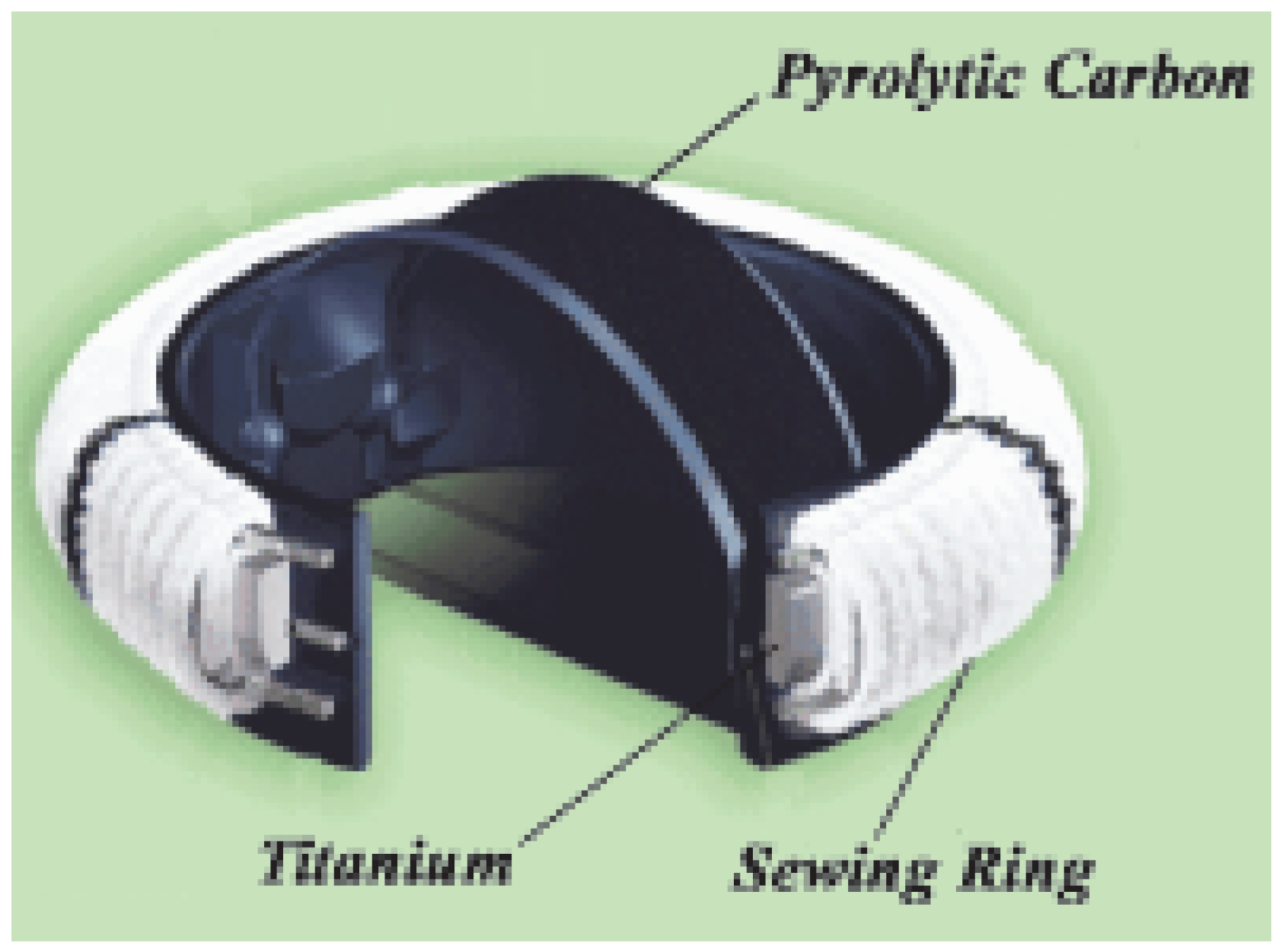
The metal components of mechanical valve prostheses are most of the time made of pyrocarbon, a very light and resistant material. A housing (made of pyrocarbon and titanium) and a sewing cuff complete the prosthesis (
Figure 2). Many designs have been experimented, starting with the cage balls in the early sixties. Nowadays, only two categories of mechanical valve prosthesis are implanted: the tilting disk and the bileaflet prosthesis (
Figure 3). These prostheses are implanted mostly in the left side (usually the systemic side) of the heart. As a matter of fact, these prostheses, prone to thrombo-embolic complications, are less suitable for the “right heart”. The advantages of the mechanical valve prostheses are their reliability, the easiness with which they can be implanted, and their low-profile. The low profile is particularly advantageous in case of mitral valve replacement in an acute situation like an endocarditis or a papillary muscle rupture. In both situations, the left ventricle presents with a normal size and a high profile prosthesis (like most bioprosthesis) can impinge in the left ventricular outflow tract. Although the tilting disc prosthesis has a large efficient opening area, most surgeons rely primarily on bi-leaflet prosthesis, because of their superior reliability. There are very many such prostheses on the market. If mild differences exist in their design and laboratory performance, their clinical outcome is roughly similar.
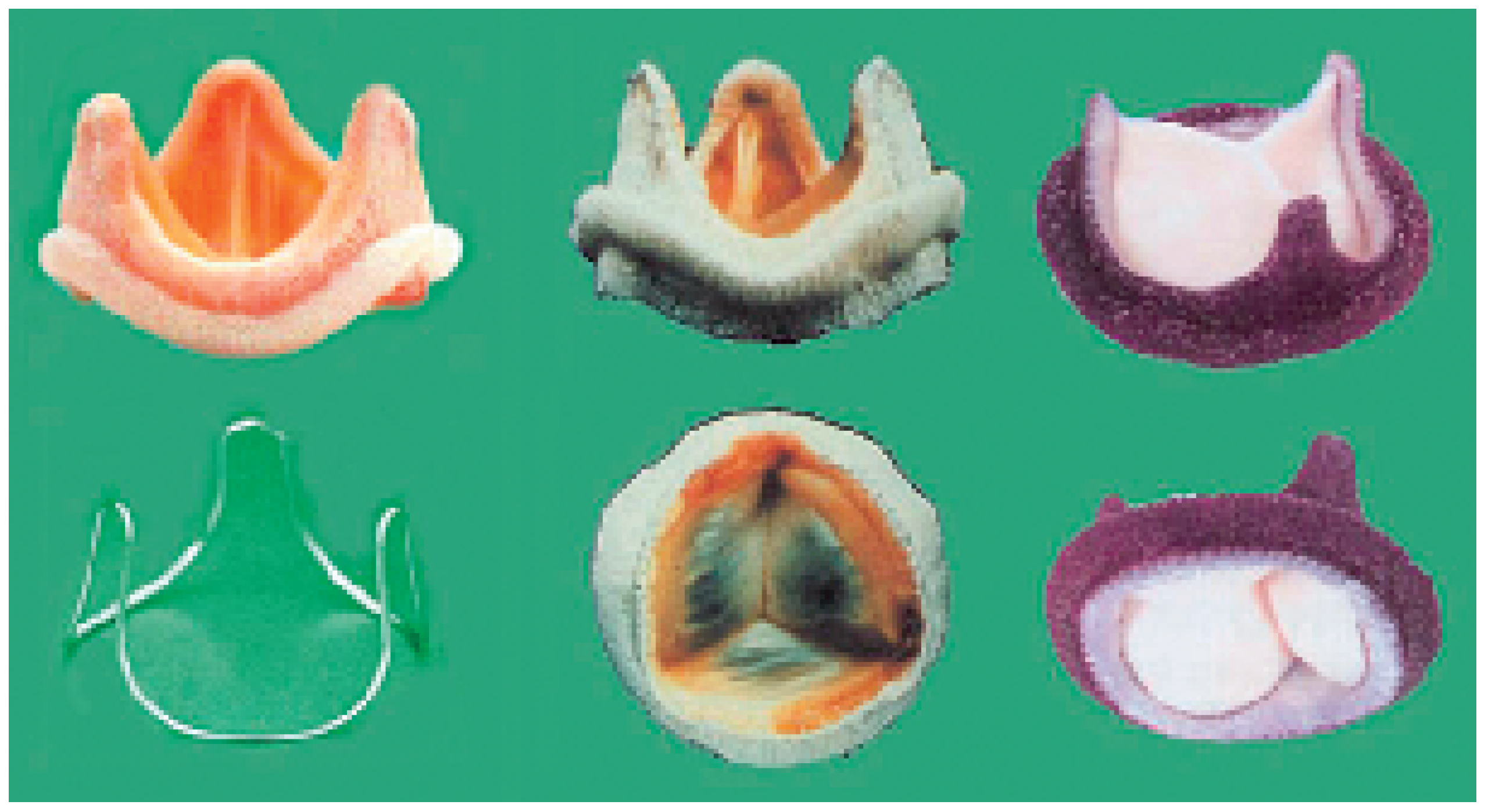
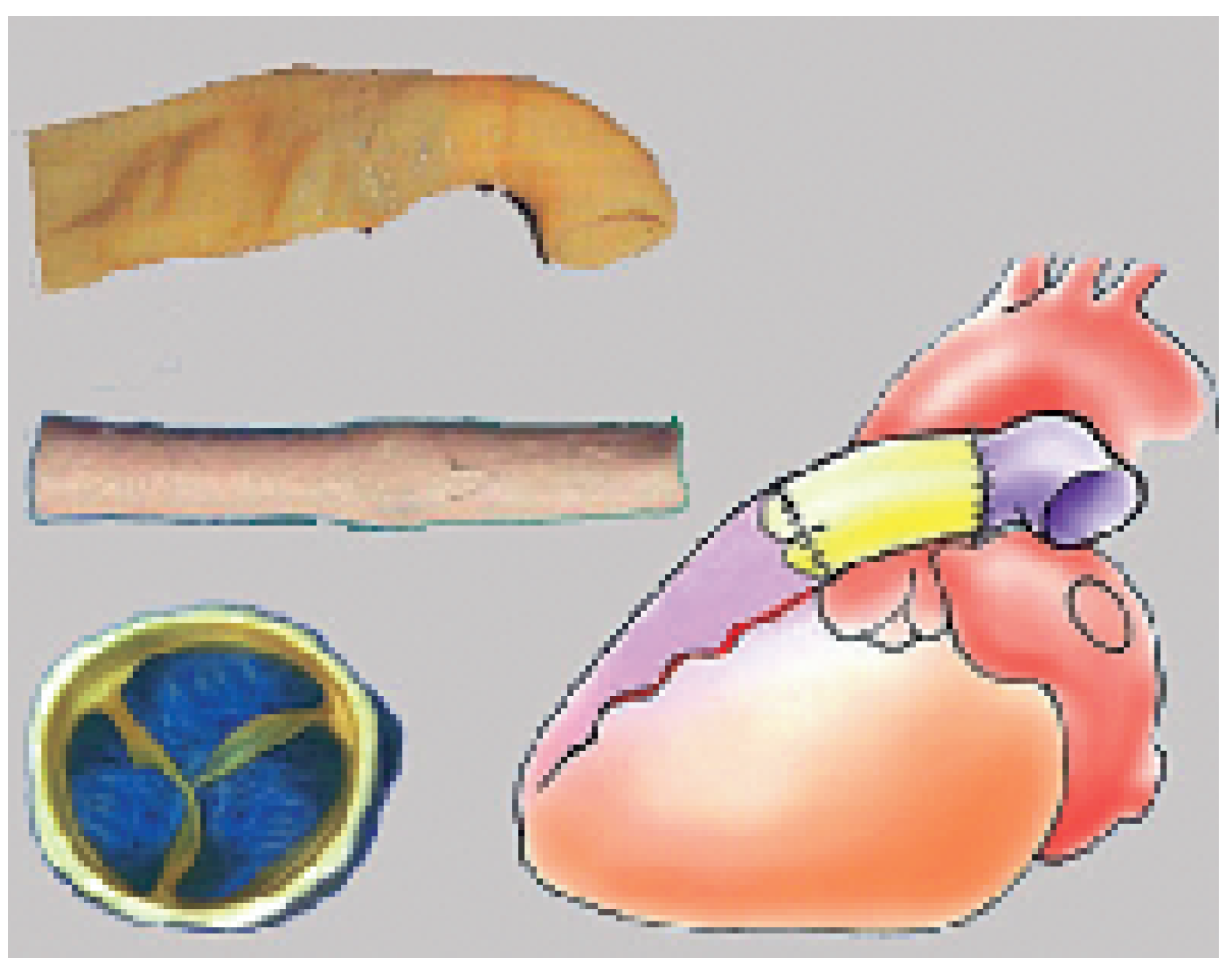
Tissue valve prostheses are usually made out of human (homografts) or animal (xenografts) cardiac valves or out of animal pericardium (xenopericardium). Major developments have been made in the last decade in the search of an ideal tissue valve prosthesis. The rate of implantation of such prostheses has steadily increased and, nowadays, these prostheses are the most commonly used. They present with three cusps and resemble an aortic valve (from which many come from) and are available as valvular prosthesis or as valved conduits.
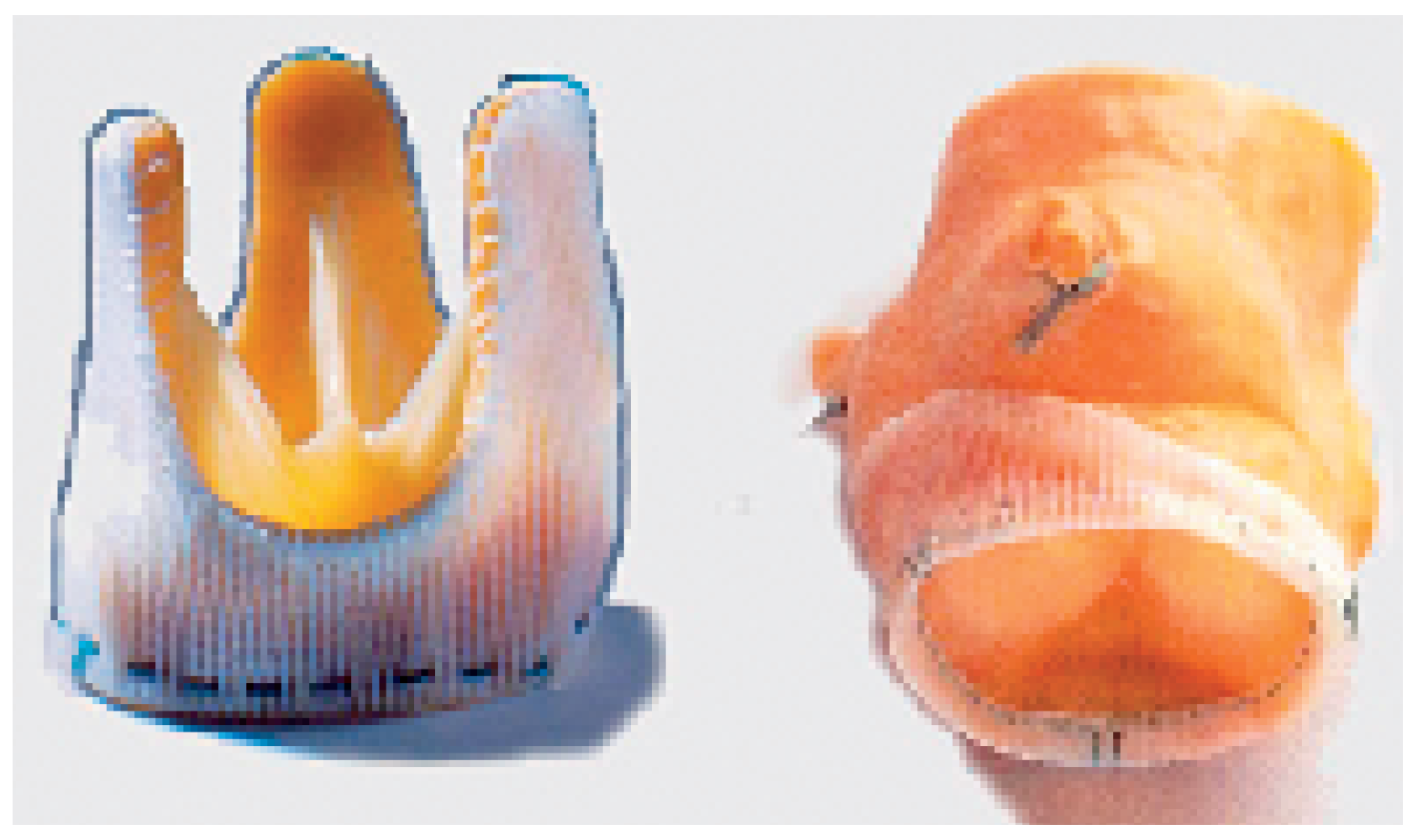
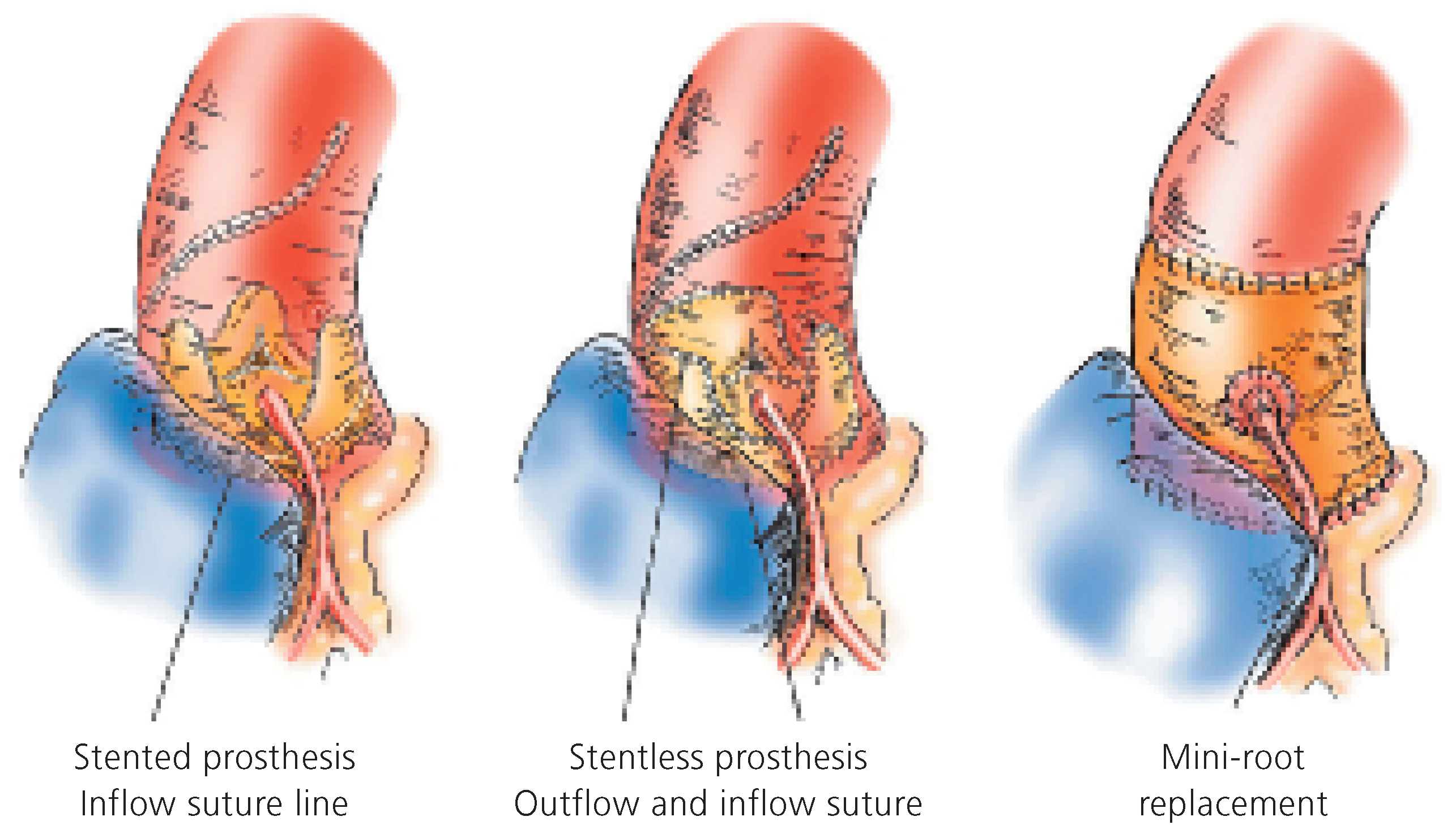
These prostheses replace the diseased valvular cusps (without replacement of the sinuses for instance) and exist in two categories: the stented and the stentless prosthesis. The stented prosthesis—the oldest, the traditional prosthesis—is made on a rigid armature (usually a metal stent) on which the cusps and a sewing ring are mounted (
Figure 4, panel left). The haemodynamic stress on the moving leaflet concentrates along its insertion line on the metal post. This is the typical area where the degenerative process starts and develops. The sewing ring, a relatively broad rim added on the external part of the prosthesis, significantly reduces the effective opening area. In spite of these haemodynamic disadvantages, these bio-prostheses remain particularly recommended (and widely used) in old patients because of the simplicity of their insertion. In this population, the haemodynamic stress created by the basal cardiac output (absence of strenuous exercise) is limited and does not lead to premature degeneration. These prostheses are usually made out of native porcine aortic valve or are totally constructed out of animal pericardium (
Figure 4, panel right). They can be used to replace anyone of the four cardiac valves. Recent long term-studies tend to give an advantage for the bio-prostheses fashioned out of pericardium, which have achieved outstanding results at 15 years [
1].
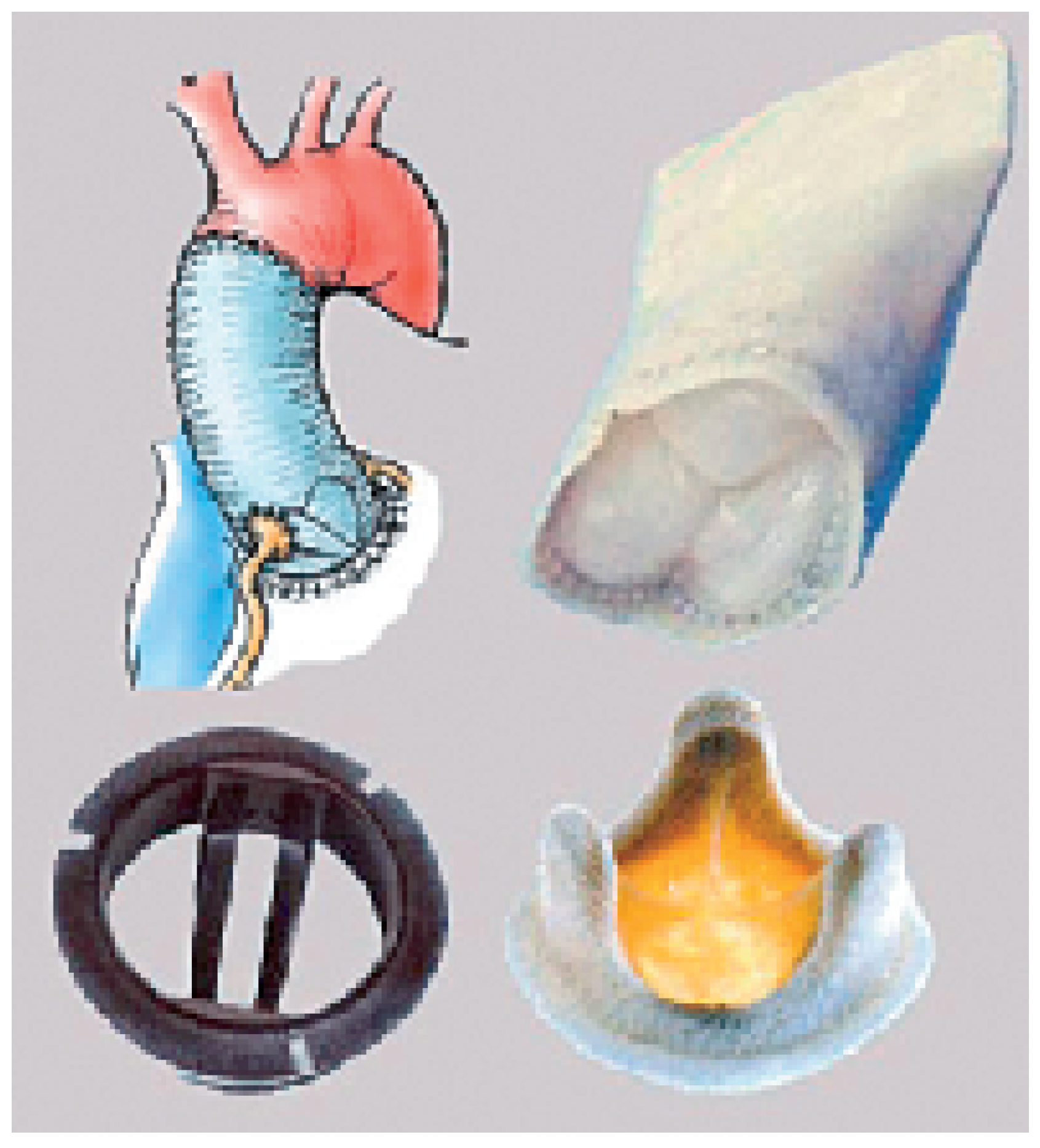
The stentless valve group is the other main group of tissue valve prostheses and consists of valvular prostheses or valved conduits (
Figure 5). These prostheses are used to replace the semi-lunar valves only (the aortic and the pulmonary valve). The principle of the stentless prosthesis compared to the stented one is the avoidance of an internal rigid stent and of a “space consuming” sewing ring. The effective orifice area is therefore substantially increased; an advantage in physically active people who multiply their cardiac output. Furthermore, the expansion of the sinuses (in which each commissure is inserted) softens the stress distributed on the cusps. With the corresponding reduction of haemodynamic stress, it is expected that degeneration will be delayed, a fact that seems to be confirmed in clinical studies. The implantation of a stentless prosthesis is, however, technically more demanding (
Figure 6). The base of the prosthesis must be sewn on the aortic annulus (the inflow suture) and the scalloped sinuses and commissures must be implanted in the aortic root (the outflow suture). The fine tri-dimensional geometry of the cusps must be preserved for proper prosthesis function. These valves, when rightly implanted, achieve excellent haemodynamic results. This property is important in physically active patients where the cardiac output can increase tremendously without producing a significant trans-valvular gradient. They are, however, contra-indicated in calcified aortic root, because of the increased risk of fragment embolisation and of aortic or coronary artery dissection.
Biological valved conduits (
Figure 1 upper left,
Figure 5 right for the aortic position,
Figure 11 for the pulmonary position) are gaining popularity, because they offer the advantages of the stentless prosthesis (reduced haemodynamic stress and large effective opening area) and have a large versatility. They are used to replace the whole aortic or pulmonary root. In doing so in the aortic position, the surgeon must temporarily explant the coronary arteries (with a button of aortic wall to facilitate reimplantation) and reimplant them in the neo-aortic root (
Figure 6, right,
Figure 10, right).
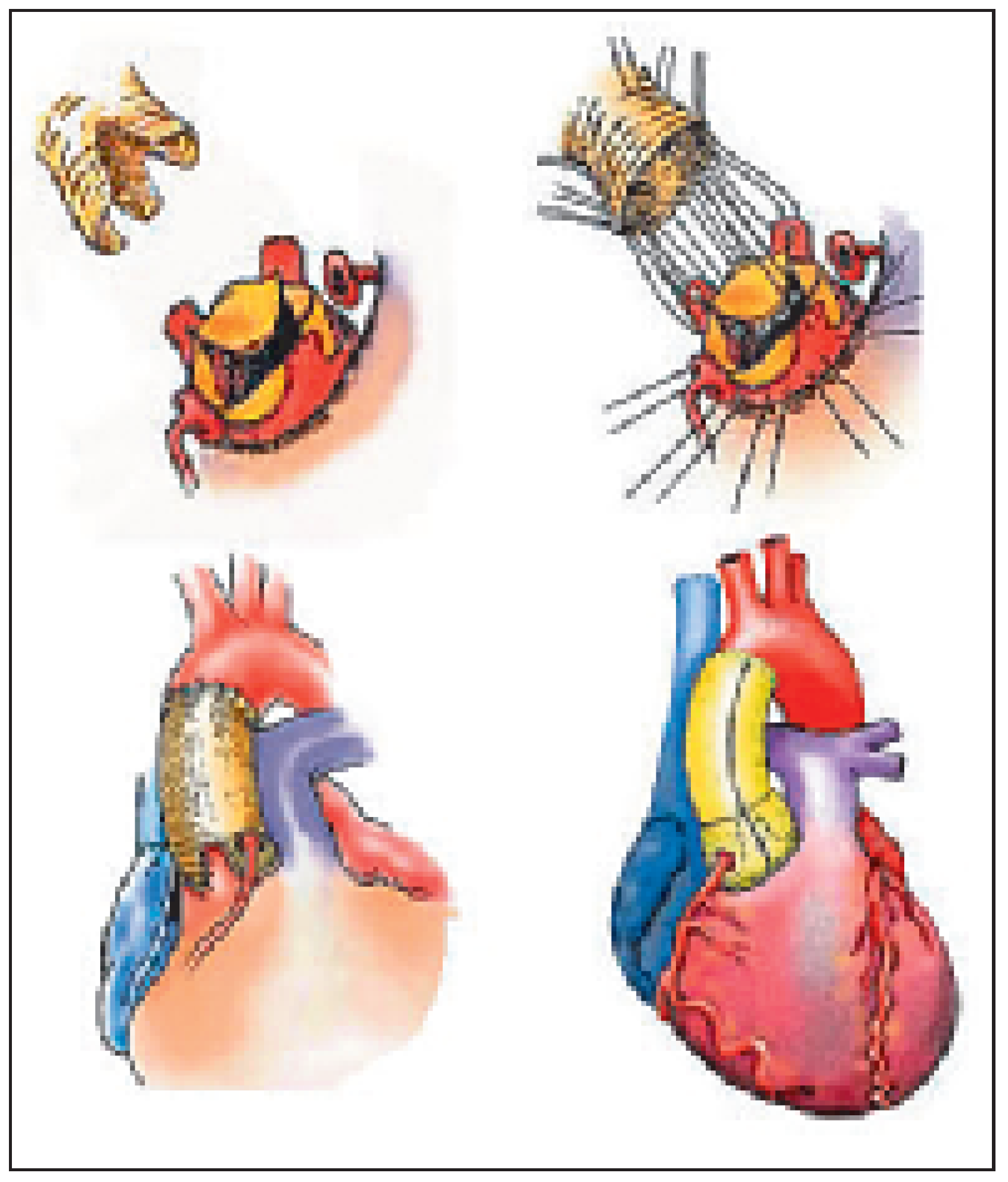
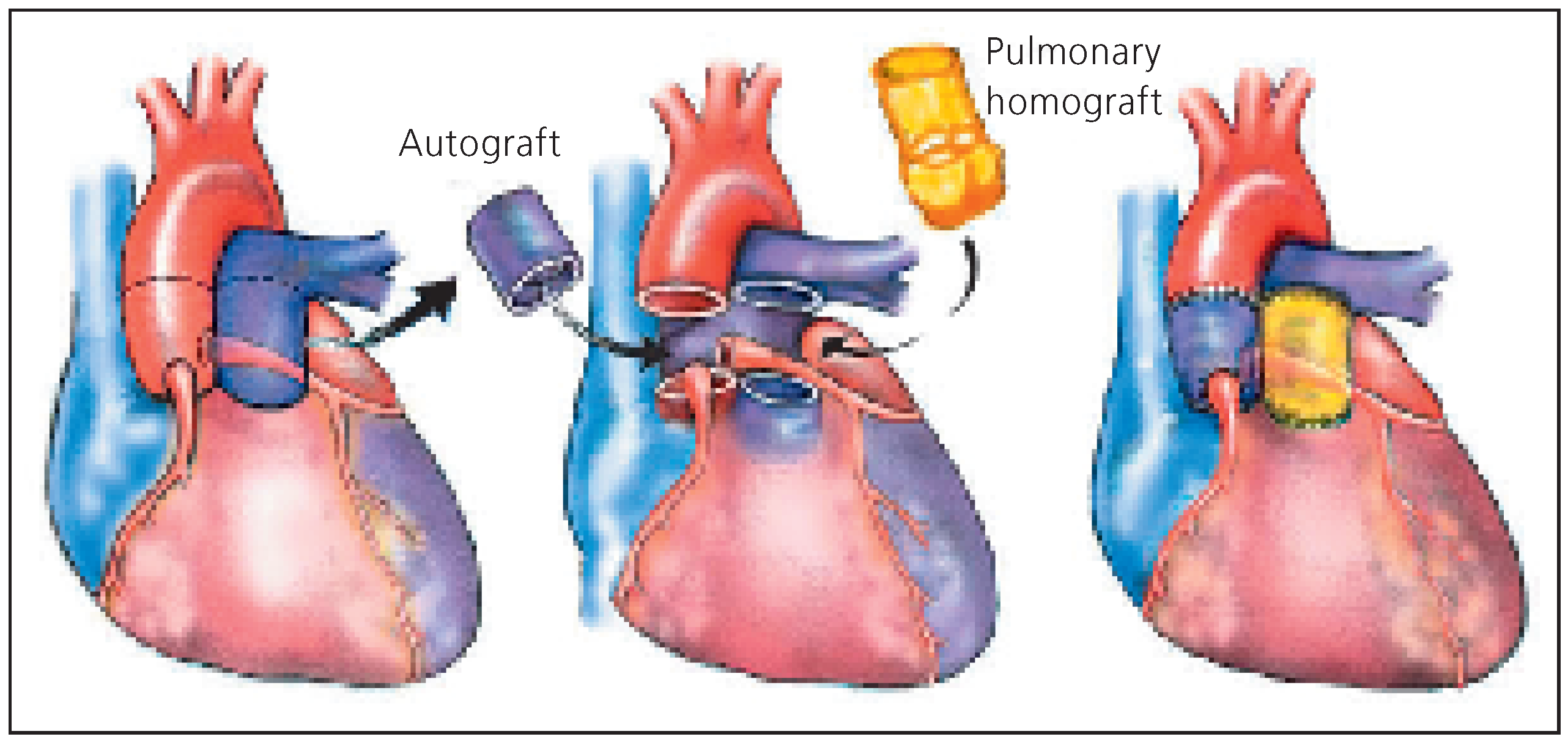
Three origins of conduits are used to replace the aortic root: the autografts, homografts and xenografts. The autograft (the mainstay of the Ross procedure) is the pulmonary root which is transferred onto the aortic annulus. The pulmonary root itself is replaced by a homograft or a xenografts (
Figure 7). The advantage of this demanding operation is that the autograft has potential for growth (the solution for children), usually sustains the systemic pressure over time and may be a definitive solution for the aortic root. The reduced haemodynamic stress on the homograft used to replace the pulmonary root explains freedom from degeneration sometimes over 25 years [
2].
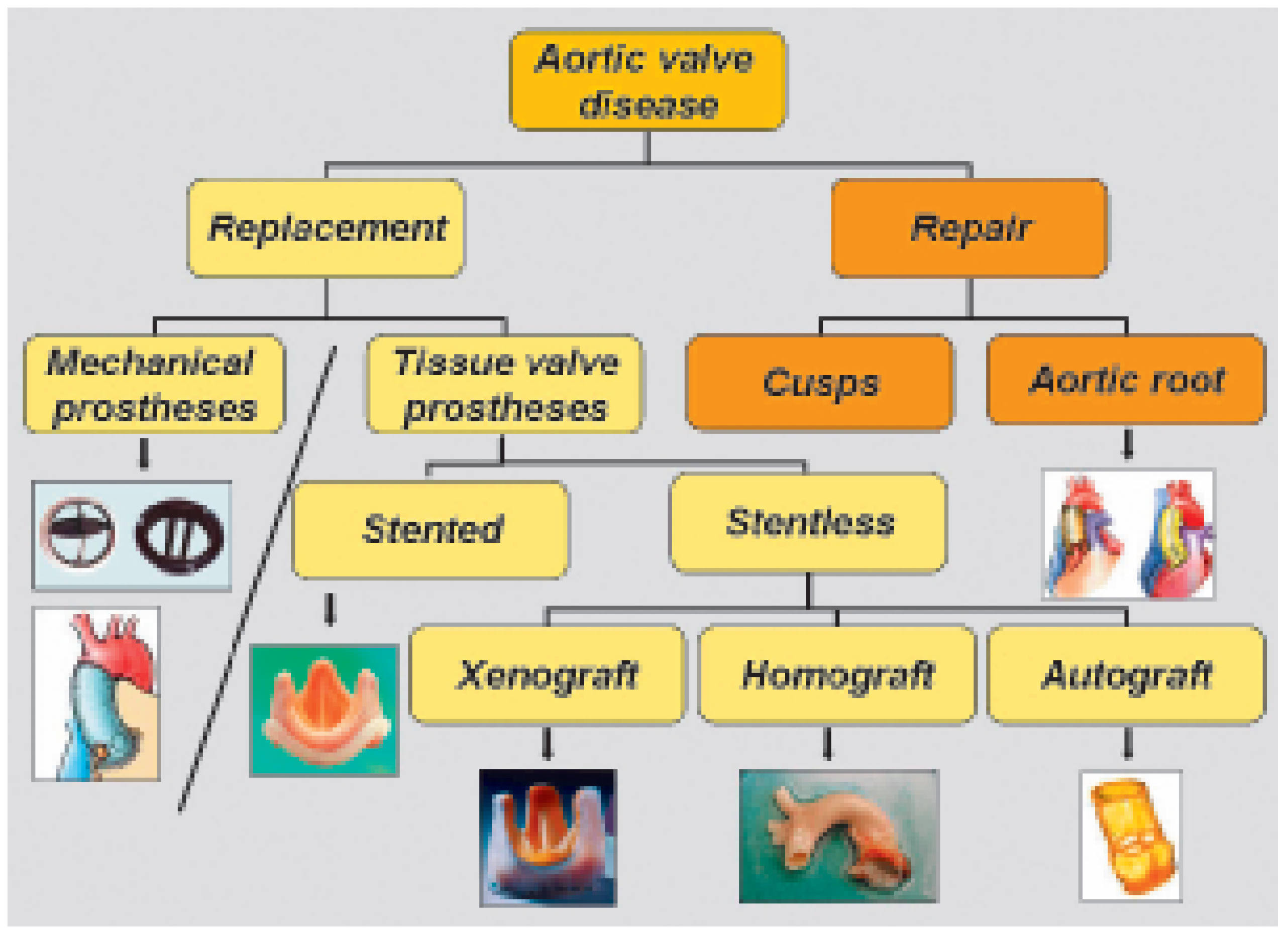
Aortic valve replacement is one of the most commonly performed cardiac operations. There is a wide variety of prostheses or procedures that can be used for this purpose (
Figure 8). Again, the choice of proper valve or operation depends on patient’s characteristic, but also on the surgeon’s preference or ability. A common concern when considering the aortic valve is the appropriate reduction of the transvalvular gradient, not only at rest (during echocardiographic evaluation) but also at exercise [
3].
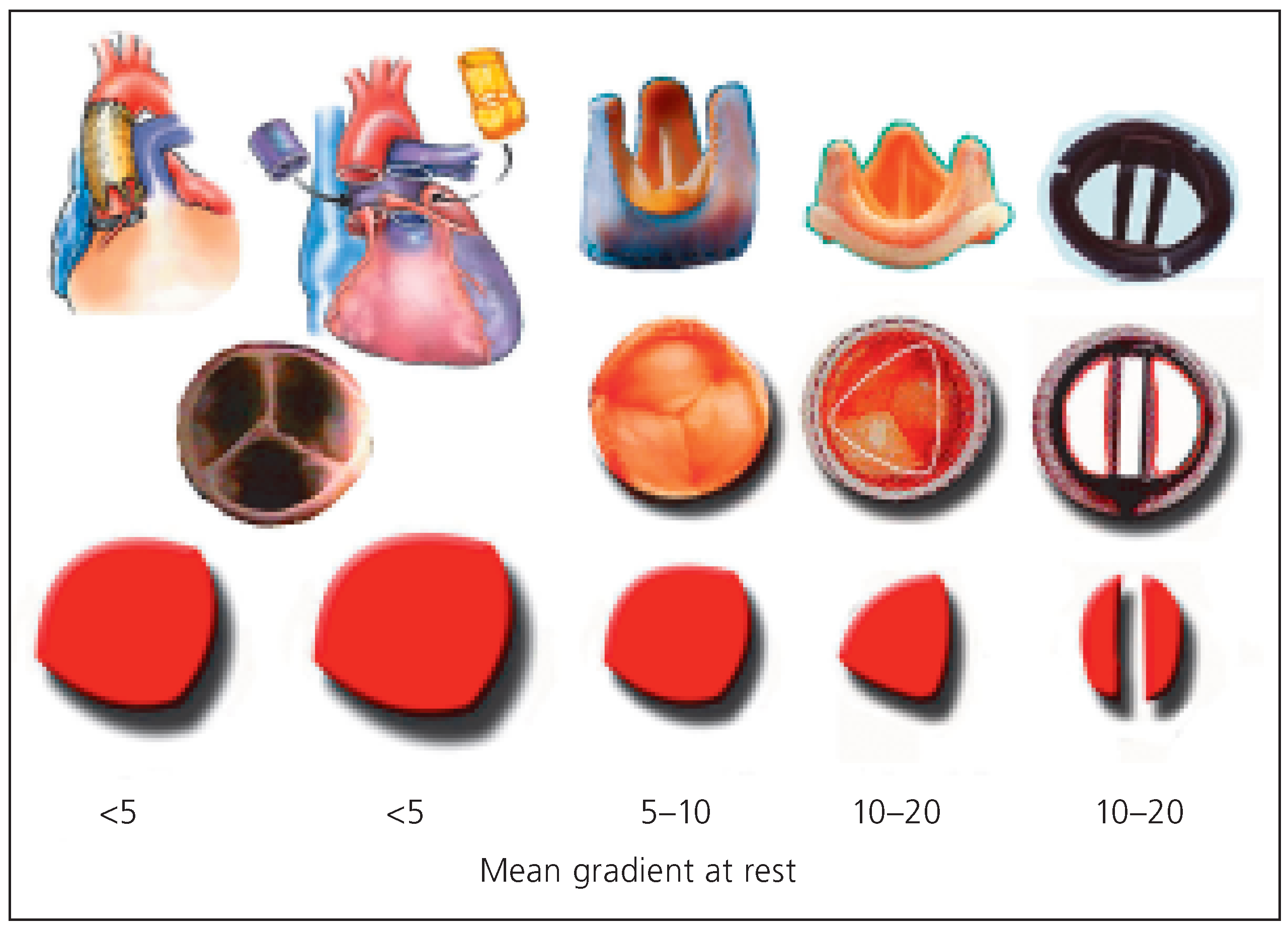
Figure 9 depicts the effective opening area for a given annular size obtained by various prostheses or procedures.
Young patients present special problems when replacement of the aortic valve is considered. The cumulative risk of thrombo-embolic and bleeding complication associated with mechanical valves and its anticoagulation is no longer insignificant when the risk is repeated over many years [
4,
5]. Young people are frequently physically active, practicing many strenuous sports. During high efforts, the transvalvular gradient rises significantly and can maintain or induce a dangerous left ventricular hypertrophy. Furthermore, young women contemplate pregnancies at this age, which is prone to be complicated by oral anti-coagulation. Finally, any prosthesis does not bear any potential for growth. For all these reasons, the
“Ross procedure” (
Figure 7) appears as one of the most attractive options in those young persons. The procedure consists in replacing the aortic root with the pulmonary root (this necessitates harvesting and reimplantation of the coronary arteries). The harvested pulmonary root is usually replaced by a pulmonary homograft [
6]. These homografts, in the low pressure system can last more than 20 years without degeneration [
2]. They are furthermore easily accessed by percutaneous techniques [
7]. The procedure is, however, technically much more demanding than a simple valve replacement and can only be performed under strict conditions and by trained teams. Because of the growth potential of the autograft, the
“Ross procedure” is the only recommend operation in children with aortic valve disease that cannot be repaired [
8,
9].
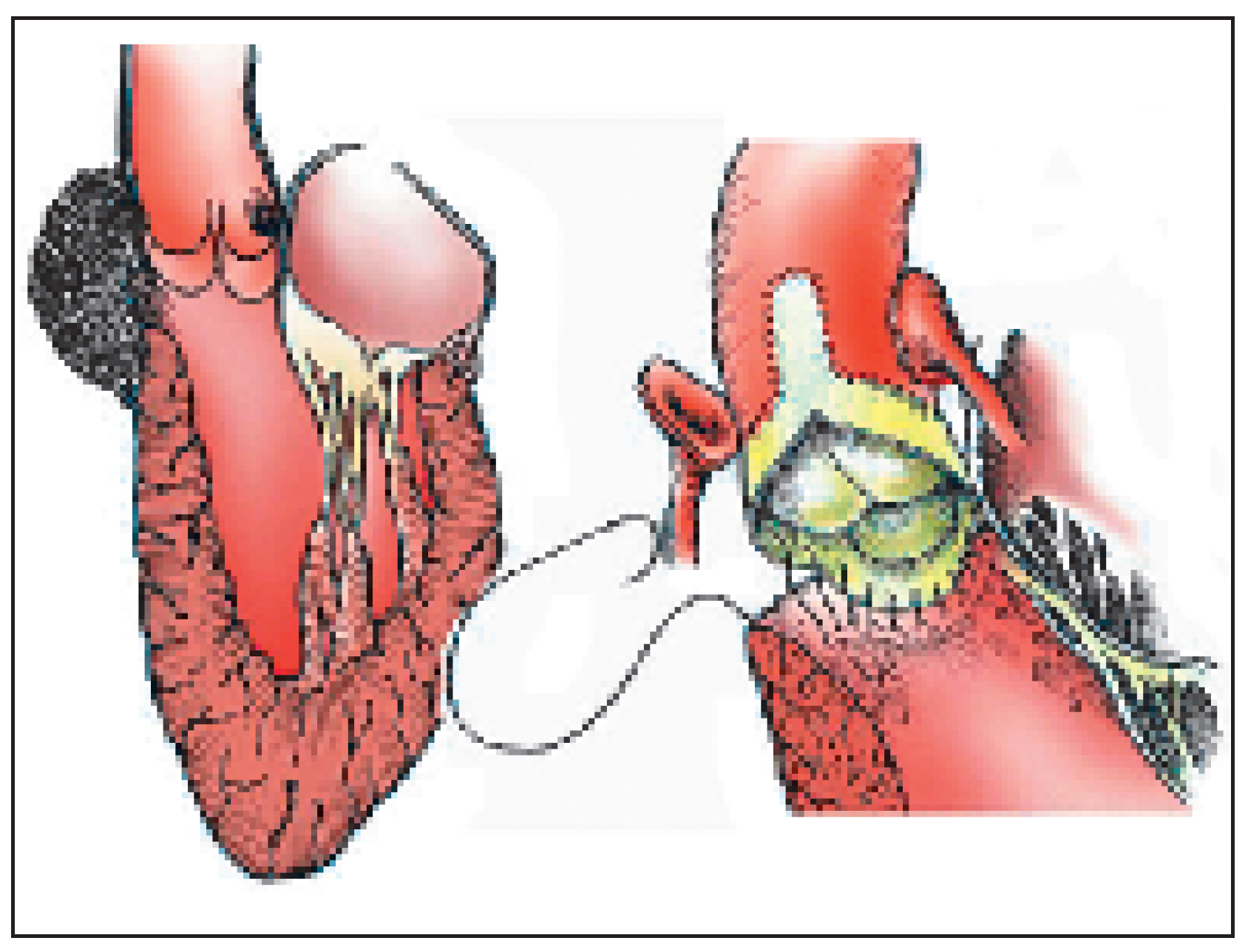
In some patients, the sinuses are dilated, but the valve cusps are still pliable and can be preserved or repaired. Replacement of the aortic root with preservation (and as needed, reparation) of the cusps (the so-called “valve preserving operation”) can be done in two ways (
Figure 10). The
“Yacoub procedure”, described in 1983, consists in replacing the sinuses by a Dacron graft shaped in three scallops [
10,
11]. Both coronary arteries are reimplanted in corresponding sinuses of the graft. This procedure can lead to excellent long term results with a normal valve function. Dilatation of the remnant of the aortic root and annulus is however possible, and is more prone to occur in patients with conjunctive tissue disease. The
“Tirone David procedure” uses the same principles. The graft, usually a Dacron graft with an enlarged distal portion simulating sinuses, is implanted directly on the aortic annulus, completely “wrapping” the aortic root. The scalloped sinuses and commissures are inserted with a complete suture line within the graft [
12]. Here too, the coronary arteries are reimplanted in their corresponding sinus. In the
“Tirone David procedure”, the aortic annulus is fixed within the Dacron prosthesis and can no longer dilate [
1].
Replacement of the aortic valve and of the aortic root is also frequently performed. Composite grafts (mechanical or biological), homografts and autografts are used for this purpose. The most common indication for such replacement is a valvular disease associated with dilatation of the proximal aorta. Two other classical indications are an acute aortic dissection with a diseased aortic valve and an acute bacterial endocarditis with annular abscess [
13,
14,
15]. In the latter condition, aortic homografts and bio-conduits present an increased resistance towards infection compared to mechanical compositegrafts, and accommodate themselves into a destructed (and frequently distorted) annulus better than a rigid prosthesis (
Figure 11). The relatively high systemic pressures (mainly the diastolic pressure) induce degeneration of a biological graft (homograft or xenograft) more rapidly than when inserted in the pulmonary position. Still, biological valve conduits are used more and more to replace the aortic root either in case of endocarditis or in simple valve replacement [
14,
16,
17]. The availability of these grafts is large, and their haemodynamic performances are excellent.
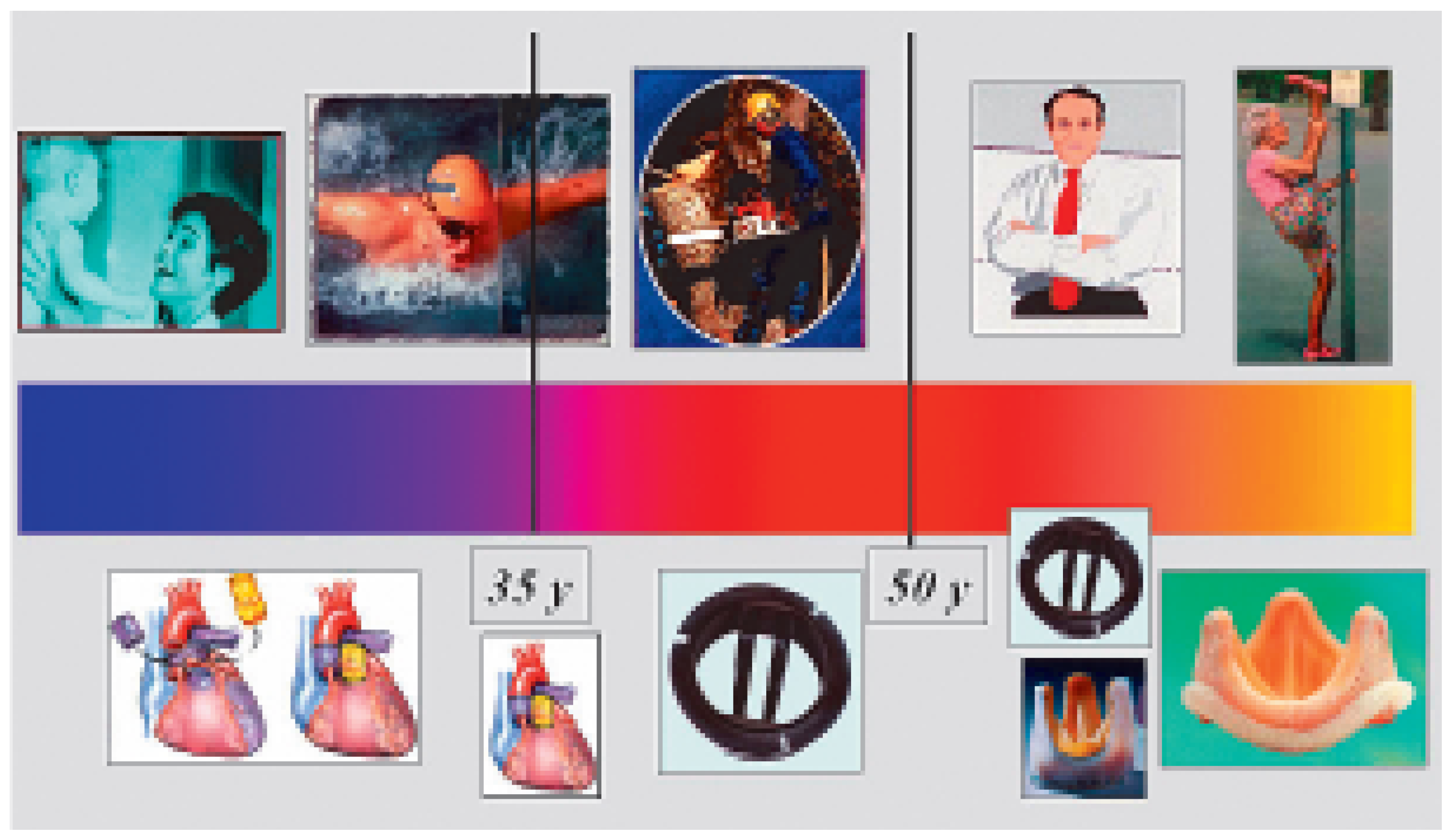
Figure 12 gives a brief overview of common possibilities in replacement of the aortic valve according to patient’s age and life style. Age is one of the most influent factors dictating the correct choice of prosthesis and/or procedure.
Figure 10.
The “Aortic valve preserving” operations: only the sinuses and part of the ascending aorta are removed (the coronary arteries are harvested and reimplanted in the Dacron graft). Left shows the “Yacoub procedure” where the Dacron graft is scalloped and inserted along the line of insertion of the valve cusps (the aortic annulus is not supported by the graft), right shows the “Tirone David procedure” where the entire aortic root is wrapped by a prosthesis. The commissures and remnant of the sinuses are entirely inserted within the graft. The aortic annulus is supported by the graft and cannot dilate.
Figure 10.
The “Aortic valve preserving” operations: only the sinuses and part of the ascending aorta are removed (the coronary arteries are harvested and reimplanted in the Dacron graft). Left shows the “Yacoub procedure” where the Dacron graft is scalloped and inserted along the line of insertion of the valve cusps (the aortic annulus is not supported by the graft), right shows the “Tirone David procedure” where the entire aortic root is wrapped by a prosthesis. The commissures and remnant of the sinuses are entirely inserted within the graft. The aortic annulus is supported by the graft and cannot dilate.
Figure 11.
Insertion of a bio-composite graft in an acute aortic valve endocarditis with annular destruction. These grafts accommodate the modified geometry of the annulus and present an increased resistance to acute bacterial infections.
Figure 11.
Insertion of a bio-composite graft in an acute aortic valve endocarditis with annular destruction. These grafts accommodate the modified geometry of the annulus and present an increased resistance to acute bacterial infections.
Figure 12.
Overview of possibilities of aortic valve replacement according to patient’s age and physical activity or lifestyle.
Figure 12.
Overview of possibilities of aortic valve replacement according to patient’s age and physical activity or lifestyle.
Usually, the pulmonary valve and the main pulmonary artery are replaced simultaneously. Homograft (either aortic or pulmonary) (
Figure 13) and xenograft conduits (biological valved conduits) are used for that purpose. Pulmonary homografts are preferred to aortic ones when the pulmonary resistance is normal because of their slower calcification rate and hence longer survival rate. Biological valved conduits are numerous, among which the bovine jugular vein has taken a prominent position over the past years [
18]. The vein of bovines has three cusps and resembles a semilunar valve (
Figure 14, lower left panel). These conduits should not be used in case of increased pulmonary resistance because of their tendency to dilate under increased pressure. Their size limitation (no conduit larger than 22 mm) restricts their application in the adult population.
The atrio-ventricular valves (mitral and tricuspid) are usually repaired. Still, in some cases of rheumatic stenosis, acute endocarditis, re-operation or rupture of a papillary muscle, a replacement of the valve is necessary. A mechanical valve is usually preferred to a biological one (which is in this case a stented bioprosthesis). Mechanical prostheses have a low profile (contrary to many stented biological ones) which does not impinge in the left ventricular outflow tract. Furthermore, the haemodynamic stress is bigger on the mitral valve (this valve sustains the systolic pressure) than on the aortic valve (which sustains the diastolic pressure) and leads to accelerated degeneration of a bio-prosthesis. Finally, reoperations on the mitral valve are more difficult to perform and more dangerous than on the aortic valve. These reasons explain the frequent choice for a mechanical prosthesis when mitral valve replacement is considered.
It is rarely necessary to replace the tricuspid valve except in severe bacterial endocarditis. The choice is usually a biological prosthesis, although mitral homografts are an excellent option, especially if an atrial fibrillation is concomitant or when subsequent infections are likely (
Figure 15). The opening area of a mitral homograft largely surpasses that of any available bio-prosthesis [
19]. The driving force to fully open a homograft is close to that required to open a normal tricuspid valve and is inferior to that required to open any classical biological prosthesis. This fact is advantageous in case of atrial fibrillation with loss of the atrial drive.