Summary
In an era of an increasing number of invasive procedures particular attention to radiation exposure and protection measures for patients and staff is warranted. Ionising radiation accounts for risk-dose-dependent stochastic effects (no threshold dose) and dose-dependent deterministic effects (threshold dose). The effective dose (ED) is a weighted sum of equivalent doses delivered to various organs to assess the stochastic risk, whereas deterministic effects are related to the entrance dose. Dosearea-product (DAP) is an indicator of the ED to the patient, which is approximately 5–20 mSv per coronary angiography (CA). The most important factors influencing DAP are fluoroscopy level, the use of cinegraphy, complexity of the procedure, and skill of the operator, which most of can be optimised to reduce radiation to the patient. The operator is not directly exposed to the X-beam, but to a considerable amount of scatter radiation. The annual ED of an interventional cardiologist consequently using a lead apron will hardly exceed the annual dose limit of 20 mSv. However, ED measurement with one or two dosimeters does not reflect the doses to susceptible unprotected parts of the body, namely the hands and the eyes, which may be affected by deterministic effects such as the development of cataract. The use of a lead glass screen placed between patient and operator markedly reduces the dose to the operator’s eyes but has almost no effect on the dose to the hands.
As shown by several recently published studies, there is a high potential to reduce DAP levels and thus to reduce radiation to the patient and to improve lead shielding with subsequently enhanced safety for staff. Unfortunately, these trials do not reflect the current practice in many catheterisation laboratories. Therefore, awareness of the problem and efforts to improve the current standard are required.
Key words: effective dose; dose-area-product; scatter radiation; lead protection
Introduction
Regarding the increasing number of patients undergoing invasive procedures such as coronary angiography (CA), percutaneous coronary intervention (PCI), occlusion of patent foramen ovale, and catheter ablation [
1], particular attention to radiation protection for patients and staff is required. The occurrence of radiation-induced skin lesions in patients [
2] as well as cataracts in operators [
3], and the debate about a higher incidence of brain cancer in interventional cardiologists [
4] has led to intense research in this field in the last years. The aim of the present review is to inform interventional cardiologists as well as referring physicians about the level of radiation exposure of patients and staff in the catheterisation laboratory and the current evidence about the importance and the usefulness of radiation protection measures.
Radiobiology and radiation protection principle
The susceptibility of different organs to radiation varies considerably. The bone marrow, gonads, intestine, thyroid gland, lung, stomach, bladder, and the skin are among the most vulnerable organs [
5]. Radiation-induced effects of concern in radiation protection fall into two general categories: deterministic and stochastic effects that are different in nature. A deterministic effect arises when enough cells in an organ or tissue are killed inducing a partial or complete loss of organ function (for example skin injuries due to disruption of the microcirculation) [
6,
7]. The severity of deterministic effects increases with the dose above a threshold dose. Deterministic effects appear at relatively high absorbed dose levels. Data on deterministic effects on human beings come from side effects of radiotherapy, from effects on early radiologists, from the effects of the atomic bombs at Hiroshima and Nagasaki and from severe radiation accidents. A modified somatic cell may still retain its reproductive capacity but may give raise to a clone of modified cells that may eventually result in cancer (for germ cells this may lead to modifications of hereditary information transmitted to future generations). These somatic and hereditary effects, which may start from a single modified cell, are called stochastic effects. At present, the main sources of information on stochastic effects are the epidemiological studies especially on the survivors of the nuclear weapon attack on Hiroshima and Nagasaki and on patients exposed to radiation for medical purposes. The uncertainty on stochastic effects remains relatively high and as a conservative measure, the probability of occurrence of a stochastic effect has been postulated as linear to dose without any threshold. One of the objectives of radiation protection is to prevent the occurrence of radiation-induced deterministic effects by adhering to dose limits that are below the established threshold. The other objective is to limit the risk of stochastic effects, to a reasonable level in relation to social needs, values, benefits gained and economic factors.
Stochastic risk is commonly based on the effective dose (ED) that relates the risk from a non-uniform exposure to the risk from an equivalent whole body exposure, whereas deterministic effects are closely related to the organ absorbed dose, eg entrance skin dose [
6,
7] in the case of fluoroscopy since it is the organ which receives the highest dose during the examination.
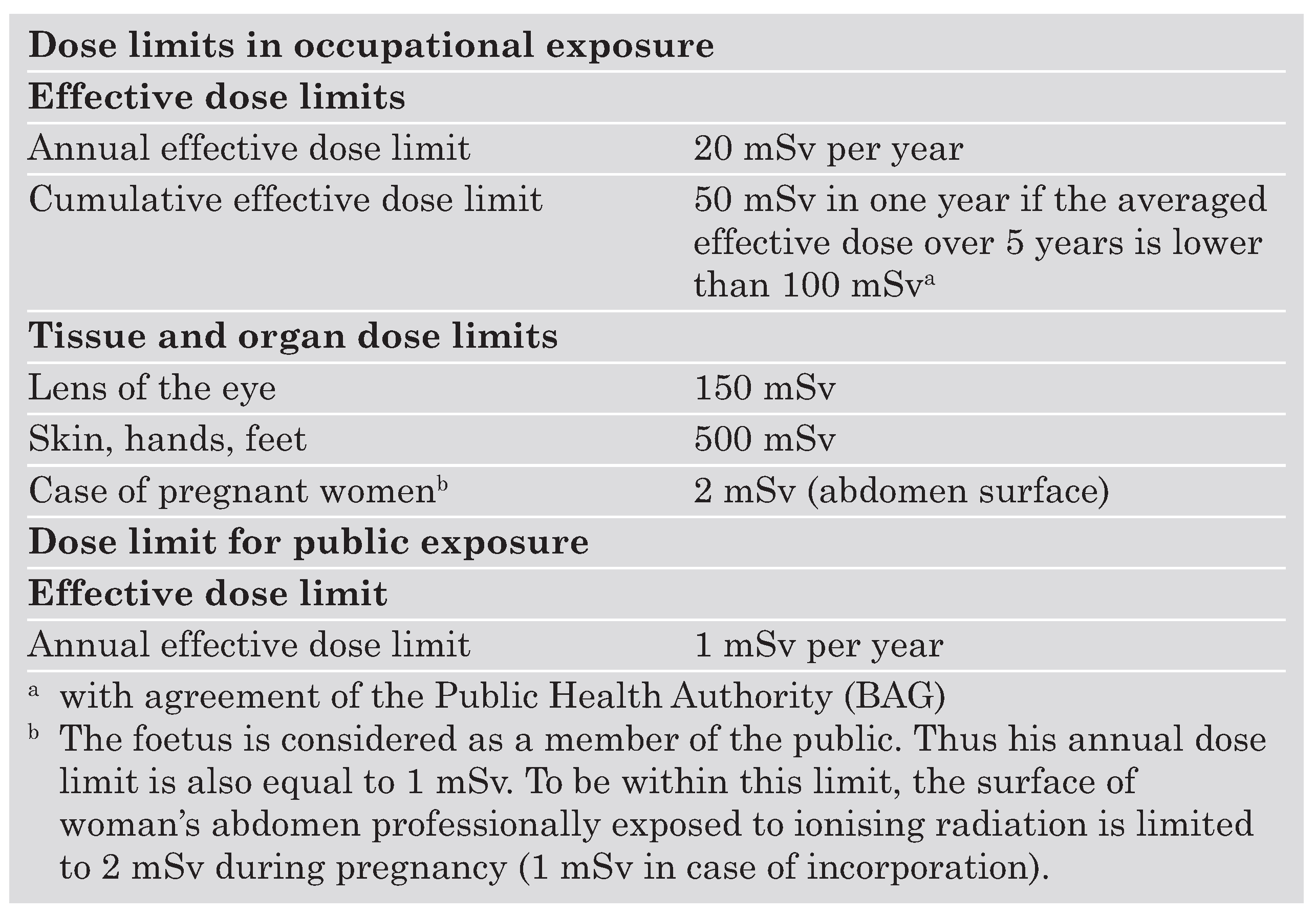
To control staff exposure dose limits have been introduced (
Table 1) [
7]. These limits do not apply for patients, however their radiation protection has to be assured by the justification and the optimisation of the procedure. Reference dose levels are also being introduced to verify that the dose delivered during the examination is compatible with the usual practice.
Definitions
The first quantity locally characterising the amount of radiation is called air kerma (K), an acronym for kinetic energy per mass unit. It quantifies the energy transferred to charged particles from non-directly ionising particles such as photons and is expressed in Gray (Gy): one Gy corresponding to the transfer of energy of one Joule per kg of air. The second quantity, which can be easily derived from air kerma is the absorbed dose, and refers to the energy deposited locally in an absorbing medium from ionising radiation. In the energy range of diagnostic radiology and for soft tissue, one can consider that air kerma and absorbed dose are numerically equivalent, even if their concepts are different. Organ dose consists of the total energy deposited in the organ per unit mass of that organ. The entrance skin dose is appropriate for characterisation of deterministic risks such as skin lesions. To assess the amount of radiations operational quantities have been introduced to take into account the geometry of the exposure. In the field of fluoroscopy and radiography, the dose-area product (DAP) or kerma-area product have been introduced. The DAP is the product of the cross section of an X-ray beam and the air kerma averaged over that cross section, and represents the amount of radiation that the patient receives. Dose-area-product can be measured at any point between the housing of the X-ray tube and the patient, since it is focal spotpatient distance independent. Measurements are routinely done with transmission ionisation chambers or can be computed by the fluoroscopy unit. The unit of this quantity is the Gray × square centimetre (Gycm2).
Not all types of radiation cause the same biological damage per unit of absorbed dose. In order to reflect the relative effectiveness of the type of radiation in producing biological damage, a radiation weighting factor wR was introduced. The product of the absorbed dose by the radiation weighting factor wR is the equivalent dose (H). The unit for equivalent dose is the Sievert (Sv). For radiations principally used in the field of medicine (ie X-rays, gamma rays, and beta particles) the radiation factor wR is equal to one; thus 1 mGy = 1 mSv. Finally, one has to take into account that not all tissues are equally sensitive to ionising radiation. Tissue weighting factors, wT, were also established by the International Commission on Radiological Protection (ICRP) to assign for a particular organ or tissues (T) the proportion of the detriment from stochastic effects (eg induction of cancer and genetic effects) related to that tissue compared to uniformed whole-body irradiation. The sum of the products of the equivalent dose to each organ or tissue irradiated (HT) and the corresponding weighting factor (wT) for that organ or tissue is called the effective dose (ED). The ED is also expressed in Sv [
5,
8,
9,
10].
Radiation to the patient
As outlined above, the ED is an important indicator of the global risk of stochastic effects of an exposed person, but it is difficult to assess in a real patient, since calculation is based on different organ doses and tissue weighting factors. However, DAP measurement allows to get an estimate of the ED delivered to the patient during a procedure. Based on experiments with anthropomorphic phantoms DAPto-ED conversion factors in the thoracic region can be approximated to 0.20 mSv Gy–1cm–2 (0.183 mSv Gy–1cm–2 according to reference [
12] or 0.185 mSv Gy–1cm–2 according to reference [
13] or 0.220 mSv Gy–1cm–2 according to references [
14,
15]). A patient’s average ED sustained during CA is approximately 5–20 mSv. To better recognise the dimension of this radiation dose, one should consider that in Switzerland radiation from medical examinations accounts for one quarter (1 mSv) of the total average annual background radiation of 4 mSv [
16]. A national survey in 1998 revealed that angiographies made up only 1% of almost one million radiological examinations, but accounted for 11% of the total dose applied. A chest X-ray (pa/ds) is associated with an ED to the patient of about 0.1 mSv, whereas corresponding levels for CA (5–20 mSv) are equivalent to 50 to 200 chest X-rays. Thus, a patient undergoing CA may acquire an ED, which is 1 to 5 times higher than the average background radiation in Switzerland [
6,
10,
17].
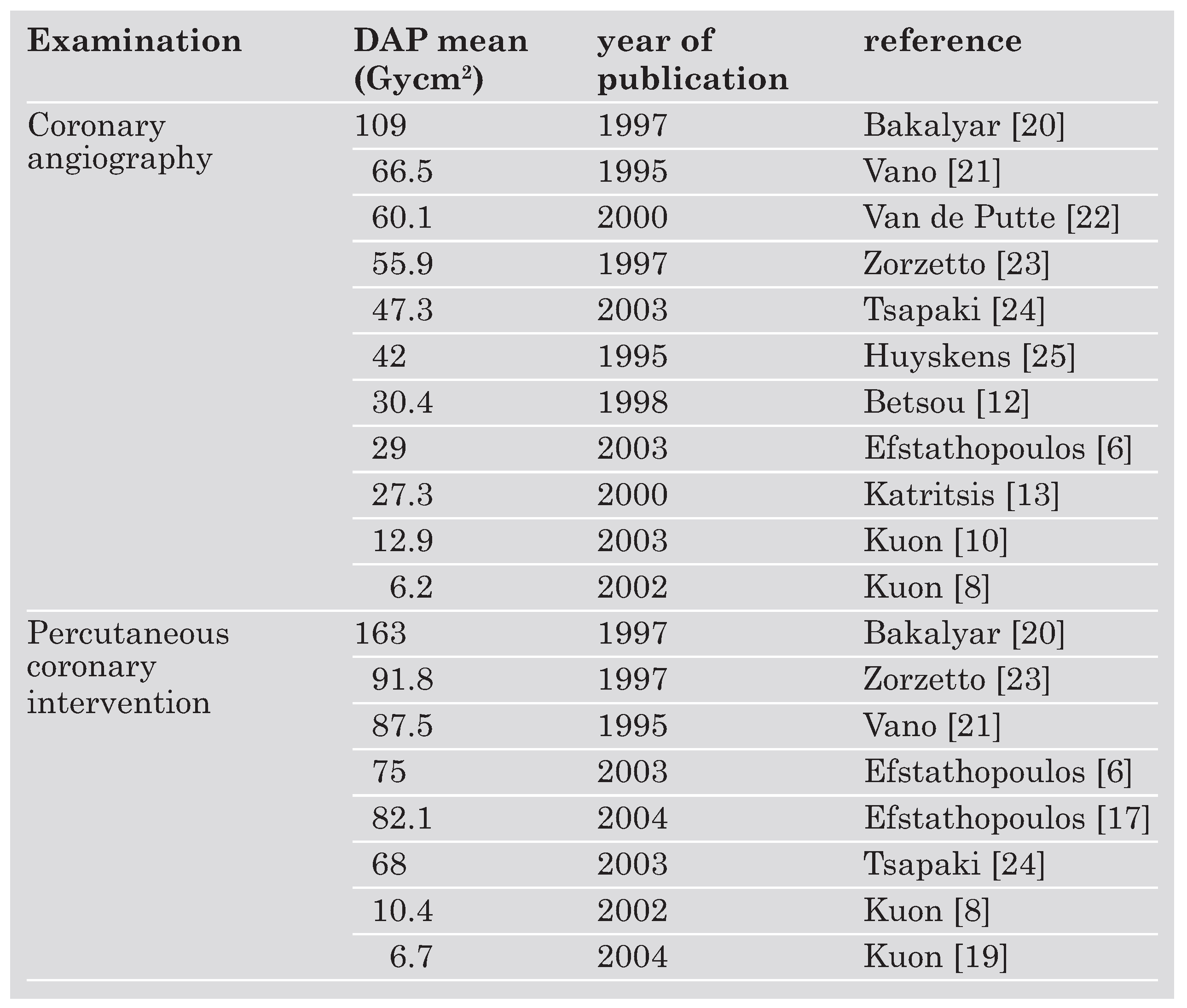
As DAP is easy to assess, most studies provide DAP levels and reductions in DAP as a surrogate for the ED to the patient and its reduction respectively. Dose-area-product depends on time and mode (higher DAP levels for higher pulse levels) of fluoroscopy and thus on duration [
11] and complexity [
18] of the procedure. Especially the number of cinegraphic frames substantially contributes to DAP. Accordingly, for PCI or CA with ad hoc PCI higher DAP levels are recorded than for CA alone [
12]. Interventions of the right coronary artery were found to require higher DAP levels than for the left coronary artery [
19], and reopening of a totally occluded coronary artery [
10], procedures including ventriculography [
11] or bypass angiography [
11,
19,
20] are associated with especially high DAP levels. However, the literature provides considerably varying DAP values for the same procedure (
Table 2) [
6,
8,
10,
12,
13,
17,
19,
20,
21,
22,
23,
24,
25] implicating that beyond the procedure per se, operator and patient related factors have substantial impact on DAP levels. Less experienced operators, especially fellows during their first year of training need higher DAP levels per procedure than more experienced cardiologists [
26], since more experienced cardiologist can significantly reduce the duration of fluoroscopy and the number of cine frames used [
24]. Furthermore, different tube angulations also have considerable impact on radiation to the patient (highest for extreme oblique angulations). This issue is discussed in more detail in the “Radiation protection of staff members” section. In addition, a weak correlation between DAP and the patient’s body mass index has been found [
10].
Beyond the ED, doses to sensitive organs of the patients are of interest, eg those to the coronary arteries. One study addressed the question to which doses the patient’s coronary arteries are exposed during CA. By using minidosimeters attached to the catheter tip Katritsis and co-workers were able to demonstrate that the dose at the coronary arteries correlated significantly with DAP [
13,
17]. However, the doses to the coronary arteries are far below those augmenting proliferative activity [
27] and are thus very unlikely to influence restenosis rate following stent implantation [
6,
13].
Reduction of radiation exposure to the patient
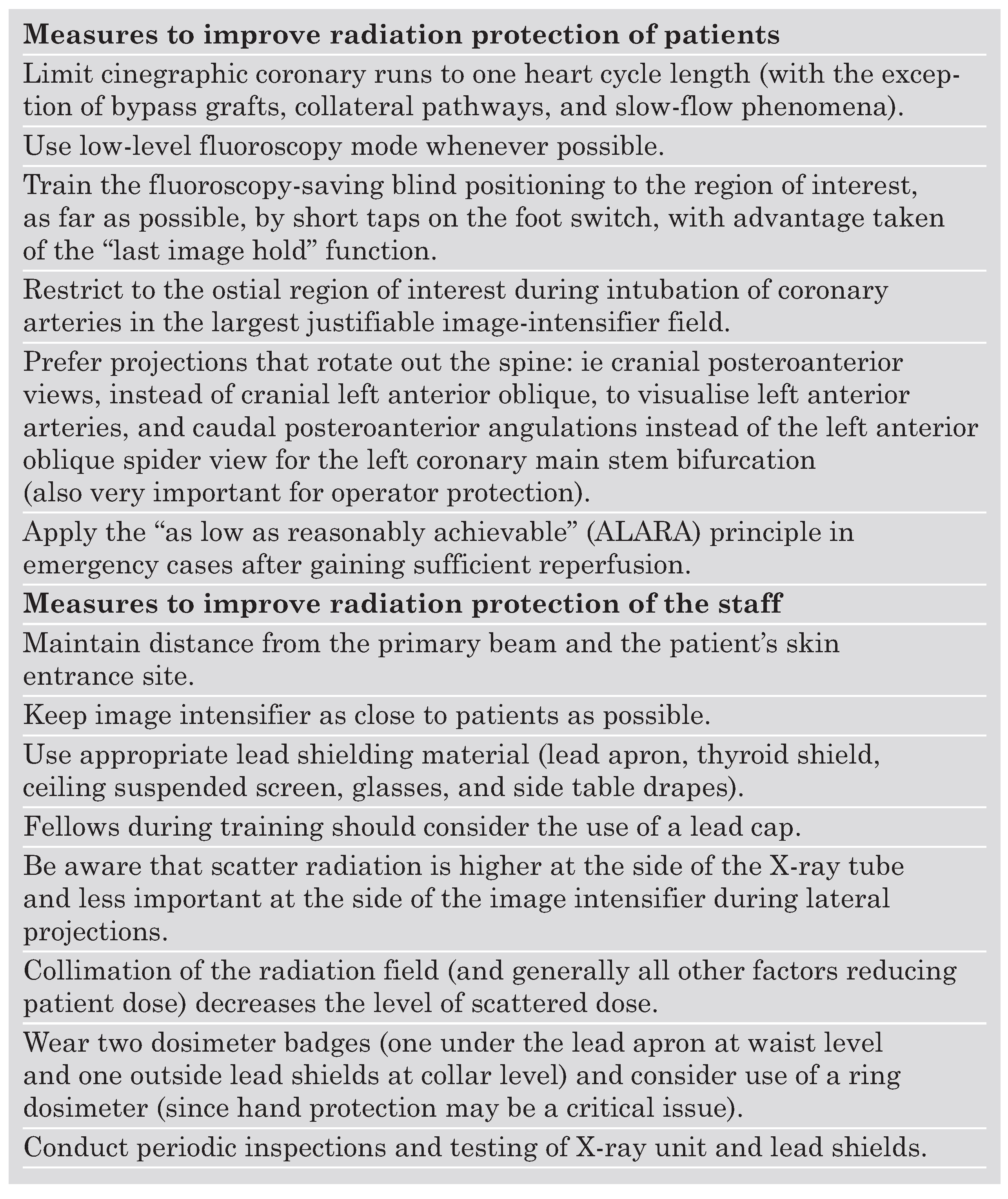
Following the principle of ALARA (as low as reasonably achievable) [
7] every effort should be made to reduce the DAP of a procedure and thus the ED to the patient undergoing CA or PCI. This goal can be primarily achieved by intensive training of the operator, which is underscored by the considerable variance of DAP levels between different centres (
Table 2), but also among operators in the same centre [
28]. The use of slower pulse frequencies (12.5 rather than 50 frames/s) and efforts to minimise fluoroscopy time as well as to choose fluoroscopy-saving positions can account for major reductions in DAP [
8,
10]. Kuon and coworkers were able to demonstrate a reduction of the previously rather low DAP level per CA from 37.1 to 12.9 Gycm2 within 12 months by (1.) limitation of cinegraphic runs, (2.) systematic use of low-level fluoroscopy, (3.) blind positioning of the region of interest, and (4.) consequent avoidance of oblique positions [
10]. A second analysis including 642 PCIs performed by the same operator over a period of three years revealed mean DAP values per procedure ranging from 6.7 to 19.4 Gycm2 depending on the number of treated vessels [
19]. These DAP levels are comparable to those required for multislice spiral computed tomography of the heart [
19].
Not only inter-operator but also significant intra-operator differences exist. Analysis of DAP levels for CA and PCI assessed at different times during the working day of one single high-volume operator revealed higher DAP levels when PCI was done in the afternoon compared to procedures performed in the morning, whereas no difference was found in only diagnostic angiographies. This difference was supposed to be due to the operator’s fatigue developing during a busy day with subsequently prolonged interventions and impact on the mode of fluoroscopy use. Thus, the authors suggest that PCI should be performed during the first 6 hours of an interventional cardiologist’s working day [
29]. Since coronary anatomy is not known before the procedure in most cases, and therefore PCI will be performed ad hoc if indicated, it will be hardly possible to schedule PCI for a given time of the day however. From the viewpoint of radiation protection of the patient the femoral rather than the radial approach is preferable, and direct stenting rather than stenting after pre-dilatation is advisable during ad hoc one-vessel PCI [
11].
Radiation exposure to the operator
The operator is not directly exposed to the Xray beam, but to a considerable amount of scatter radiation reflected from the patient (primary) and to a lesser extent from the walls of the laboratory (secondary). The scatter radiation levels are highest at the X-ray tube side where the beam enters the patient. According to the inverse square law the operator’s distance from the patient’s skin entrance site is crucial. This law states that the level of scatter radiation is inversely proportional to the distance squared. Therefore, the operator’s position and body height have major impact on the amount of scatter radiation to different parts of the operator’s body. A right-handed operator performing the procedure via the right femoral artery has his left body side turned towards the patient. Therefore the left side of the body, especially the hands, which are at the level where the X-ray beam enters the patient, are exposed to the highest level of scatter radiation. The left hand was reported to receive the two-fold dose as compared with the right hand during cardiac catheterisation [
30]. The left eye is also exposed to higher doses than the right eye [
30]. Not surprisingly, a tall operator receives a lower eye dose than a small operator since the distances from the eyes to patient entrance site can considerably vary depending upon the operator’s body height.
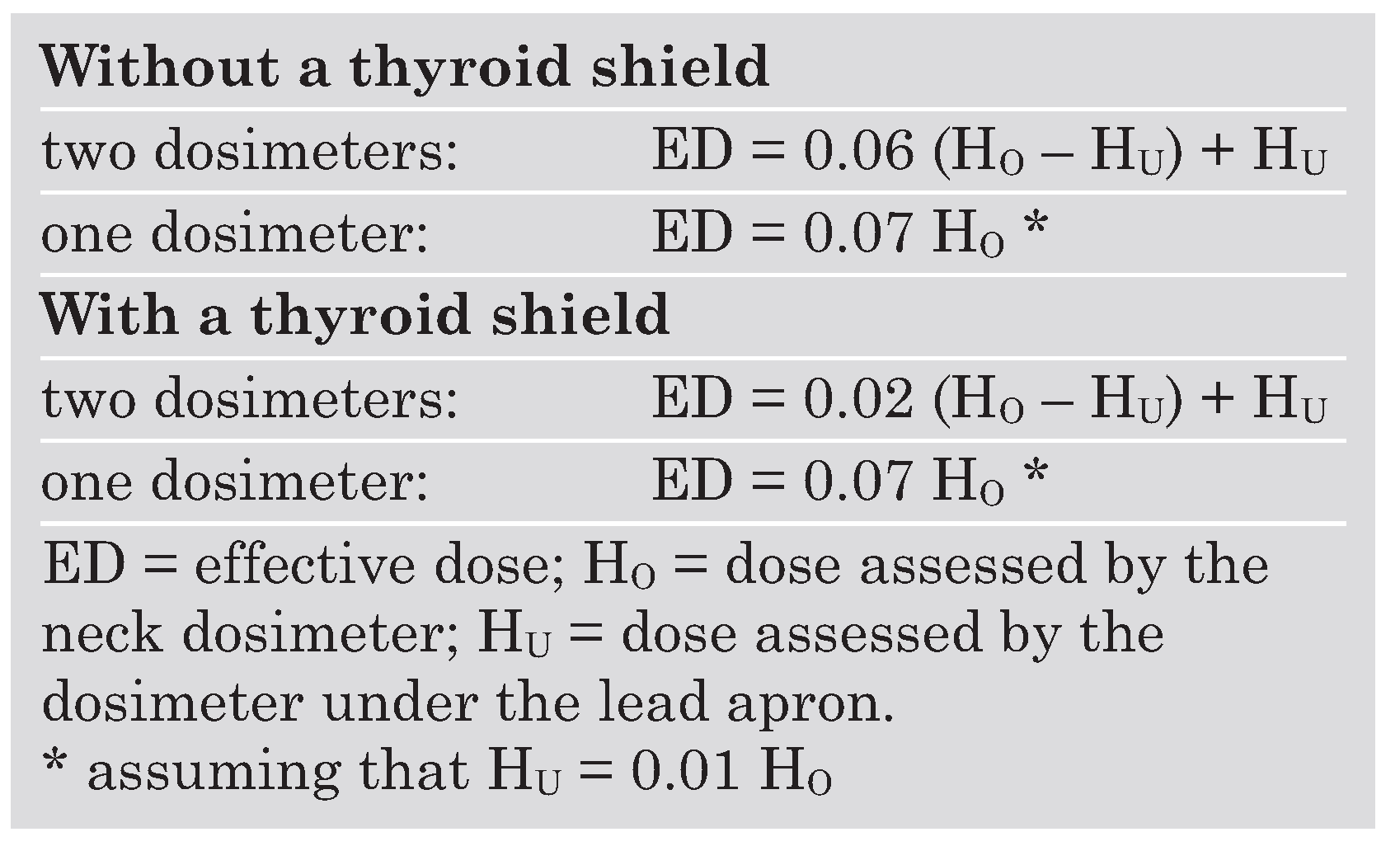
In most catheterisation laboratories the daily use of a dosimeter is mandatory. If an operator is wearing either (1.) one dosimeter over the lead apron at the neck level or (2.) two dosimeters, one under the lead apron at the waist level, and one over the apron at the neck level, the ED can be roughly estimated according to the equations given in
Table 3 [
31,
32]. Interventional cardiologists were reported to receive an annual average ED of 46.2 mSv without protection devices [
33], which is reduced to 1.7 mSv by basic protection such a lead apron and a thyroid shield. For investigational purposes, the ED per procedure is more informative. The literature provides EDs to the cardiologist per procedure ranging from 0.2 to 18.8 μSv [
32]. Even if we assume a rather high caseload of 1000 angiographies per year, the annual threshold level of 20 mSv will be hardly exceeded. Moreover, a recent study from a state-of-the-art Greek centre reported estimated EDs to the operator of only 0.04–0.05 mSv/year [
6]. Unfortunately, these ED data do not reflect the reality in many catheterisation laboratories. Many operators do not only inconsequently use protection devices, but also do not regularly wear the dosimeter to avoid problems with authorities if high radiation levels are recorded [
30].
In addition, the concept of the two-dosimeter-based ED calculation is useful to estimate an operator’s stochastic risk but does not take in account the radiation effects to unprotected parts of the body such as the extremities and the head. These doses escape when relying on dosimeter readings and ED calculations as outlined above unless special dosimeters are employed (eg ring dosimeters or dosimeters attached to eye glasses). The dose to eye lens does not contribute to the stochastic risk, but is critically important for development of cataract. The fact that the legal (in Switzerland and EU) annual limit of 500 mSv for the hands can be exceeded by less than one minute of exposure to unattenuated fluoroscopy beam per month [
34], and reports on eye lens doses up to 900 mSv per year over several years and consecutive lens injuries in non-optimised interventional radiology laboratories [
3] as well as the debate about a higher incidence of brain cancer in interventional cardiologists [
4] highlight the importance of both better entrance dose assessment and protection of head and hands.
Radiation protection of staff members
Staff radiation protection can not be handled independently from patient protection, since they correlate in many ways. As a general rule, the operator lowers his own level of radiation exposure, if he can reduce radiation exposure of the patient. As the operator is primarily affected by scatter radiation, a reduction of the ED to the patient will result in lower radiation exposure of the operator since scatter radiation decreases. Therefore, any measures to reduce DAP will add to a lower radiation exposure not only to the patient, but also to the operator [
35].
Beyond DAP reduction (as described in the “Reduction of radiation exposure to the patient” section) lead shielding is critically important for the operator to protect himself. Recent studies assessing the entrance doses to different parts of the operator’s body by several dosimeters have provided useful information about the effectiveness of protection measures. To better compare staff entrance doses per procedure under different conditions, a quotient of the entrance dose divided by DAP is frequently used to incorporate possible differences in the duration and complexity of the procedure. This DAP-standardised local dose provides information about the effectiveness of protection measures apart from DAP reduction [
32].
Published data on mean entrance doses to the operator’s unprotected eyes and hands range between 120 and 400 μSv, and 240 to 510 μSv per coronary intervention, respectively [
36]. An operator with a comparatively high caseload of 1000 procedures per year may reach the recommended occupational limits of 150 mSv for the lens of the eye and 500 mSv for the hands. The effect of eye protection devices is clearly documented in the literature. The use of 1.0 to 1.5 mm-lead-equivalent overcouch shields was reported to lower the dose to the eyes to 38% [
8], whereas in more recent studies the DAP-standardised eye lens dose has been shown to be reduced by a factor of about 20 by a lead glass screen [
9,
37,
38].
In a pivotal study, Kuon and co-workers [
8] combined maximal efforts to reduce DAP with optimised lead shielding. By using an articulated ceiling screen (60 × 75 cm) lengthened by a flap beside the table (21 × 80 cm), longitudinal protection adjacent to the table (60 × 80 cm) enhanced by a top shield (60 × 20 cm), a foot-switch shield, and a cover (60 × 80 cm) wrapped around the patient’s thighs (50 × 80 cm, each shield 1.0 mm lead equivalent) and by wearing glasses, apron, and a collar of 0.5 mm lead equivalence the operator was exposed to only 0.8% of typical radiation levels in advanced cardiac catheterisation laboratories.
The use of a 0.5 mm lead equivalent cap reduces the radiation dose to the operator’s head (by a factor 30) even more efficiently compared to a 1.0 mm lead equivalent transparent lead glass screen [
9]. The superiority of the cap despite a smaller lead equivalent was attributed to secondary scatter reflected from the laboratory wall [
9]. High-volume cardiologists and fellows during training may enhance their safety by wearing a lead cap instead of using only a lead screen. The only disadvantage of the lead cap is its weight (1140 g [
9]), which might be associated with discomfort when wearing it. Since the lead screen has almost no impact on the dose to the hands [
38], the use of gloves is theoretically advisable. However, gloves may significantly impair the operator’s skill and thus the quality and the speed of the examination thereby preventing most cardiologists from their use. Unfortunately, there is no optimal protection tool for the hands, and therefore awareness of the operator is required to avoid any unnecessary fluoroscopy to the hands. As a rule, if an operator’s hands are visible on the monitor, then practices should be altered [
39].
Furthermore, the angulation of the X-ray tube influences the amount of scatter radiation to the operator. Radiation levels have been found to be highest for the left anterior oblique (LAO) position, whereas in posterior-anterior (PA) and right anterior oblique (RAO) angulations much lower levels have been detected [
8,
9,
36]. In a recently published paper, Kuon and co-workers have shown that the standard view for the left coronary main stem (LAO 60°/20°–) is associated with a 7.6-fold increase in dose to the operator (and 2.6-fold for the patient) as compared to an alternative less frequently used angulation (caudal PA 0°/30°–). Similarly, the typical angulation for visualisation of the bifurcation into the left anterior descending artery and the diagonal branch (LAO 60°/20°+) produces a five-fold increase in scatter dose level to the operator (and 2.5-fold for the patient) as compared to the cranial PA 0°/30° view. The authors therefore emphasise that LAO views 060° with cranial or caudal angulation 020° should be avoided, and they present a list of suggestions how to replace typical tube angulations by alternative angulations without significant loss of information: for almost any target structure an appropriate RAO or PA angulation can be used instead of the standard LAO angulation [
36].
Data about radiation exposure to assisting operators, nurses and technicians involved in invasive cardiological procedures are rare. The assisting operator was found to acquire almost the same ED as the primary operator [
6]. Care should be taken that assistants are placed at the operator’s right side or at the foot of the table and not at his left side to avoid unnecessary radiation exposure. In a recently published small study (20 CA, 20 CA + PCI) dosimeter readings for the radiographer and the nurse were below the detectable range, and therefore the authors concluded that minimal radiation hazard could be assumed for them [
6]. However, DAP levels were comparatively low, and more important dosimeter readings for the operators were extremely low (estimated annual effective dose 0.04–0.05 mSv). The results of his study do certainly not correspond to the current practice, and should not lead to the dangerous assumption that protection measures are not required for assisting operators, nurses and technicians. In another study, the maximal annual ED for a nurse was estimated at 0.14 mSv [
23].
Cancer risk
Efstathopoulos and co-workers [
6] calculated the total risk for developing fatal cancer by multiplying the ED by factors proposed by the ICRP [
5] and found a risk of 28 × 10–5 for patients undergoing CA compared to 78 × 10–5 for those undergoing PCI. In a second study they calculated a risk of 83 × 10–5 for patients subjected to PCI, which implicates that for every 100 000 patients undergoing PCI, 83 patients should develop a fatal cancer within the next 40 years [
16].
A study published in 1997 addressed the question whether the operator is exposed to an increased cancer risk. Assuming a lifetime ED of 68 mSv for an operator wearing a lead apron and a thyroid shield performing 1500 procedures per year, a lifetime cancer risk of 0.3% was calculated [
33]. Data from a more recent study in CA and PCI suggest much lower annual EDs of 0.04–0.05 mSv leading to a calculated lifetime probability of fatal cancer between 1.6 and 2.0 × 10–6 for the primary operator per year [
6]. Again, one has to be aware that most studies were rather small, and that most of the recorded ED levels were very low and might not reflect the “real world”.