Summary
Interventional management of aortic dissection and other pathologies of the descending aorta by use of stent-grafts evolved slowly in anticipation of the risk of paraplegia from spinal artery occlusion, a complication known to occur in up to 18% after surgery. With ongoing technical improvement a large series of type B dissection cases has now been successfully treated by endovascular stent-graft placement using the concept of sealing the most proximal entry tears to the dissecting process without neurological sequelae. Recent studies have demonstrated that closure of the entry tear is essential to depressurise the false lumen, reconstruct the aortic wall and reduce total aortic diameter. Entry tear closure promotes both thrombus formation in the false lumen and remodeling of the entire aorta. Various observational studies and registries have shown that the use of a customised stent-graft is an effective method to exclude an enlarging and aneurysmal dilated false lumen by sealing of the proximal entry tear. The absence of a distal reentry tear is desirable but not a prerequisite. Besides dissection, however, focal true (or false) aneurysm of the descending thoracic aorta represents another interesting target for endovascular repair instead of open surgical repair.
Similarly, penetrating aortic ulcers, often originating from a localised intramural haematoma of the aorta, is evolving as a new attractive indication for stent-grafting, both in emergency and elective scenarios.
Moreover, partial or complete rupture occuring as a result of deceleration trauma appears to be amenable to either emergent or delayed endovascular management. Stabilisation of the disrupted aorta with stent-graft has proven beneficial, with reconstruction of the inner lining by virtue of the endoprothesis to prevent enlargement, aneurysm formation and eventual rupture.
Background
Conventional treatment of
Stanford type A (De Bakey type I, II) dissection consists of surgical reconstruction of the ascending aorta with complete or partial resection of the dissected aortic segment; thus in type A dissections interventional endovascular strategies have no clinical application except to relieve critical malperfusion prior to surgery by distal fenestration in cases of thoracoabdominal extension (De Bakey type I) and ischaemic complications. Various scenarios of malperfusion syndrome are amenable to endovascular management. These include static or dynamic (by intima invagination) collapse of the aortic true lumen, static or dynamic occlusion of one or more vital side branches, or enlarging false aneurysm due to patent proximal entry tear. Stent-graft placement aims at remodeling of the thoracic descending aorta by sealing one (or multiple) proximal entry tears with a Dacron-covered stent, thus initiating thrombosis of the false lumen [
1,
2,
3,
4]. In addition reconstruction of a collapsed true lumen might result in reestablishment of sidebranch flow (
Figure 1).
Although peripheral pulse deficits can be acutely reversed with surgical repair of the dissected thoracic aorta in approximately 90%, patients with mesenteric or renal ischaemia do not fare well. Mortality of patients with renal ischaemia is 50 to 70% and as high as 87% with mesenteric ischaemia [
5,
6,
7]. The surgical mortality rates in patients with peripheral vascular ischaemic complications are similar to those with mesenteric ischaemia, reaching an 89% in-hospital mortality rate [
8,
9,
10,
11]. Operative mortality of surgical fenestration varies form 21 to 61%, which encouraged percutaneous interventional management by endovascular balloon fenestration of a dissecting aortic membrane to treat mesenteric ischaemia, a concept discussed as a niche indication in such complicated cases of malperfusion [
10,
11,
12].
The interventional management of
Stanford type B (De Bakey type III) dissection and the use of stent-grafts evolved slowly in anticipation of the risk of paraplegia from spinal artery occlusion as seen in up to 18% after open surgery [
11,
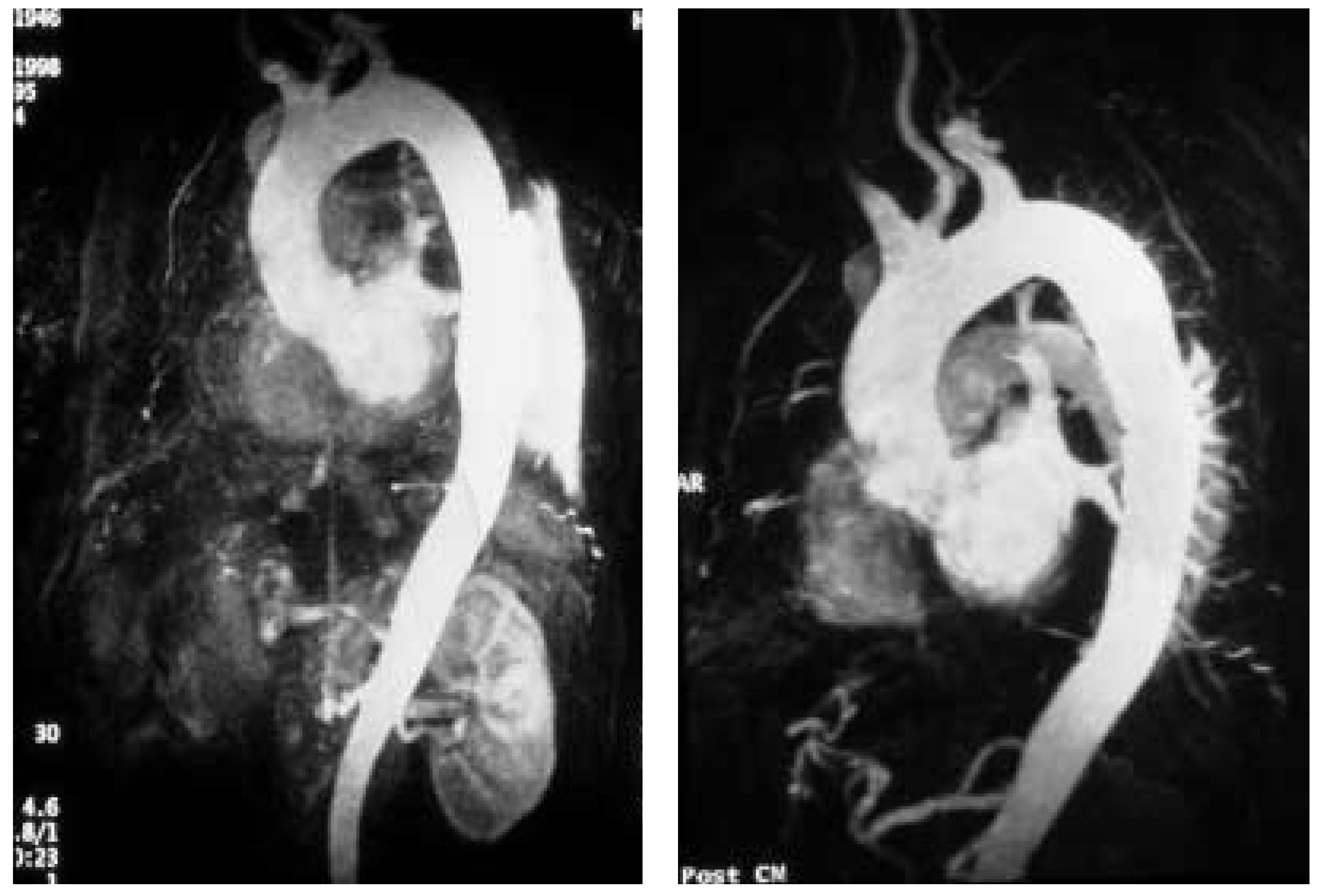
12]. However, with further technical improvement a large series of cases has now been successfully treated by endovascular stent-graft placement covering entry tears in the descending aorta and even in the aortic arch. Recent studies have demonstrated that closure of proximal entry tears is essential to reconstruct the aortic wall and reduce total aortic diameter. Entry tear closure promotes depressurisation of false lumen, thrombus formation in the false lumen (
Figure 2) and remodeling of the entire aorta [
2,
3,
12]. In the near future combined surgical and interventional procedures even for proximal dissection are on the horizon [
13,
14,
15].
Indications for fenestration and stent-graft placement
The exact role of percutaneous fenestration and stent-grafting in the treatment of
aortic dissection continues to evolve. There appears to be a role for stent-graft placement in the treatment of static or dynamic obstruction of aortic branch arteries, because static obstruction of a branch can be overcome by placing endovascular stents in the ostium of the compromised side branch, and dynamic obstruction may benefit from stents in the aortic true lumen with or without additional balloon fenestration or sidebranch stenting. In classic aortic dissection, successful fenestration leaves true lumen pressure unchanged [
16]. Sometimes bare stents must be deployed from the true lumen into side branches in order to buttress the flap in a stable position [
17]. In chronic dissection where fenestration of a fibrosed dissecting membrane may result in collapse of the connection between true and false lumen, a stent may be necessary to keep the fenestration open. A rare use of fenestration is to create a reentry tear for the dead-end false lumen back into the true lumen with the aim to prevent thrombosis of the false lumen and compromise of branches fed exclusively from the false lumen or jointly from the false and true lumen, a concept, however, that lacks clinical proof of benefit. Conversely, fenestration may increase the long-term risk of aortic rupture because a large reentry tear promotes flow in the false lumen and provides the basis for aneurysmal expansion of the false lumen. There is also a risk of peripheral embolism from a patent but partly thrombosed false lumen [
17,
18].
The most effective method to exclude an enlarging and aneurysmal dilated false lumen is the sealing of proximal entry tears with a customised stent-graft; the absence of a distal reentry tear is desirable for optimal results but not a prerequisite. Adjunctive treatment by fenestration and/or ostial bare stents may help establish flow to compromised aortic branches. Compression of the true aortic lumen cranial to the main abdominal branches with distal malperfusion (so called pseudo-coarctation) may also be corrected by stent-grafts that enlarge the compressed true lumen and improve distal blood flow [
2,
3,
10,
12]. Depressurisation and shrinking of the false lumen is the most beneficial result to be gained, ideally followed by complete thrombosis of the false lumen and remodeling of the entire dissected aorta, and in rare occasions even in retrograde type A dissection [
14]. Similar to previously accepted indications for surgical intervention in type B dissection, scenarios such as intractable pain with descending dissection, rapidly expanding false lumen diameter, extraaortic blood collection as a sign of imminent rupture or distal malperfusion syndrome are accepted indications for emergent stent-graft placement [
15,
17,
18,
19]. Moreover, late onset of complications such as malperfusion of vital aortic side branches may justify endovascular stent-grafting of an occlusive lamella (or fenestration) to improve distal true lumen flow as a first option. Only after an unsuccessful attempt surgery may still be employed considering that surgical repair failed to prove superiority over interventional treatment even in uncomplicated cases; in complicated cases the concept of endoluminal treatment is currently replacing open surgery in advanced experienced centers [
1,
2,
3,
17,
18,
19,
20]. A summary of treatment options is listed in
Table 1.
Besides dissection, focal
true (or false) aneurysm of the descending thoracic aorta represents another interesting target for endovascular repair instead of open surgical repair. A number of studies have not only documented feasibility and safety, but also favorable shortand midterm outcomes especially in multimorbid patients subjected to elective stentgraft treatment with circumscript thoracic aneurysm that are considered surgical candidates based on pathoanatomical features [
21,
22,
23,
24]. With the most recent technical refinements of endovascular devices previous shortcomings of homemade stent-grafts are likely to be overcome [
25]. Treatment of descending thoracic aneurysms—if anatomically suitable—by endovascular stent-grafting is the primary management consideration today [
26].
Penetrating aortic ulcers, often originating from a localised intramural haematoma of the aorta, currently evolve as a new attractive and benevolent indication for stent-grafting, both in emergency and elective scenarios. Few reports have documented successful sealing of ulcers by use of commercial stent-grafts in an endovascular approach. While aortic ulcers may only occasionally progress rapidly to generate a medical emergency, traumatic aortic rupture or partial or even complete transection of the descending thoracic aorta is always a truely emergent life-threatening condition.
Partial or complete rupture almost always occurs as a result of deceleration trauma usually as a result of a car accident or fall from height. The aorta ruptures at the fixation points and may just be contained by the adventitia. Emergent or better delayed (but timely) endovascular management by stabilisation of the disrupted aorta with a suitable stent-graft is considered beneficial, with reconstruction of the thoracic aorta (
Figure 3) by virtue of a new inner lining (by the endoprothesis) that prevented enlargement, aneurysm formation and eventual rupture, and enabled self healing of the transected aortic tissues [
27,
28,
29].
The technical goal in percutaneous balloon fenestration is to create a new tear in the dissection flap between the true and false lumen. Fenestration is performed from the smaller (usually the true lumen) into the larger or false lumen. Most commonly a Roesch-Uchida, Brockenborough or Colopinto needle is used for fenestration close to the compromised side branches. After the needle and a stiff wire are advanced from the true into the false lumen at the desired location, a balloon catheter of 12–15 mm diameter and 20–40 mm length is advanced and pulled back after inflation to create a transverse tear. If intravascular ultrasound (IVUS) is not available, a pulmonary balloon catheter can be used as a targeting object for piercing the dissecting membrane with the needle [
16].
Aortic stent-grafts are primarily used to correct compression of the supplying true lumen cranial to major aortic branches and to increase distal flow. Moreover, proximal communications should be sealed to depressurise the false lumen, direct flow to the true lumen and induce thrombosis in the false lumen with fibrotic transformation and subsequent remodeling of the aortic wall. Stent-graft placement across the origin of the celiac, superior mesenteric and renal arteries is strongly discouraged for empiric reasons.
Based on the measurements obtained during angiography, TEE, CT, MRI, or IVUS, customised stent-grafts should be used in covering up to 16 cm of dissected aorta and the major tear. The procedure is best performed in the catheterisation laboratory using digital angiography and under general anesthesia. The femoral artery is the most popular access-site and can usually accommodate a 24 F stentgraft system. Using the Seldinger technique a 260 cm stiff wire is placed over a pigtail catheter navigated with a soft wire in the true lumen under both fluoroscopic and transoesophageal ultrasound guidance. In complex cases with multiple reentries in the abdominal aorta the “embracement-technique” with the use of two pig-tail catheters is useful. Apig-tail catheter which has been installed in the true aortic lumen via the left brachial artery, picks up the femoral pig-tail catheter in the true lumen of the abdominal aorta and pulls it up into the aortic arch (
Figure 4). This procedure ensures definite positioning of the stiff guide wire in the true lumen, which is essential for correct deployment of the stent-graft. Carefully advanced over the stiff wire the launching of the stent-graft is performed with blood pressure briefly lowered to 50–60 mm Hg by infusing sodium nitroprusside to prevent dislodgement. After deployment short inflation of a latex balloon may be used to improve apposition of the stent struts to the aortic wall, but only if proximal sealing of thoracic communications is incomplete. Both Doppler-ultrasound and contrast fluoroscopy are instrumental for documenting the immediate result or initiating adjunctive maneuvers. For thoracic aortic aneurysm or ulcers the navigation of wires and instruments is markedly easier, but meticulous imaging with ultrasound and fluoroscopy is equally important.
A frequent anatomical consideration is the close vicinity between the origin of the left subclavian artery (LSA) and the degenerative aneurysm or the primary tear (in type B dissections). For this reason complete coverage of the ostium to the LSA has to be accepted at times to utilise endovascular devices in aortic pathologies adjacent to the LSA. According to observational evidence prophylactic surgical maneuvers are not imperatively required for safety reasons, but may be relegated to an elective measure after an endovascular aortic intervention when intolerable signs or symptoms of ischaemia occur [
30]. However, prior to intentional LSA occlusion attention has to be paid to potential supra-aortic variants and pathologies during pre-interventional imaging and vascular staging.
With both fenestration maneuvers and bare stents in sidebranches compromised flow can be restored in more than 90% (range 92 to 100%) of vessels obstructed from aortic dissection. The average 30 day mortality rate is 10% (range 0 to 25%) and additional surgical revascularisation is rarely needed [
31]. Most patients remain asymptomatic over a mean follow-up time of about one year. Fatalities related to the interventional procedure may occur as a result of nonreversible ischaemic complications, progression of the dissection or complications of additional reconstructive surgical procedures on the thoracic aorta [
1,
2,
3,
17,
20,
31]. Potential problems may arise from unpredictable haemodynamic alterations in the true and false lumen after fenestration and side branch stenting. These alterations can result in loss of previously well perfused arteries, or in loss of initially salvaged side branches.
Recent reports suggest that percutaneous stent-graft placement in the dissected aorta is safer and produces better results than surgery for type B dissection. Paraplegia may occur after use of multiple stent-grafts but still appears to be a rare phenomenon, especially when the stented segment does not exceed 16 cm. Results of short-term follow-up are excellent with a 1-year survival rate of >90%; tears can be readapted and aortic diameters generally decrease with complete thrombosis of the false lumen. This suggests that stent placement may facilitate healing of the dissection, sometimes of the entire aorta, including abdominal segments (
Figure 1). However, late reperfusion of the false lumen has been observed occasionally underlining the need for stringent follow-up imaging. In some patients, follow-up imaging has revealed tears that had initially been overlooked, but required additional stents.
Both acute and midterm results in patients with thoracic aortic aneurysm, ulcers and traumatic aortic lesion are encouraging; although lifetime is more or less a matter of comorbidities. While the endovascular maneuvers usually ensure complete occlusion of the aneurysmal sac or ulcer, selected patients are usually older with various comorbidities that are more likely to limit life expectancy [
25,
26]. However, better patient selection, avoidance of endoleak and further technical refinement will improve outcomes even for those patients that would never be accepted for open surgical repair.
In view of the fact that post-procedural mortality rate seems largely dependent on the severity and duration of ischaemia before the interventional procedures (eg, in one report half of the 30 day mortalities were due to irreversible damage sustained before the endovascular treatment) timely percutaneous stent treatment seems highly desirable [
32]. Considering the excess mortality of acute type A dissection with malperfusion of peripheral branches, percutaneous intervention for relief of malperfusion has been suggested before surgical repair, a concept, however, not settled yet. In addition, patients can develop a systemic inflammatory reaction after stent-graft implantation. This may present as an elevated C-reactive protein in concert with fever. Both signs disappear within days or weeks spontaneously or with transient non-steroidal treatment as aortic remodeling progresses.
Long-term follow-up and adjunctive treatment
The long-term approach to patients with successful initial treatment of acute aortic dissection or aneurysm begins with an appreciation that such patients have a systemic illness which variably predisposes their entire aorta and potentially its larger branches to dissection, aneurysm and rupture. Systemic hypertension, advanced age, aortic size, and presence of patent false lumen are all factors of higher risk, as is the entire spectrum of connective tissue diseases (most importantly the Marfan syndrome). All post-stent patients merit extremely aggressive medical therapy, follow-up visits, and serial imaging. It has been estimated that nearly a third of patients surviving initial treatment for acute dissection will experience dissection extension, aortic rupture, or require surgery for aortic aneurysm formation within 5 years of presentation. Furthermore, this risk is substantial in the first few months after initial therapy [
33].
Treatment with effective beta blockade is the cornerstone of medical therapy. By lowering both blood pressure and contractility, beta blockers have been shown to retard aortic expansion in Marfan’s syndrome [
34] and prevent expansion of chronic abdominal aortic aneurysms [
35]. Observational studies suggest similar unique benefits in aortic dissection when compared to other antihypertensive agents; guidelines recommend progressive uptitration of dosage to achieve a blood pressure <135/80 mm Hg in usual patients and <130/ 75 mm Hg in those with Marfan’s syndrome [
35,
36,
37].
Serial imaging of the aorta is an essential component of long-term surveillance and follow-up of patients with aortic aneurysm (before and after surgery or stent-graft placement) in Marfan’s disease and in all cases of chronic dissection. Choice of imaging modality is dependent on institutional availability and expertise as well as extent of aortic involvement, and the age of the patient. Previous recommendations suggest follow-up imaging and examination at 1, 3, 6, 9, and 12 months following discharge, and annually thereafter [
38,
39]. This aggressive strategy underlines the observation that both hypertension and aortic expansion/dissection are common and not easily predicted in the first months following hospital discharge. Further, imaging is not confined simply to the region of initial involvement since both dissection and aneurysm formation may occur anywhere along the entire length of the aorta.
Progression to an ascending aortic diameter of 4.5 cm is an indication for elective surgical repair in patients with Marfan’s syndrome. For non-Marfan patients, aorta’s exceeding 5.5 to 6.0 cm warrant repair, as does distal aortic expansion to >6.0 cm in any patient. As with non-dissecting aneurysms, rate of growth and size of the aorta are both important factors to consider when it comes to prophylactic vascular surgery. While an ascending aortic aneurysm of 5.0 cm may merit urgent repair in a young patient with Marfan’s syndrome [
40], an aneurysm stable at 5.0 cm for 3 years in an elderly person with well controlled blood pressure is unlikely to rupture.
Patients who have been treated with surgery and/or endovascular stent grafting warrant similar follow-up to those whose initial treatment was limited to medical treatment. Aortic expansion, dissection, and rupture both at surgical suture site and at a distance are common in survivors of type A dissection. Meticulous attention to blood pressure control and serial imaging are just as relevant to operative survivors as for patients in early stages of aortic pathology.