Effect of Ezetimibe Co-Administered with Statin Therapy in Swiss Outpatients †
Summary
Introduction
Patients and methods
Patients and lipid parameters
Statistics
Safety and adverse events
Results
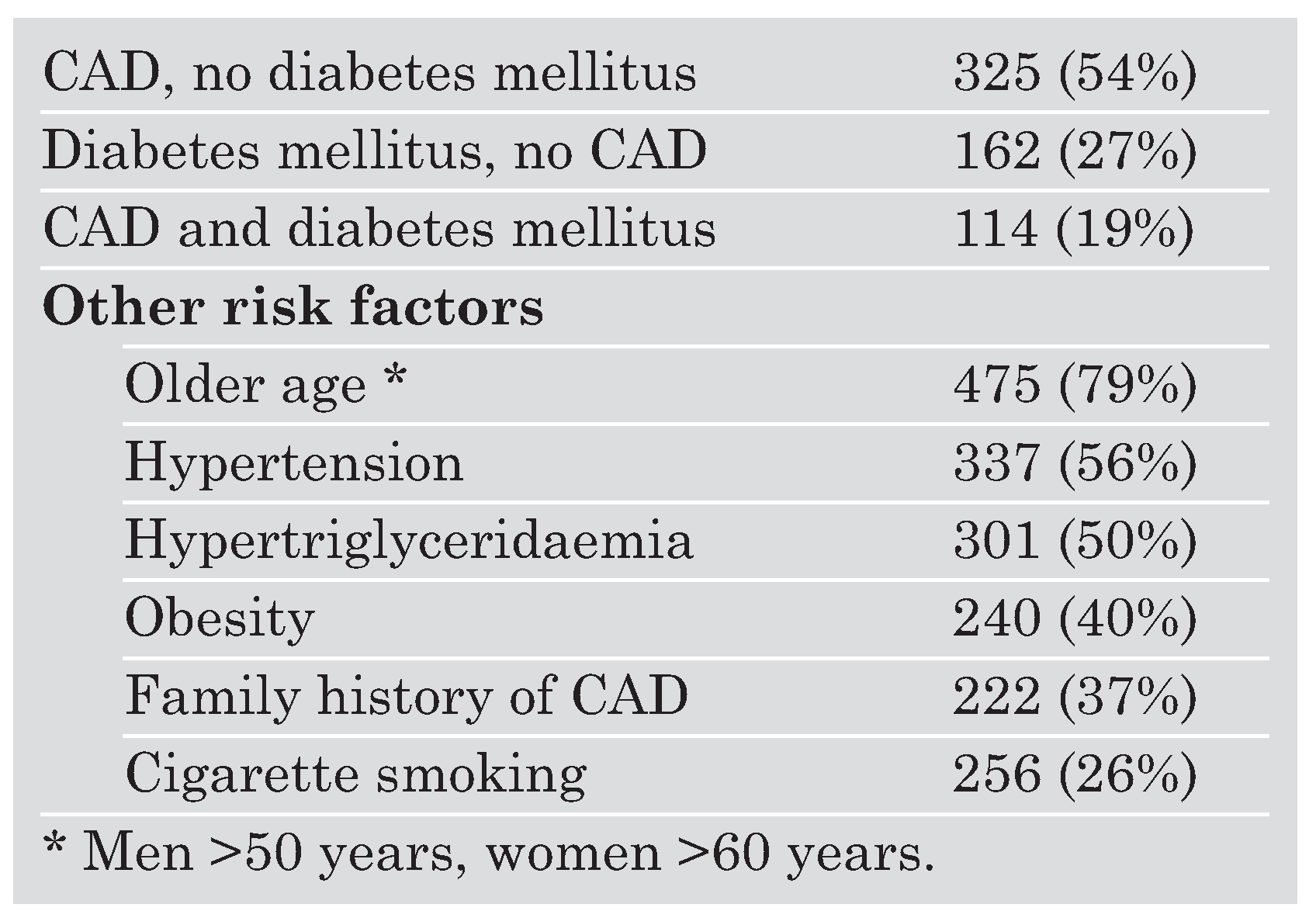
Baseline characteristics
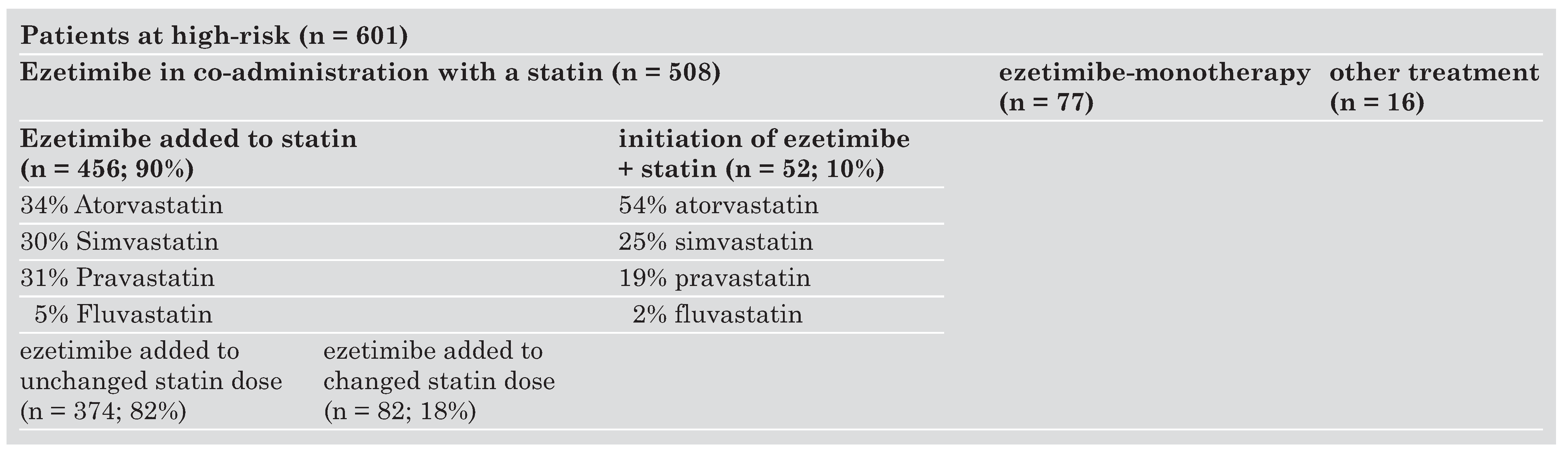
Statin doses
Effects of ezetimibe initiated in co-administration with a statin in high-risk patients
Effects of ezetimibe added to established statin therapy in high-risk patients
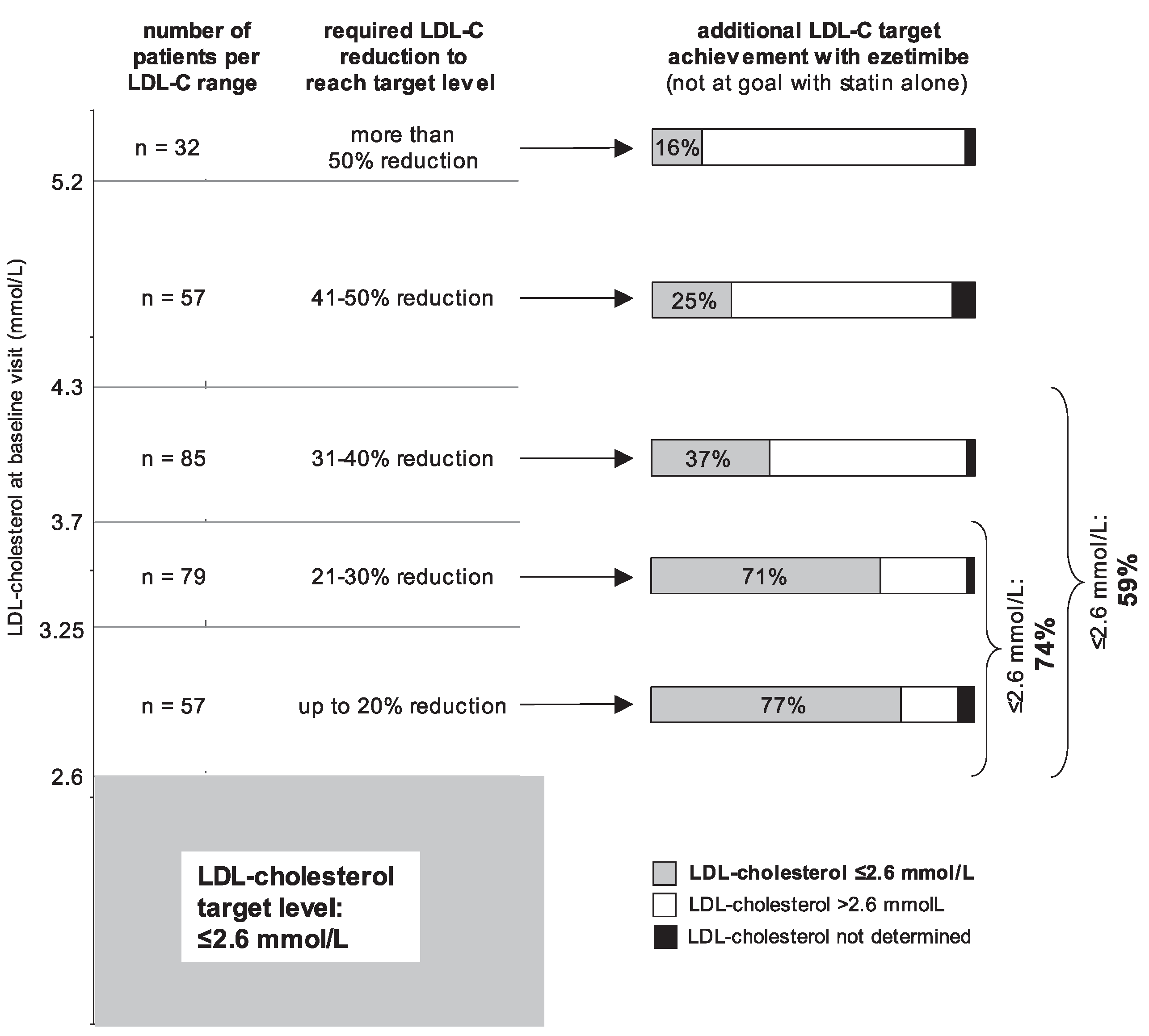
Cholesterol target achievement with ezetimibe added to established statin therapy in high-risk patients
Safety and adverse events
Discussion
Conclusions
Acknowledgments
References
- Murray, C.J.; Lopez, A.D. Mortality by cause of for eight regions of the world: Global burden of disease study. Lancet 1997, 349, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Heart disease and stroke statistics-2004 Update, American Heart Association, Dallas. Available online: http://www.american-heart.org/presenter.jhtml?identifier=1928.
- Steinberg, D.; Witztum, J.L. Lipoproteins and atherogenesis. Current concepts JAMA 1990, 264, 3047–3052. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Alternative approaches to cholesterol-lowering therapy. Am J Cardiol 2002, 90, 1135–1138. [Google Scholar] [CrossRef]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: A randomised placebocontrolled trial. Lancet 2002, 360, 7–22. [Google Scholar] [CrossRef]
- Sever, P.S.; Dahlöf, B.; Poulter, N.R.; Wedel, H.; Beevers, G.; Caulkield, M.; et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): A multicentre randomised controlled trial. Lancet 2003, 361, 1149–1158. [Google Scholar]
- Serruys, P.W.J.C.; De Feyter, P.; Macaya, C.; Kokott, N.; Puel, J.; Vrolix, M.; et al. for the Lescol Intervention Prevention Study (LIPS) investigators. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention. JAMA 2002, 287, 3215–3222. [Google Scholar] [CrossRef]
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389. [Google Scholar]
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Rader, D.J.; Rouleau, J.L.; Belder, E.; et al. for the Pravastatin or Atorvastatin Evaluation and Infection Therapy—Thrombolysis in Myocardial Infarction 22 Investigators. Comparison of intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004, 1495–1504. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tuzcu, M.E.; Schoenhagen, P.; Brown, E.G.; Ganz, P.; Vogel, R.A.; et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis. A randomized controlled trial. JAMA 2004, 291, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C. Statin therapy after acute myocardial infarction: Are we adequately treating high-risk patients? Curr Atheroscler Rep 2002, 4, 99–106. [Google Scholar] [CrossRef]
- Pearson, T.A.; Laurora, I.; Chu, H.; Kafonek, S. The Lipid Treatment Assessment Project (L-TAP): A multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch Intern Med 2000, 160, 459–467. [Google Scholar] [CrossRef]
- Stone, N. Combination therapy: Its rationale and the role of ezetimibe. Eur Heart J Supplements 2002, 4 (Suppl J), J21–24. [Google Scholar] [CrossRef]
- Bays, H. Ezetimibe. Expert Opin Investig Drugs 2002, 11, 1587–1604. [Google Scholar] [CrossRef]
- Altmann, S.W.; Davis, H.R.; Zhu, L.; Yao, X.; Hoos, L.; Tetzloff, G.; et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef]
- Van Heek, M.; Davis, H. Pharmacology of ezetimibe. European Heart J Supplements 2002, 4 (Suppl J), J17–20. [Google Scholar]
- Dujovne, C.A.; Ettinger, M.P.; McNeer, J.F.; Lipka, L.J.; LeBeaut, A.P.; Suresh, R.; et al. for the Ezetimibe Study Group. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholes-terolemia. Am J Cardiol 2002, 90, 1092–1097. [Google Scholar] [CrossRef]
- Gagné, C.; Bays, H.E.; Weiss, S.R.; Mata, P.; Quinto, K.; Melino, M.; et al. for the Ezetimibe Study Group. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol 2002, 90, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Houri, J.; Notarbartolo, A.; Melani, L.; Lipka, L.J.; Suresh, R.; et al. for the Ezetimibe Study Group. Effect of ezetimibe co-administered with atorvastatin in 628 patients with primary hypercholesterolemia. A prospective, randomized, double-blind trial. Circulation 2003, 107, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H.; McGarry, T.; Bettis, R.; Melani, L.; Lipka, L.J.; LeBeaut, A.; et al. for the Ezetimibe Study Group. Ezetimibe co-administered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol 2002, 40, 2125–2134. [Google Scholar] [CrossRef]
- Melani, L.; Mills, R.; Hassmann, D.; Lipetz, R.; Lipka, L.; LeBeaut, A.; et al. for the Ezetimibe Study Group. Efficacy and safety of ezetimibe co-administered with pravastatin in patients with primary hypercholesterinemia: A prospective, randomized, double-blind trial. Eur Heart J 2003, 24, 717–728. [Google Scholar] [CrossRef]
- Kerzner, B.; Corbelli, J.; Sharp, S.; Lipka, L.J.; Melani, L.; LeBeaut, A.; et al. for the Ezetimibe Study Group. Efficacy and safety of ezetimibe co-administered with lovastatin in primary hypercholesterolemia. Am J Cardiol 2003, 91, 418–424. [Google Scholar] [CrossRef]
- Knopp, R.H.; Gitter, H.; Truitt, T.; Bays, H.; Manion, C.V.; Lipka; et al. for the Ezetimibe Study Group. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur Heart J 2003, 24, 729–741. [Google Scholar] [CrossRef]
- Battegay, E.; Bertel, O.; Darioli, R.; Gutzwiler, F.; Keller, U.; Mordasini, R.; et al. für die Schweizerische Gesellschaft für Kardiologie, Arbeitsgruppe «Lipide und Atherosklerose». Empfehlungen 1999 zur Behandlung des Risikofaktors Cholesterin. Schweiz Ärztezeitung 1999, 80, 549–552. [Google Scholar]
- Noseda, G.; Riesen, W.; Darioli, R.; Mordasini, R. LDL-Cholesterin 2,6 mmol/L—Neue Zielwertempfehlung der AGLA für die Behandlung von Patienten mit hohem Risiko. Schweiz Ärztezeitung 2003, 84, 302. [Google Scholar]
- Arzneimittel-Kompendium der Schweiz 2004.
- Gagné, C.; Gaudet, D.; Bruckert, E.; for the Ezetimibe Study Group. Efficacy and safety of ezetimibe co-administered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterinemia. Circulation 2002, 105, 2469–2475. [Google Scholar] [CrossRef]
- Gaudiani, L.; Lewin, A.; Meneghini, L.; Perevozskaya, I.; Tobert, J.; Plotkin, J.; et al. Efficacy and safety of ezetimibe in co-administration with simvastatin versus simvastatin alone in thiazolidinedione-treated patients with diabetes mellitus. Abstract (presented ACC 2004).
- Feldmann, T.; Koren, M.; Insull WJr McKenney, J.; Schrott, H.; Lewin, A.; et al. Treatment of high-risk patients with ezetimibe plus simvastatin co-administration versus simvastatin alone to attein National Cholesterol Education Program Adult Treatment Panel III low-density lipoprotein cholesterol goals. Am J Cardiol 2004, 93, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Blazing, M.A.; King, T.R.; Brady, W.E.; Palmisano, J. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am J Cardiol 2004, 93, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O.; Lerman, L.O.; Napoli, C.; Lerman, A. Statin effects beyond lipid lowering—Are they clinically relevant? Eur Heart J 2003, 24, 225–248. [Google Scholar] [CrossRef] [PubMed]
- Sager, P.T.; Melani, L.; Lipka, L.; Strony, J.; Yang, B.; Suresh, R.; et al. Effect of coadministration of ezetimibe and simvastatin on high-sensitivity C-reactive protein. Am J Cardiol 2003, 92, 1414–1418. [Google Scholar] [CrossRef]
- Ridker, P.M. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: From concept to clinical practice to clinical benefit. Am Heart J 2004, 148, S19–26. [Google Scholar] [CrossRef] [PubMed]


 |
 |
 |
© 2005 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Maeder, M.; Blank, R.; Feucht, J.; Darioli, R.; Rickli, H. Effect of Ezetimibe Co-Administered with Statin Therapy in Swiss Outpatients. Cardiovasc. Med. 2005, 8, 399. https://doi.org/10.4414/cvm.2005.01131
Maeder M, Blank R, Feucht J, Darioli R, Rickli H. Effect of Ezetimibe Co-Administered with Statin Therapy in Swiss Outpatients. Cardiovascular Medicine. 2005; 8(11):399. https://doi.org/10.4414/cvm.2005.01131
Chicago/Turabian StyleMaeder, Micha, Robert Blank, Julia Feucht, Roger Darioli, and Hans Rickli. 2005. "Effect of Ezetimibe Co-Administered with Statin Therapy in Swiss Outpatients" Cardiovascular Medicine 8, no. 11: 399. https://doi.org/10.4414/cvm.2005.01131
APA StyleMaeder, M., Blank, R., Feucht, J., Darioli, R., & Rickli, H. (2005). Effect of Ezetimibe Co-Administered with Statin Therapy in Swiss Outpatients. Cardiovascular Medicine, 8(11), 399. https://doi.org/10.4414/cvm.2005.01131




