Summary
Background: An elevated interleukin-6 (IL-6) serum level was described as a diagnostic marker for a myxoma. This study tried to assess the value of IL-6 determination to differentiate myxomas from non-myxomatous tumours in patients with an intracardiac mass.
Methods: From 1993 to 2001, patients with an intracardiac mass by echocardiography were prospectively included. There were 32 patients: 19 consecutive patients with a cardiac myxoma (all histologically confirmed), and 13 randomly selected patients with non-myxomatous cardiac masses including seven with a cardiac tumour and six with an intracardiac thrombus. Serum IL-6 and C-reactive protein (CRP) levels were compared between groups and correlated with tumour size measured by 2D echocardiography. Additionally, two patients with relapsing myxomas were studied with serial IL-6 determinations and echocardiography.
Results: There was no difference in tumour size between myxomas (57 ± 55 mL) and nonmyxomatous masses (50 ± 116 mL; p = 0.83). Average IL-6 levels were 11.6 ± 7.9 pg/mL (range: 0.3–29.5) in 19 patients with myxomas and 16.2 ± 15.5 pg/mL (range: 0–43.7) in 13 with a non-myxomatous heart tumour (p = 0.28). IL-6 levels were elevated in 15 patients with myxomas resulting in a sensitivity of 79% (95% confidence interval CI 54–94%) and in 10 with non-myxomatous tumours resulting in a specificity of 23% (95% confidence interval 5–54%; p = 0.67). There was no significant difference between CRP levels between the different groups (p = 0.77). There was no correlation between serum IL-6 or CRP with tumour size in any of the groups (p >0.05). ROC analysis showed an area under the curve for IL-6 of 0.47 (95% CI 0.24–0.70) and for CRP 0.50 (95% CI 0.26–0.74). In the two patients with relapsing myxomas, however, there was no relapse without an increase in IL-6.
Conclusions: An elevated IL-6 or CRP serum level in a patient with an intracardiac mass is not specific for myxoma or another type of intracardiac tumour. The value of IL-6 as an additive marker to echocardiography in the detection and follow-up of patients with suspected or resected myxomas has yet to be proven.
Introduction
The exact diagnosis of an intracardiac mass is essential for an optimal patient management. Most common causes include a benign tumour, a primary or metastatic malignant tumour, a vegetation or a thrombus. Of the primary tumours involving the heart, 75% are benign and 25% are malignant [
1]. Cardiac myxomas are the most common primary heart neoplasm comprising 40% of the tumours in most pathological series [
2]. Myxomas are usually suspected based on a typical echocardiographic criteria [
3].
Although features of the echocardiography study and the patient’s history suggest a particular tumour type, none are specific enough to warrant a reliable diagnosis before a biopsy. Additional predictive markers would be helpful.
Atrial myxomas have a strong association with prominent constitutional symptoms such as fever, anaemia and arthralgia, suggestive of inflammatory disorders [
4]. Recently, myxomas have been shown to produce interleukin-6 (IL-6), as evidenced by the observation that serum levels may become undetectable after resection of the tumour [
4]. IL-6 has been demonstrated in myxoma by immunohistochemistry, and cultured myxoma cells in vitro produce IL-6 mRNA and IL-6 protein [
5,
6,
7]. Production and release of the cytokine IL-6 may mediate the systemic inflammatory response and elevation of C-reactive protein levels (CRP) seen in patients with cardiac myxoma. Several case reports and few studies of myxoma patients with high serum IL-6 levels have been published. So far, no study compared the accuracy of serum IL-6 and CRPdetermination in myxomas and non-myxomatous intracardiac masses.
Our study therefore addressed the question whether serum levels of IL-6 and CRP can differentiate myxomas from non-myxomatous tumours or thrombi in patients with an intracardiac mass. Possible correlations between tumour size and IL-6- and CRP-levels were tested, and the value of IL-6 as a marker for recurrence after surgical excision was examined.
Methods
Patients
Between January 1993 and November 2001, all consecutive patients undergoing echocardiography at the University Hospital of Zurich were prospectively screened. Inclusion criteria were: an intracardiac tumour by echocardiography and determination of IL-6 and CRP-levels within seven days of the echocardiographic exam. There were 32 patients. Patients were assigned to the myxoma group or the control group of non-myxomatous tumours according to the histology and underlying diagnosis. Histology was obtained in 22 of the 32 patients (69%). In four patients, there was metastatic cancer and the tumour was assumed to be a metastasis of the already known cancer and no histology was obtained. In 6 other patients there was a thrombus in the akinetic or dyskinetic segments of known prior myocardial infarction and again, no histology was obtained.
Constitutional symptoms were defined as follows: fever with no other cause, weight loss of >5% of body weight and/or flushing.
Determination of serum interleukin-6 and C-reactive protein levels
Serum IL-6 levels were determined by a commercial ELISA kit according to the manufacturer’s instructions (Amersham). Sensitivity was 0.5 pg/mL, the upper limit of a normal serum level of IL-6 was 3.1 pg/mL. CRP levels were measured by a turbidimetric assay (Wako CRP HR II) with the use of an automatic analysis system (Hitachi 747).
Follow-up examinations
A 51-year-old female with recurrent malignant myxoma and a 42-year-old male with a Carney-complex which is defined as a combination of heart myxoma with primary nodular adrenal cortex dysplasia, spot-shaped skin pigmentation and myxoma-like tumours in other locations [
8] were followed with serial IL-6 determinations and echocardiography studies.
Echocardiography
Complete 2D and Doppler-echocardiographic exam was performed in all patients using one of the following echocardiography machines (Hewlett Packard 500, Hewlett Packard 1500, Hewlett Packard 5500 and Acuson Sequoia). Tumour volume was obtained by echocardiography according to the area length method in two orthogonal planes (average).
Statistical analysis
Continuous variables were indicated as means ± standard deviations. Nominal variables were given as percentages. The software package used was SPSS 11.0 (SPSS Inc.). Nonparametric tests including Spearman rank correlation, Mann Whitney U test as well as Fisher’s exact test were used where appropriate. A p-value <0.05 was considered as significant. Sensitivities and specificities are presented with exact 95% confidence intervals (from: Scientific Tables by Geigy, Volume on statistics, 8th edition. Basle; 1980). Discriminative power of possible markers was evaluated using ROC analysis.
Results
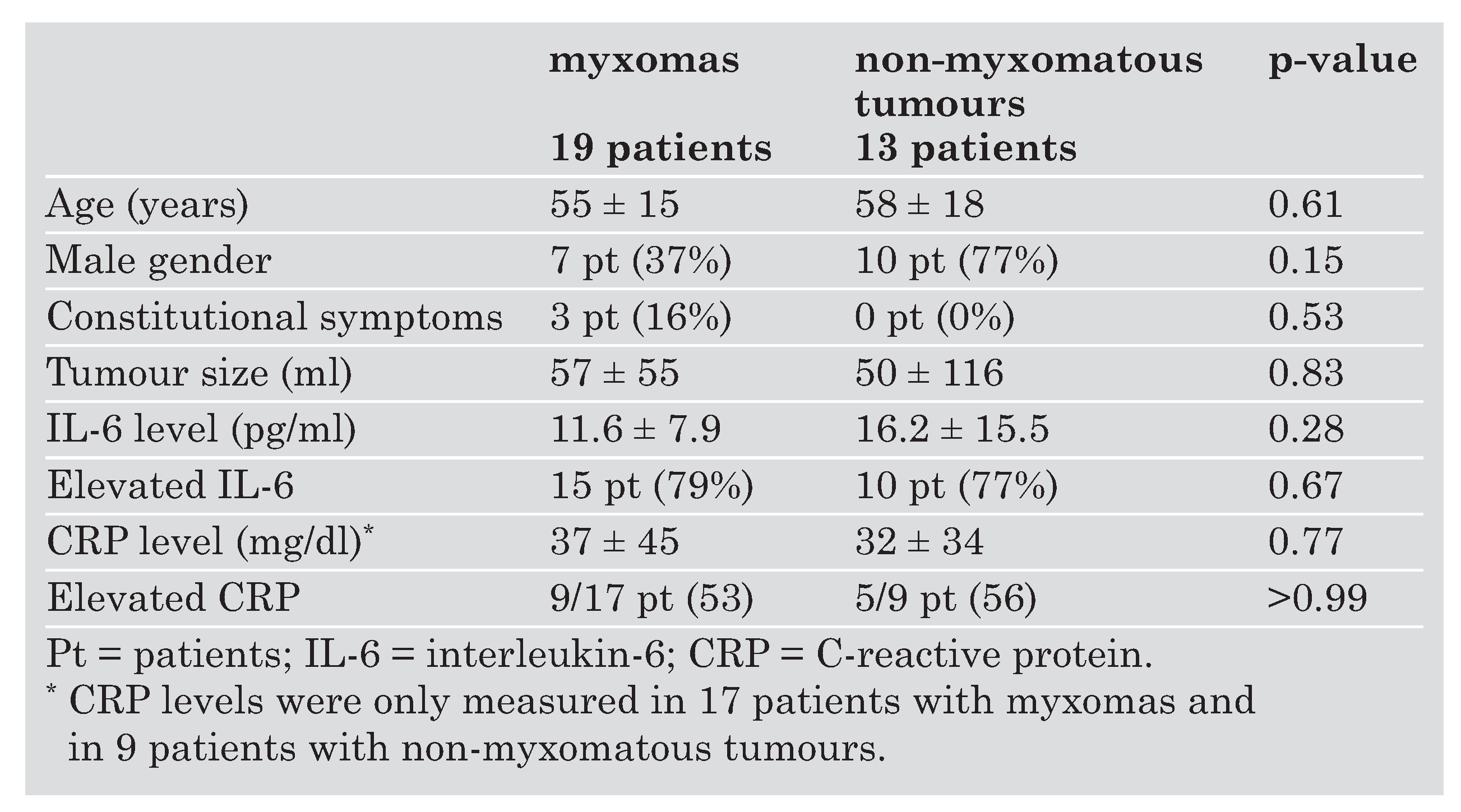
The clinical characteristics, tumour size, IL-6 and CRP levels of 32 patients who were included in the study are summarised in
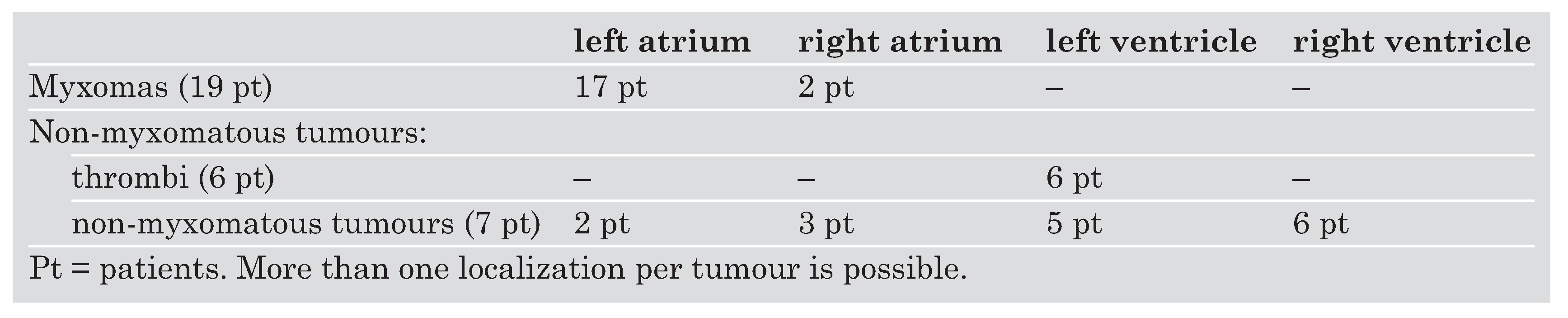
Table 1. There were 19 patients with myxomas. The non-myxomatous group included seven patients with non-myxomatous tumours and six patients with an intracardiac thrombus due to transmural infarction. Intracardiac localisation of myxomas and non-myxomatous tumours is shown in
Table 2. Non-myxomatous tumours included papillary fibroelastoma (2 patients), malignant lymphoma invading the heart, metastasis of a melanoma, sarcoma, one patient with a lipoma and a nonspecified granulomatous inflammatory tumour (one patient each).
Data of 19 patients with a cardiac myxoma were compared with those of the 13 patients with non-myxomatous intracardiac mass lesions. There was no significant difference in size, IL-6 levels or CRP levels (
Table 1). The subgroup of patients with intracardiac thrombi had smaller tumour volumes (6.2 ± 3.5 mL; range: 1–12) as compared to myxoma patients (57 ± 55 mL; range: 1–192; p = 0.04). IL-6 levels in the six patients with an intracardiac thrombus were 11.5 ± 11.9 pg/mL (range: 0–27.5) which was not significantly different from myxoma patients with 11.6 ± 7.9 pg/mL (range: 0.3–29.5; p = 0.97). Sensitivity of elevated IL-6 levels for detection of myxomas was 79%, (95% Confidence interval CI 54–94%) and specificity 23%, (95% CI = 5–54%).
Only three of 19 myxoma patients had constitutional signs such as fever, weight loss or flush. One patient in the myxoma group had an infected myxoma with consecutive endocarditis of the mitral valve. He developed a subclavian steal syndrome due to massive embolism and secondary inflammation of arteries in the area of the brachiocephalic truncus. In the non-myxomatous tumour group, two patients (a patient with melanoma and a patient with granulomatous inflammation) had also subacute myocardial infarction as another cause for elevated CRP- and IL-6 levels. A patient with a melanoma had an IL-6 value of 181.3 pg/mL and a CRP of 3 mg/l. The patient with granulomatous inflammation showed a different inflammatory pattern with very low IL-6 value of 1.7 pg/mL and a very high CRP level of 91 mg/mL. Two patients with myocardial infarction revealed elevated CRP- and IL-6 levels, with the IL-6 levels being 13.2 respectively 23.4 pg/mL.
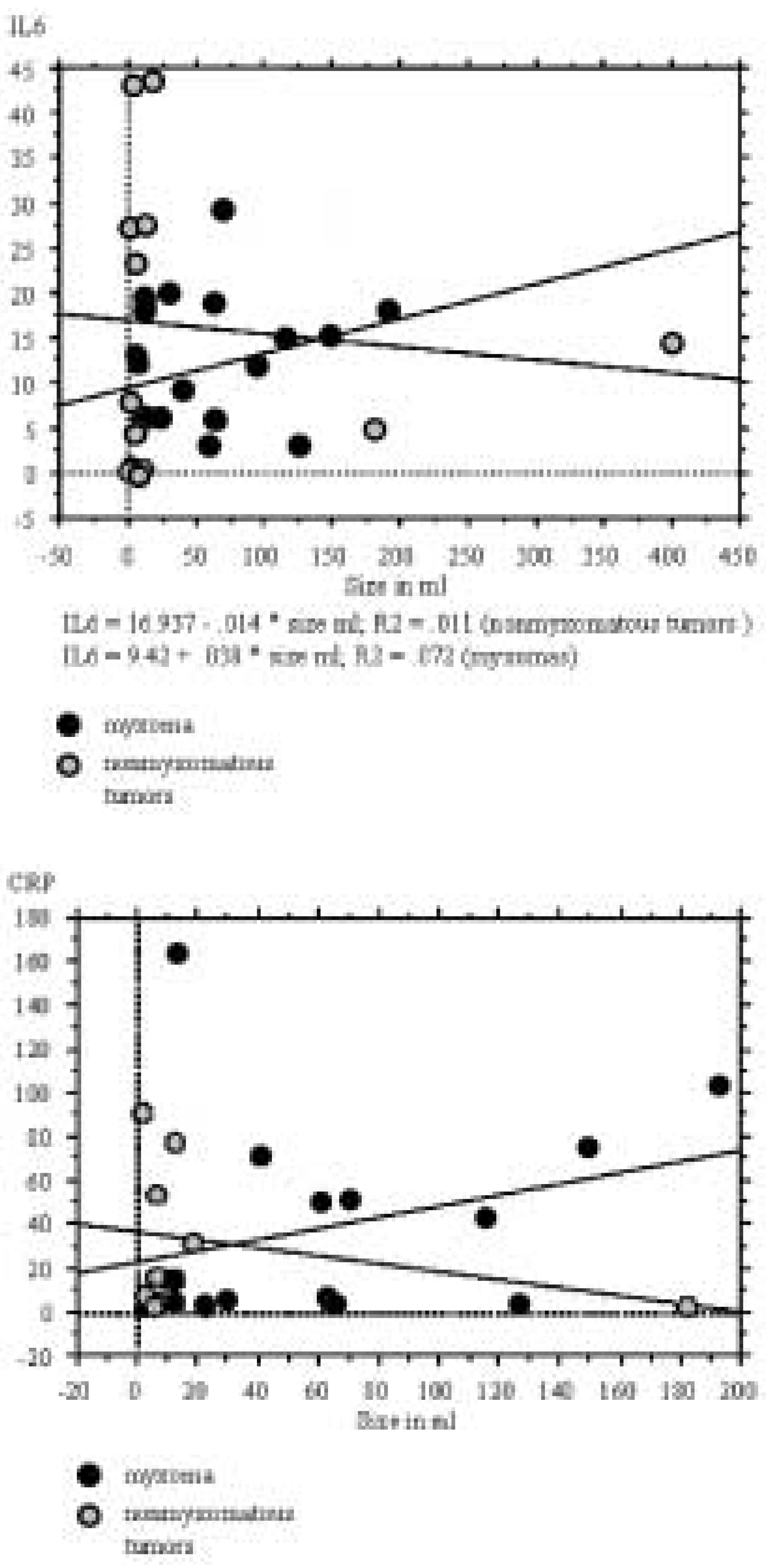
In the intracardiac thrombus subgroup, one patient had chronic obstructive pulmonary disease-associated pneumonia during sampling of IL-6- and CRP-levels. There was no IL-6 serum level elevation in three of the 19 myxoma patients, tumour volumes in these patients ranged between 1–125.6 mL. In eight patients with a myxoma, the CRP level was below 10 mg/l. Neither IL-6 nor CRP correlated with tumour size in each of the groups analysed (p >0.05) as shown in
Figure 1A and B. As shown in
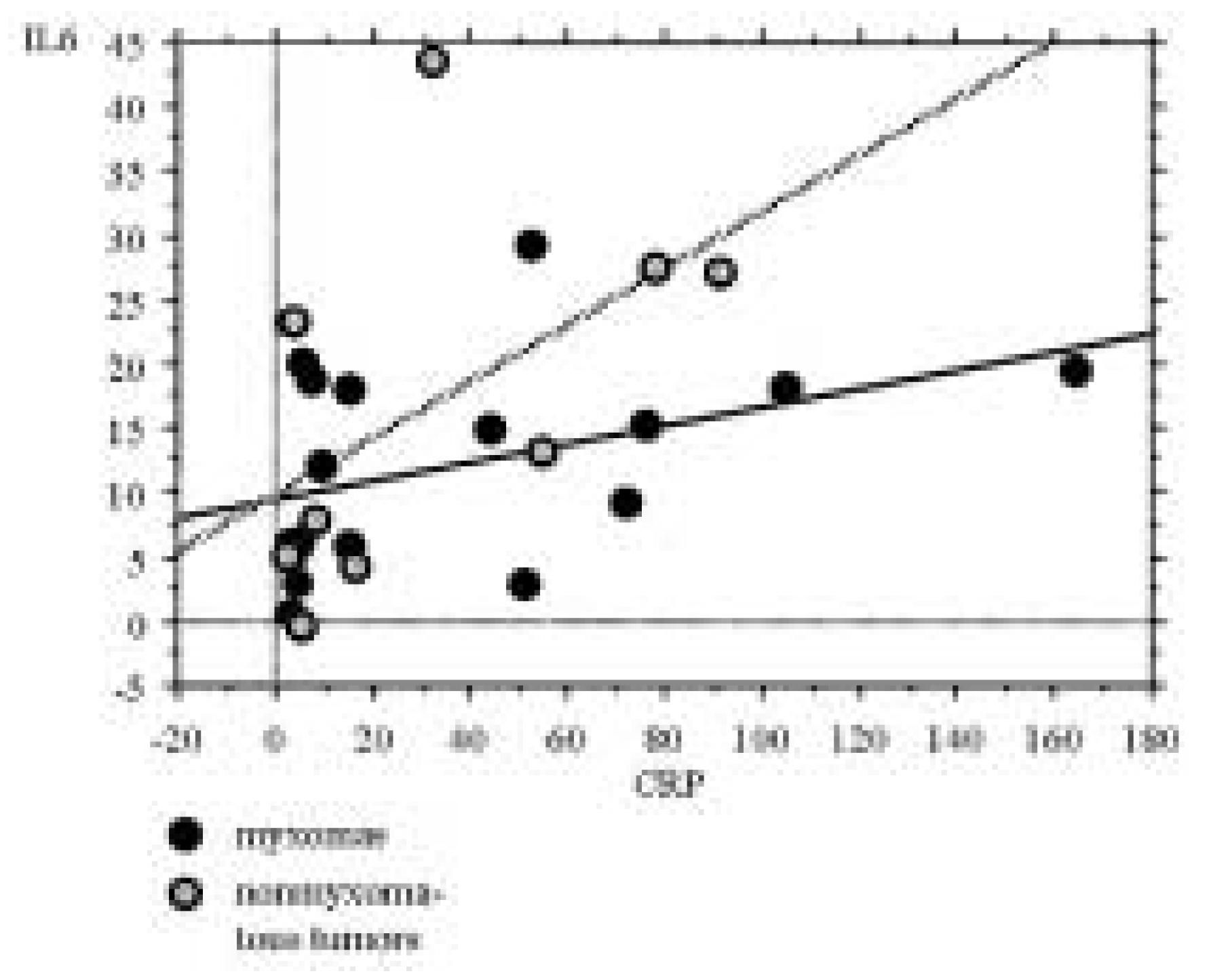
Figure 2, there was no strong correlation between IL-6- and CRP levels in either of the groups (p >0.05). The patients with the low IL-6 levels didn’t correspond to those with low CRP values. The IL-6 level could be elevated without relevant CRP elevation and vice versa.
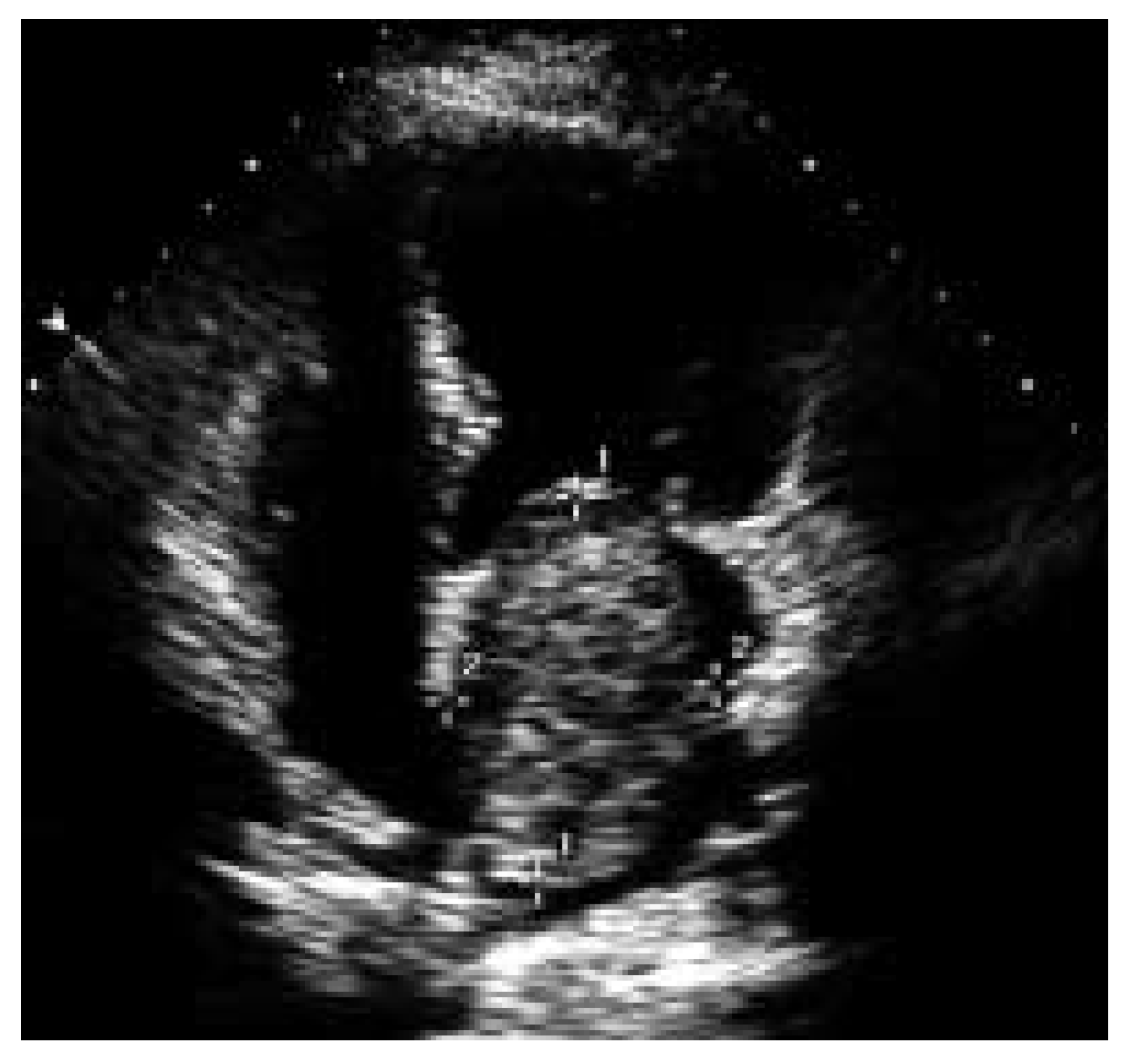
An echocardiographic example of large myxoma in a 57-year-old patient in whom only minor elevations of CRP and IL-6 levels were present is shown in
Figure 3.
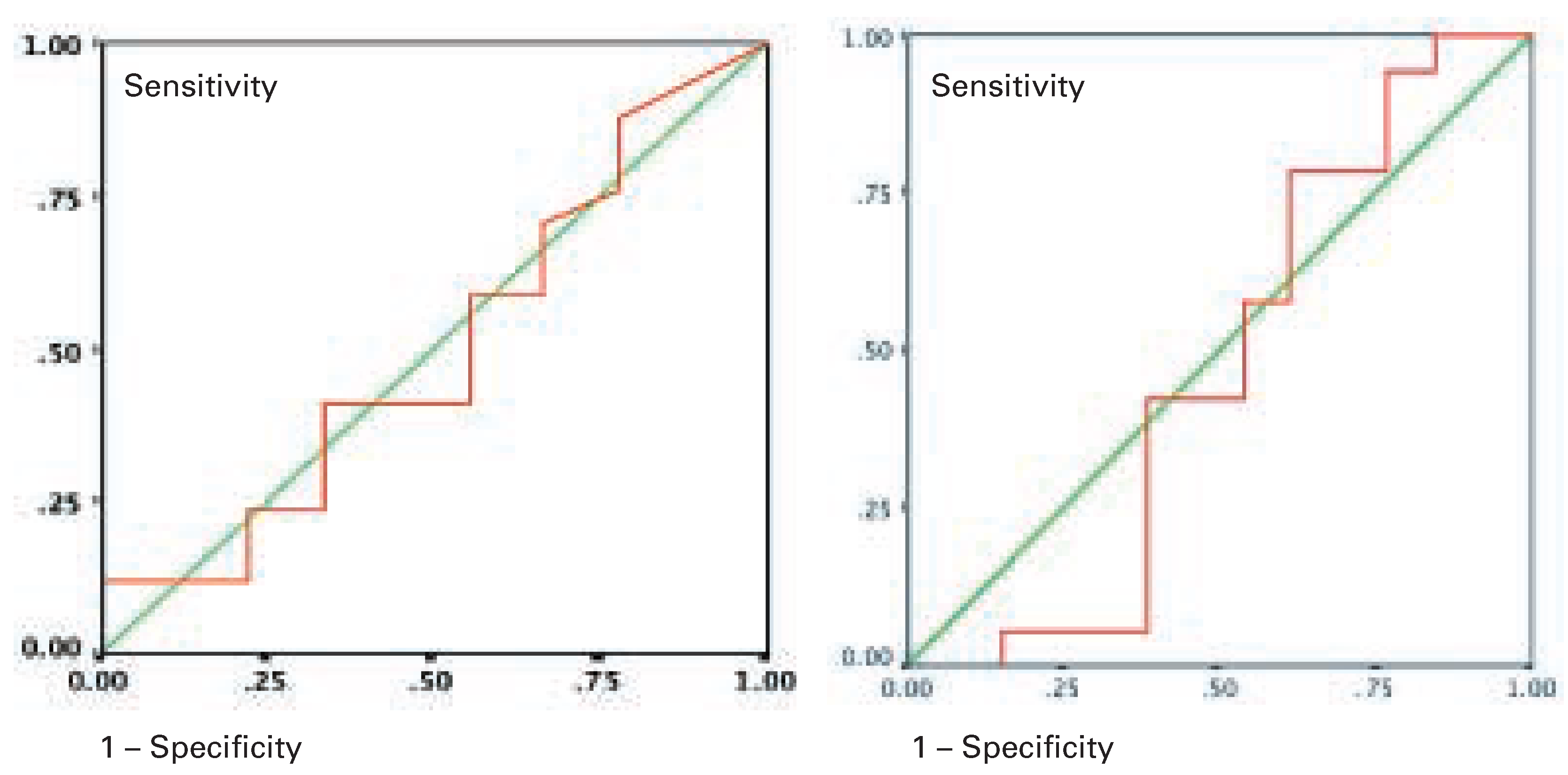
Results of the ROC analysis are shown in
Figure 4A,B. The area under the ROC curve or IL-6 (
Figure 4A) and CRP (
Figure 4B) is 0.47 respectively 0.50 and shows a rather low excellent predictive value. A sensitivity of 79% with a specificity of 23% is very close to the diagonal.
Follow-up studies
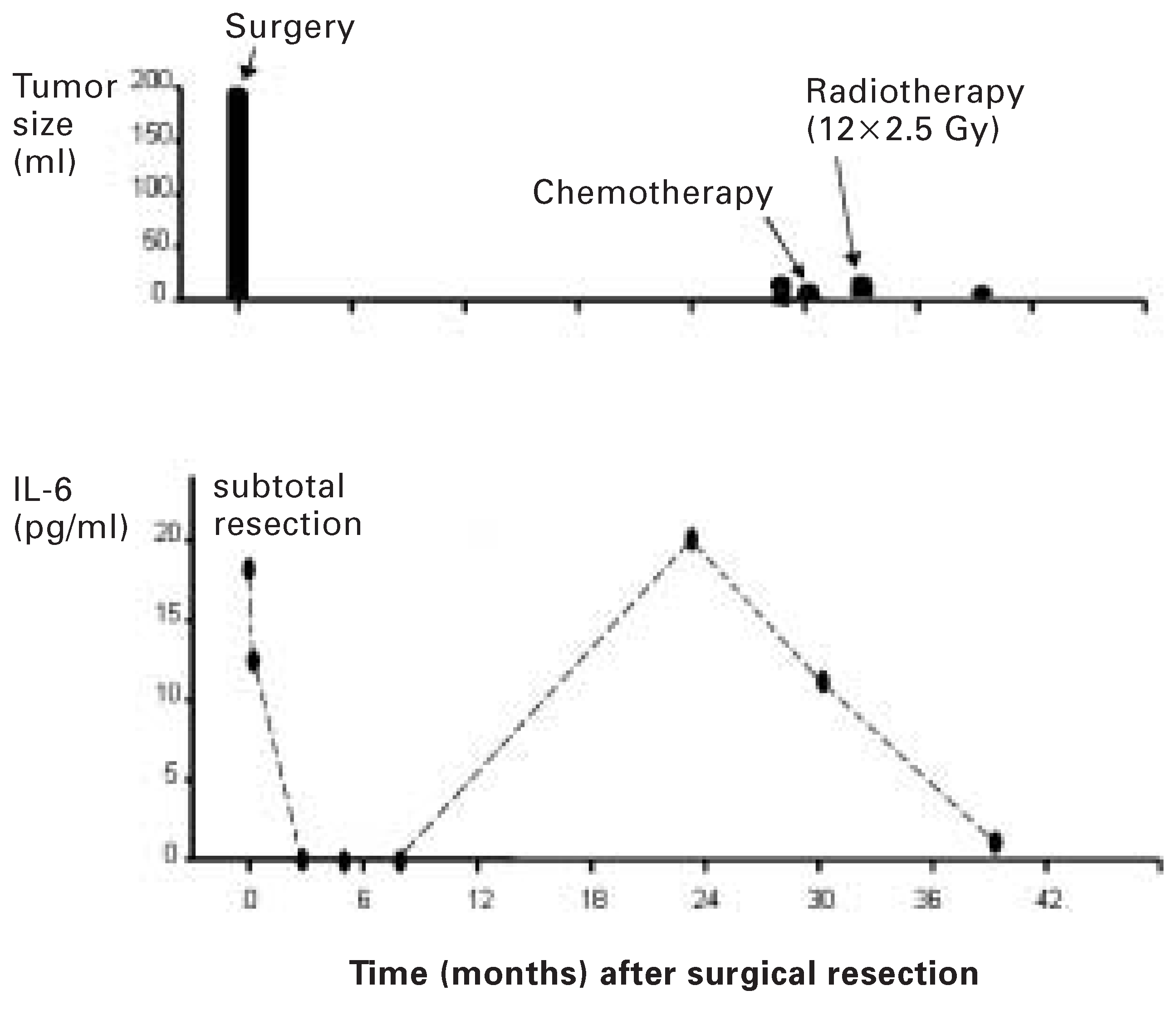
In the two patients with relapsing myxomas, recurrence was predicted by an increase in IL-6. A 51-year-old female patient with a myxoma had a preoperative IL-6 level of 18.2 pg/mL and a tumour size of 192 mL. Then, she underwent resection of the myxoma. Histological examination revealed malignant myxoma. Serial postoperative IL-6 levels were below the detection limit. After 23 months IL-6 rose to 20 pg/mL. By echocardiography, tumour relapse could only be seen seven months later at a size of 7.2 mL (
Figure 5). After chemotherapy with holoxan and famorubicine and radiotherapy, IL-6 levels were undetectable. 30 months after surgery, tumour volume did show further increase to 14.4 mL. With radiotherapy, there was regression of the tumour size to 4.8 mL.
A 47-year-old male patient had familial myxoma syndrome (Carney-complex). This patient had a normal IL-6 level of 0.8 pg/mL with a myxoma volume of 2.3 mL, which was the second recurrence of a myxoma in the right atrium. It was excised. Elevated IL-6 levels were measured on several occasions. Thirty months later, several elevated IL-6 levels with a peak of 1614 pg/mL were measured without echocardiographic evidence for cardiac recurrence of a myxoma in a total follow-up time of 4 years. However, at 6 years of follow-up, he died of metastastic myxoma with no cardiac recurrence.
Discussion
Echocardiography is the initial diagnostic procedure of choice in a cardiac tumour, and provides sufficient information concerning tumour size, site and attachment to allow the surgeon to proceed to excision. Although echocardiographic features may suggest a particular tumour type, none are specific enough to warrant a reliable diagnosis. A serum marker specific for myxoma would help in the management. In our study, however, neither IL-6 nor CRP levels were helpful or specific enough to differentiate cardiac myxomas from non-myxomatous cardiac masses. Thus, for the exact diagnosis of an intracardiac tumour, clinical history and excision of the tumour remain the most helpful diagnostic steps.
Other imaging modalities
Scanning by computer tomography and magnetic resonance imaging MRI can also demonstrate intracardiac tumours, and may provide additional diagnostic information as compared to echocardiography [
9,
10], however, large series with prospective comparisons between echocardiography and magnetic resonance imaging are lacking so far. Magnetic resonance imaging seems to be very specific in the rare cases of lipoma where the diagnosis on the basis of the characteristic imaging qualities of fat is feasible [
11].
IL-6 levels in myxomas
Precise and fast diagnosis of cardiac myxomas would be important because of the propensity for embolisation [
12]. Discovery of a myxoma is considered an indication for semi-urgent excision with surgery providing cure in most instances [
13,
14]. The differential diagnosis of myxomas includes other benign tumours, primary or metastatic malignant tumours, vegetations, or thrombi. Recent case reports and small series were describing elevated serum IL-6 levels in patients with myxoma [
4,
5,
6,
15,
16,
17,
18]. In one study, IL-6 was elevated in 14 of 17 myxoma patients, and it correlated with clinical findings of autoimmunity [
7]. In another small study, IL-6 was proposed as a diagnostic marker of myxomas as it was elevated in all patients [
19]. In a small series of six patients with myxoma, a significant correlation was found between tumour size and serum IL-6 levels [
15]. This prompted us to test serum IL-6 as a predictive marker for myxoma in the evaluation of cardiac masses. Our results do not confirm earlier findings from smaller series that in patients with myxoma serum IL-6 levels are elevated and correlate with tumour size [
15]. In our patients, there was no correlation between serum IL-6 and CRP levels with tumour size. IL-6 levels were elevated in 79% of our myxoma patients but not in all. Cardiac myxoma is an endocardial neoplasm that arises from multipotential mesenchymal cells as demonstrated by positive reactions for mesenchymal and epithelial markers [
20,
21]. Bigger tumours would theoretically lead to higher IL-6 levels. We could not confirm this, in our patients there was no correlation of IL-6 with tumour size.
CRP levels and other markers in myxoma patients
IL-6 is a potent B cell differentiation factor, and it is a strong hepatocyte-stimulating factor inducing the release of acute-phase proteins such as CRP [
22]. However, in our 19 myxoma patients there was not a significant correlation between tumour size and CRP levels. Are there other cytokines different from IL-6 which would qualify as markers for myxomas? Soeparwata et al. [
15] in a series of 6 patients with non-familial myxoma have analysed preoperative plasma samples for IL-1b, IL-1 receptor antagonist, IL-2, soluble IL-2 receptor, IL-6, soluble IL-6 receptor, IL-8, tumour necrosis factor receptor I and II, and granulocyte macrophage colony stimulation factor (CSF), but with the exception of tumour necrosis factor a none correlated with myxoma size. We have not determined tumour necrosis factor a in our patients.
Comparison of myxomas with non-myxomatous tumours
We compared for the first time in the literature IL-6 levels of myxoma patients with those of a control group of patients with non-myxomatous intracardiac mass lesions. Not surprisingly for a multifunctional cytokine, IL-6 levels in the control group were also elevated, but again, there was no correlation with mass size, suggesting an IL-6 source different from the heart lesion. The seven patients with nonmyxomatous cardiac tumours had elevated IL-6 serum levels in a comparable range as myxoma patients, and there was no statistically significant difference between the groups. The six patients with intracardiac thrombi had slightly lower IL-6 levels as compared to myxomas, but without a relevant difference and clearly above the normal range. Elevated serum IL-6 levels in non-myxoma patients can be due to several reasons, since high IL-6 levels have been described in a variety of clinical conditions, such as infection, sepsis, rheumatoid arthritis, myocardial infarction or severe heart failure [
22]. Whereas in most of these conditions IL-6 is produced by lymphoid or non-lymphoid cells outside the heart, nonmyxoma patients have been described with cardiac IL-6 production. Examples are syndromes resembling polymyositis and other connective tissue disorders, and in one case systemic amyloidosis with an elevated serum amyloid A protein that fell after excision of the tumour [
16,
17,
18,
19,
20,
21,
22,
23]. Myxoma patients are hypergammaglobulinaemic and display various kinds of autoantibodies whose levels diminish after excision of the myxoma [
27]. Recently, a case report of a patient with a cardiac tumour and an elevated serum IL-6 level of 12 pg/mL was reported [
28]. A myxoma was expected, but histological examination of the surgically resected tumour revealed a malignant fibrous histiocytoma in the left atrium. By immunohistochemistry IL-6 production by malignant histiocytoma cells could be demonstrated, and the tumour extract contained 170 pg IL-6/g tissue, suggesting constitutive production of IL-6 in malignant fibrous histiocytoma [
29]. Also, cardiac expression of IL-6 has been shown in non-neoplastic conditions, for example in left ventricles of dilated cardiomyopathy and in nonmyocyte cells of rat hearts after infarction. Thus, high IL-6 levels in patients with intracardiac mass lesions are not specific for myxomas.
IL-6 and constitutional symptoms
Does IL-6 classify as a possible mediator for the constitutional and inflammatory symptoms associated with myxomas? It has been suggested that constitutional signs result when IL-6 levels exceed a certain threshold [
29]. Parissis et al. found in 14 of 17 myxomas IL-6 mRNA-expression and all 14 patients with elevated IL-6 levels had constitutional signs, in contrast to 3 patients with normal serum IL-6 [
7]. In our study 16 of 19 patients had elevated IL-6 levels but only 3 of 14 of them reported constitutional signs (2 patients with infection were excluded). The average level of IL-6 in our 3 patients with constitutional symptoms was 15.7 ± 3.6 pg/mL which was not different from the IL-6 levels in the 14 patients without constitutional symptoms and without infection with 11.2 ± 8.6 (p = 0.39). Therefore it seems, that IL-6 levels alone are not the only explanation for constitutional symptoms in myxoma patients.
One patient in our myxoma group presented with bacteremia and endocarditis, a complication in myxomas which has been described in several case reports [
30,
31,
32]. Since elevated IL-6 levels in patients with bacterial endocarditis have been reported [
33], we would have predicted an even higher serum IL-6 level in this patient, but in relation to a tumour size of 40.5 mL a IL-6 serum level of 9.4 pg/mL was not particularly high.
Follow-up after excision of a myxoma
Recurrence of a myxoma after surgical removal is very low (4.7% in 526 cases reported in the literature), except in the case of familial, multiple or Carney complex myxoma [
34]. The Carney complex is characterised by multiple lentigines and blue nevi, peripheral, myxoid tumours (cutaneous myxoma or myxoid mammary fibroadenoma), endocrine overactivity (primary pigmented adrenocortical disease with Cushing’s syndrome, testicular Sertoli cell tumours, or pituitary adenomas), psammomatous melanotic schwannoma, and multiple, recurrent cardiac myxomas [
8]. If there is a recurrent nonfamilial myxoma, incomplete excision, tumour implantation or misdiagnosis of a mesenchymal sarcoma must be considered [
35]. Few authors propose regular, echocardiographic follow-up examinations in patients in whom a myxoma has been excised [
36]. Our myxoma series contains two patients with recurrent myxoma, a 53-year-old female with malignant myxoma and a 47-yearold male with Carney complex. Both were monitored by serial echocardiography studies and serum IL-6 determinations. Relapse of the malignant myxoma was correctly heralded 7–24 months prior to echocardiographic confirmation by rising IL-6 levels. We can thus not exclude that IL-6 may be useful as a marker for follow up of myxoma patients at risk for recurrence, such as familial myxomas.
Limitations
This study involved only a relatively small number of patients. Despite the small number of patients, however, it seems, that IL-6 levels are not specific; therefore this conclusion from this small study is valid.
Conclusions
An elevated IL-6 serum level in a patient with an intracardiac mass is not specific for cardiac myxoma and not helpful in the differentiation from another cardiac tumour or cardiac thrombus. However, on the basis of the observation in two patients, we can not definitely exclude that IL-6 may be used as an additive marker to echocardiography in the follow up of patients to exclude relapsing myxomas.