Abstract
Vascular disease in diabetes is accompanied by decreased levels of the vasodilators, nitric oxide (NO) and prostacyclin, and increased levels of vasoconstrictor eicosanoids, which enhance the progression of the disease. The vascular dysfunction caused by short term exposure to elevated glucose and the long term effects of diabetes are similar, suggesting that the alteration in endothelial factors in diabetes primarily results from exposure of endothelial cells to elevated glucose. Undoubtedly hyperlipidaemia contributes as well. Increased formation of reactive oxygen species is an important feature of the diabetic endothelial cell phenotype. This results in part from uncoupling of endothelial nitric oxide synthase such that it generates superoxide anion in addition to NO, forming peroxynitrite, a damaging molecule. Peroxynitrite inactivates prostacyclin synthase leading to the accumulation of inflammatory and prothrombotic eicosanoids. This not only helps to explain the impairment of endothelial vasodilator mechanisms, but also increased progression of vascular disease. Many of these cellular abnormalities can be prevented by adequate scavenging of oxygenderived free radicals or by blocking the actions of the eicosanoids at TP receptors. Exposure to elevated glucose also gives rise to oxidants in smooth muscle, and recent studies indicate that oxidation of cysteine thiols under these conditions may prevent physiological NO signaling. As a result the responsiveness to NO is impaired and accounts in part for abnormal endotheliumdependent vasodilatation.
Introduction
Nitric oxide (NO) is the principal vasodilator that regulates smooth muscle tone. However, its release from endothelial cells also regulates smooth muscle migration and growth, as well as the function of other nearby cells including platelets, leukocytes, fibroblasts, and parenchymal cells. In diabetes mellitus, as with other cardiovascular risk factors, oxidants arise that interfere with the synthesis, diffusion, and action of NO on target proteins (fig. 1). All these factors contribute to endothelial dysfunction. In diabetic endothelial cells, oxidants may arise from endothelial nitric oxide synthase (eNOS), NADPH oxidase, or mitochondria. In the setting of inflammation or established vascular disease, neutrophil myeloperoxidase may contribute. These oxidants inhibit endothelial function, by impairing the synthesis of NO, and in the case of superoxide anion (O2 –), rapidly reacting with NO. The reaction between NO and O2 is key, because it is very rapid, and not only impairs the diffusion of NO to target cells, but also forms the very reactive product, peroxynitrite (ONOO ) which reacts with proteins, lipids, and DNA. Furthermore, oxidants arise in target cells exposed to elevated glucose and impair physiological function, via actions on ion channels and transporters important in NO function.
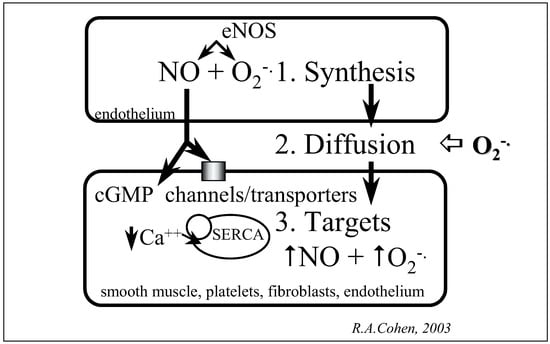
Figure 1.
Oxidant mechanisms interfering with nitric oxide function in vascular diseases.
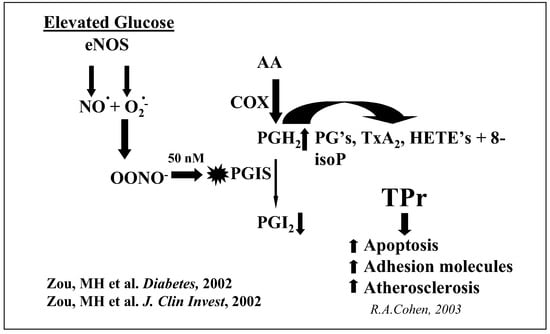
Figure 2.
Endothelial NOS uncoupling inactivates PGI synthase and increases TP receptor stimulation.
Actions in endothelial cells
The release of NO from eNOS is regulated by the redox state of tetrahydrobiopterin and the zinc-thiolate center that binds the eNOS dimers together. In endothelial cells exposed acutely to oxidants or to hyperglycemia for several days in culture, eNOS becomes uncoupled such that it generates O2– in addition to NO [1, 2]. The catalytic activity of NO synthase increases as evidenced by NADPH consumption, but NO synthesis from arginine is decreased as indicated by less formation of citrulline [2]. Evidence that eNOS is impaired may be obtained by determining the status of the zincthiolate center that binds the two dimers. If the thiols that bind together eNOS dimers are oxidized to disulfides, the dimers will separate into monomers under reducing conditions. Increased monomers of eNOS thus formed are detected in increased quantities in endothelial cells exposed in culture to high glucose, as well as in kidney and heart of diabetic atherosclerotic mice, indicating effects on the microvasculature [2].
Formation of ONOO– by uncoupled eNOS may further exacerbate the functional abnormalities within endothelial cells as demonstrated by tyrosine nitration of prostacyclin synthase [1, 2]. This enzyme is inactivated by concentrations of ONOO– as low as 50 nanomolar. We have suggested that prostacyclin synthase inactivation is an important pathophysiological event in diabetes, because it decreases the production of prostacyclin, and also results in the accumulation of eicosanoids including the prostacyclin synthase substrate prostaglandin endoperoxide (PGH2), thromboxane A2, HETE’s, and isoprostanes (fig. 2). Stimulation of the thromboxane receptor, termed the TP receptor, is a common feature of all these products. Our studies indicate TP receptor stimulation leads to increased endothelial cell adhesion molecule expression and apoptosis [1]. These adverse events accelerate atherogenesis as demonstrated by the antiatherogenic effects of treatment with a TP receptor antagonist in apolipoprotein E deficient mice [3]. In a recent study of hyperlipidaemic and hyperglycaemic streptozotocin-induced diabetic apolipoprotein E deficient mice, treatment with a TP antagonist prevented the 2–3 fold enhancement [4] of atherosclerotic lesion caused by diabetes.
Actions in smooth muscle cells
Smooth muscle vasodilation to the NO donors, sodium nitroprusside or nitroglycerin, is usually normal in many, but not all studies of endothelial dysfunction associated with cardiovascular risk factors. This has suggested to some that the major cause for impaired vascular function is the endothelial cell. However, the vasodilation to sodium nitroprusside has been reported to be abnormal in type 2 diabetes [5]. In addition, my colleagues and I have demonstrated that even when the response to these NO donors are normal in the setting of hypercholesterolaemia [6, 7] or diabetes [8,9,10], responsiveness to authentic NO is impaired to a degree similar to that of endothelium-dependent vasodilators. This is most likely due to the fact that unlike authentic NO, the NO-donors exert their effects almost solely via the stimulation guanylyl cyclase, production of cyclic GMP, and protein kinase G whose role is preserved in disease. In contrast, authentic NO exerts its effects both via cyclic GMP-dependent and independent effects mediated by ion channels [11] and transporters [12] (fig. 3). Recently, we have described a novel molecular mechanism by which NO decreases intracellular calcium and smooth muscle relaxation by stimulation of the sarco/endoplasmic reticulum ATPase (SERCA) [12]. Oxidants impair the function of this calcium ATPase.
The endothelial dysfunction is associated with impaired relaxations to authentic NO, even if the arteries are denuded of endothelium before being exposed to elevated glucose. Under these circumstances, we have found increased oxidation of cysteine thiols on SERCA. Both the activity of SERCA and the level of free cysteine thiols were decreased by approximately 50% after 6-hour exposure to elevated glucose. This suggests that oxidants formed in smooth muscle in response to elevated glucose rapidly oxidize cysteine thiols and inhibit the activity of SERCA as well as potentially other proteins. This transporter also accounts for decreased NO-induced relaxation in hypercholesterolaemia induced atherosclerosis [6]. The molecular mechanism by which NO stimulates calcium uptake involves the reversible S-glutathiolation of thiols on SERCA by intracellular NO-derived oxidants [13]. Furthermore, our studies indicate that the effects of low physiological concentrations of NO are impeded by chronic effects of higher concentrations of oxidants that arise in diseased arteries [10, 13]. As an example, exposure of isolated arteries to high concentrations of glucose impair endothelial vasodilation [9].
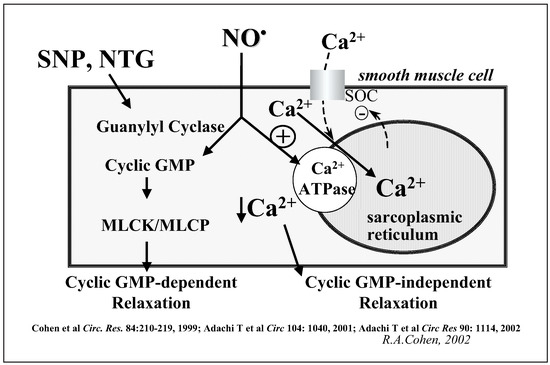
Figure 3.
Role of cyclic GMP, calcium, and SERCA in NO-induced relaxation.
Conclusions
Cardiovascular risk factors are associated with increased levels of oxidants in vascular cells that result in the impairment of vasodilation, and that likely accelerates vascular disease. Endothelial cells and smooth muscle cells are both involved, though in different ways. In endothelial cells eNOS thiols and tetrahydrobiopterin becomes oxidized and the enzyme activity becomes uncoupled. This results in production of ONOO– which contributes importantly to the cellular dysfunction caused by hyperglycaemia. Inactivation of prostacyclin synthase by ONOO– results in TP receptor activation and signaling that increases adhesion molecule expression, apoptosis, and atherogenesis. In smooth muscle, oxidants also arise that impair the physiological vasodilator response to NO.
Acknowledgments
The author would like to acknowledge the contribution of his collaborators and co-workers who have participated in the work described in this review. The work was supported by funding from the National Institutes of Health HL31607–21, HL68758, and HV28178, as well as by a Center grant funded by the Juvenile Diabetes Foundation.
References
- Zou, M.H.; Shi, C.; Cohen, R.A. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H2 receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes 2002, 51, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Shi, C.; Cohen, R.A. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Inves 2002, 109, 817–26. [Google Scholar] [CrossRef]
- Cayatte, A.J.; Du, Y.; Oliver-Krasinski, J.; Lavielle, G.; Verbeuren, T.J.; Cohen, R.A. The thromboxane receptor antagonist S18886 but not aspirin inhibits atherogenesis in apo E-deficient mice evidence that eicosanoids other than thromboxane contribute to atherosclerosis. Arterioscler Thromb Vasc Biol 2000, 20, 1724–8. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.A.; Xu, S.; Maitland, K.; Cayatte, A.J.; Lavielle, G.; Verbeuren, T.J. The TP receptor antagonist, S18886, prevents the enhancement of atherogenesis by diabetes mellitus in apolipoprotein E deficient mice. Circulation 2001, 104, II118. [Google Scholar]
- Williams, S.B.; Cusco, J.A.; Roddy, M.A.; Johnstone, M.T.; Creager, M.A. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 1996, 27, 567–74. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Matsui, R.; Xu, S.; Kirber, M.; Lazar, H.L.; Sharov, V.S.; et al. Antioxidant improves smooth muscle sarco/endoplasmic reticulum Ca(2+)-ATPase function and lowers tyrosine nitration in hypercholesterolemia and improves nitric oxideinduced relaxation. Circ Res 2002, 90, 1114–21. [Google Scholar] [CrossRef]
- Adachi, T.; Matsui, R.; Weisbrod, R.M.; Najibi, S.; Cohen, R.A. Reduced sarco/endoplasmic reticulum Ca2+ uptake activity can account for the reduced response to NO, but not sodium nitroprusside, in hypercholesterolemic rabbit aorta. Circulation 2001, 104, 1040–5. [Google Scholar] [CrossRef][Green Version]
- Tesfamariam, B.; Jakubowski, J.A.; Cohen, R.A. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2–TxA2. Am J Physiol 1989, 257, H1327–33. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, B.; Brown, M.L.; Deykin, D.; Cohen, R.A. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest 1990, 85, 929–32. [Google Scholar] [CrossRef]
- Adachi, T.; Weisbrod, R.M.; Trucillo, M.P.; Xu, S.; Sethuraman, M.; Sharov, V.S.; et al. Specific thiol oxidation can account for inactivation of the sarco/endoplasmic reticulum calcium ATPase and reduced NO-induced relaxation caused by high glucose. Circulation 2003, 108, IV–134. [Google Scholar]
- Bolotina, V.M.; Najibi, S.; Palacino, J.J.; Pagano, P.J.; Cohen, R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle cells. Nature 1994, 368, 850–3. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.A.; Weisbrod, R.M.; Gericke, M.; Yaghoubi, M.; Bierl, C.; Bolotina, V.M. Mechanism of nitric oxide-induced vasodilatation. Refilling of intracellular stores by sarcoplasmic reticulum Ca2+-ATPase and inhibition of store-operated Ca2+influx. Circ Res 1999, 84, 210–9. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Weisbrod, R.M.; Pimentel, D.; Heibeck, T.; Sharov, V.S.; Schoneich, C.; et al. Peroxynitrite activates sarco/endoplasmic reticulum calcium ATPase via S-glutathiolation and accounts for NO-induced arterial relaxation. Circulation 2003, 108, IV–282. [Google Scholar]
© 2004 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.