Flecainide Induced ST Segment Changes Can Mimic Pathological ECG Changes in Patients Undergoing Exercise Testing: A Case Report
Abstract
Introduction
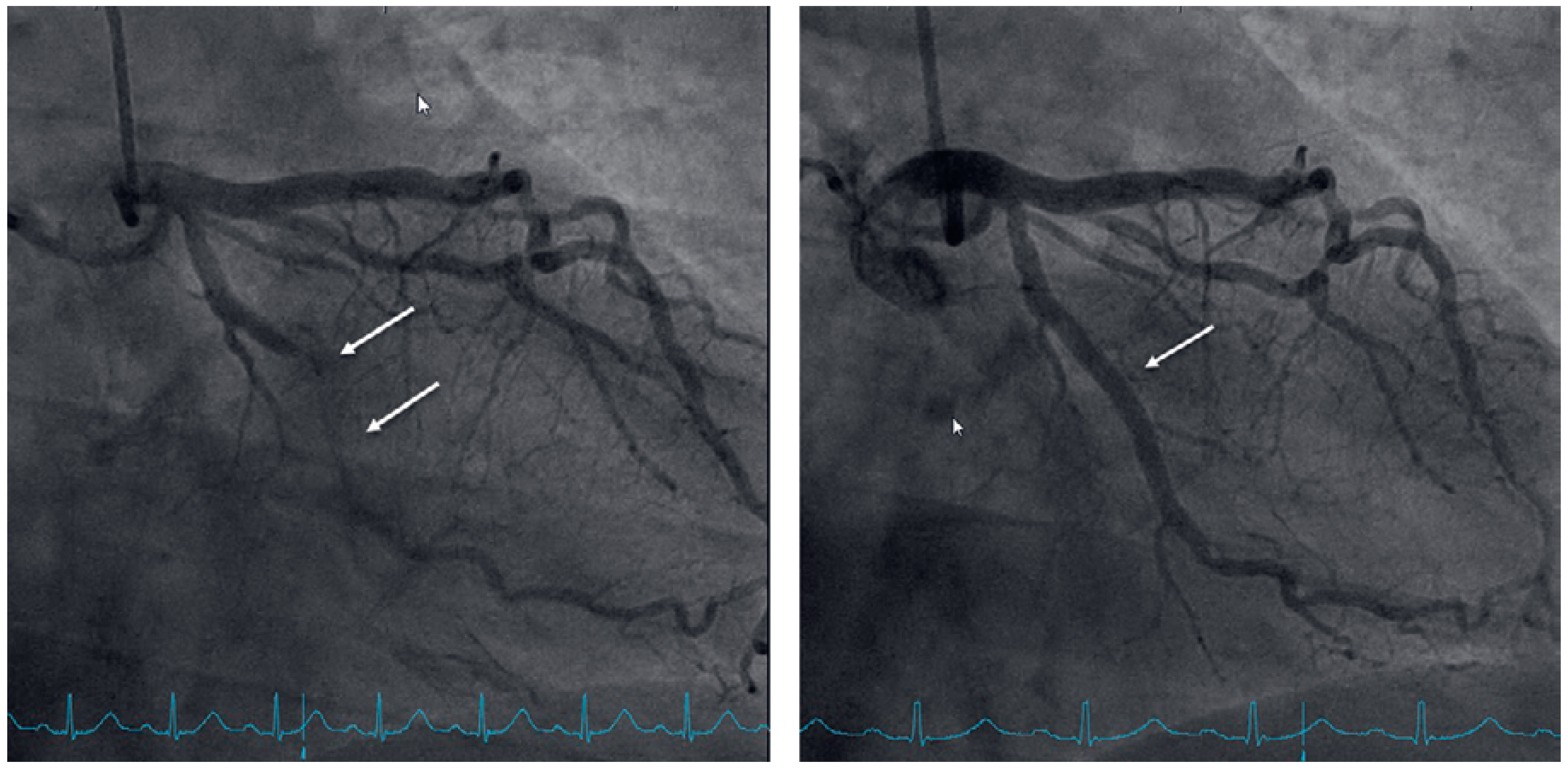
Case presentation
Discussion
Learning points
- Class IC antiarrhythmic drugs inhibit sodium channels and may therefore prolong the PR interval and lead to QRS widening.
- QRS widening in patients on class IC antiarrhythmic drugs undergoing exercise testing may mimic cardiac ischaemia.
- Flecainide is contraindicated in patients with coronary artery disease or structural heart disease.
Disclosure statement
References
- Giardina, E.G. Major side effects of class I antiarrhythmic drugs: Up To Date. Available from: https://www.uptodate.com/contents/ major-side-effects-of-class-i-antiarrhythmic-drugs.
- Alzahrani, K.; Alzahrani, T. StatPearls Flecainide; StatPearls Publishing, 2020. [Google Scholar]
- Vik-Mo, H.; Ohm, O.J.; Lund-Johansen, P. Electrophysiologic effects of flecainide acetate in patients with sinus nodal dysfunction. Am J Cardiol. 1982, 50, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.A.; Lau, C.P.; Tai, Y.T.; Wu, B.Z. Effects of flecainide on exercise hemodynamics and electrocardiography in patients without structural heart disease. Clin Cardiol. 1995, 18, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Vallurupalli, S.; Pothineni, N.V.; Deshmukh, A.; Paydak, H. Utility of Routine Exercise Testing to Detect Rate-Related QRS Widening in Patients Without Structural Heart Disease on Class Ic Antiarrhythmic Agents (Flecainide and Propafenone). Am J Cardiol. 2015, 116, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Dan, G.A.; Martinez-Rubio, A.; Agewall, S.; Boriani, G.; Borggrefe, M.; Gaita, F.; et al. Antiarrhythmic drugs-clinical use and clinical decision making: a consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). Europace. 2018, 20, 731–732an. [Google Scholar] [PubMed]
- Bordier, P.; Garrigue, S.; Bernard, V.; Haissaguerre, M.; Douard, H.; Broustet, J.P.; et al. Flecainide-induced Increase in QRS Duration and Proarrhythmia during Exercise. Clin Drug Investig. 1997, 13, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guha, A.; Buck, B.; Patel, D.; Snider, M.J.; Boyd, M.; et al. Initiation and outcomes with Class Ic antiarrhythmic drug therapy. Indian Pacing Electrophysiol J. 2018, 18, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, W.; Segawa, K.; Ito, H.; Tanaka, S.; Yoshimoto, N. Class IC antiarrhythmic drugs, flecainide and pilsicainide, produce ST segment elevation simulating inferior myocardial ischemia. J Cardiovasc Electrophysiol. 1998, 9, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Echt, D.S.; Liebson, P.R.; Mitchell, L.B.; Peters, R.W.; Obias-Manno, D.; Barker, A.H.; et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991, 324, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.S.; Noseworthy, P.A.; Yao, X.; Sangaralingham, L.R.; Shah, N.D. Gastrointestinal Safety of Direct Oral Anticoagulants: A Large Population-Based Study. Gastroenterology. 2017, 152, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; et al. ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef] [PubMed]
- Hellestrand, K.J.; Bexton, R.S.; Nathan, A.W.; Spurrell, R.A.; Camm, A.J. Acute electrophysiological effects of flecainide acetate on cardiac conduction and refractoriness in man. Br Heart J. 1982, 48, 140–148. [Google Scholar] [CrossRef] [PubMed]


© 2021 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Kruzik, M.; Padrutt, M.; Stehli, J.; Brunckhorst, C.; Schmied, C.; Niederseer, D. Flecainide Induced ST Segment Changes Can Mimic Pathological ECG Changes in Patients Undergoing Exercise Testing: A Case Report. Cardiovasc. Med. 2021, 24, 184. https://doi.org/10.4414/cvm.2021.02178
Kruzik M, Padrutt M, Stehli J, Brunckhorst C, Schmied C, Niederseer D. Flecainide Induced ST Segment Changes Can Mimic Pathological ECG Changes in Patients Undergoing Exercise Testing: A Case Report. Cardiovascular Medicine. 2021; 24(4):184. https://doi.org/10.4414/cvm.2021.02178
Chicago/Turabian StyleKruzik, Matthias, Maria Padrutt, Julia Stehli, Corinna Brunckhorst, Christian Schmied, and David Niederseer. 2021. "Flecainide Induced ST Segment Changes Can Mimic Pathological ECG Changes in Patients Undergoing Exercise Testing: A Case Report" Cardiovascular Medicine 24, no. 4: 184. https://doi.org/10.4414/cvm.2021.02178
APA StyleKruzik, M., Padrutt, M., Stehli, J., Brunckhorst, C., Schmied, C., & Niederseer, D. (2021). Flecainide Induced ST Segment Changes Can Mimic Pathological ECG Changes in Patients Undergoing Exercise Testing: A Case Report. Cardiovascular Medicine, 24(4), 184. https://doi.org/10.4414/cvm.2021.02178



