Bradyarrhythmias
Abstract
Introduction
Clinical presentation
Intrinsic versus extrinsic causes of bradyarrhythmias
Diagnosis
Electrographic features of common bradyarrhythmias
Sinus node disease
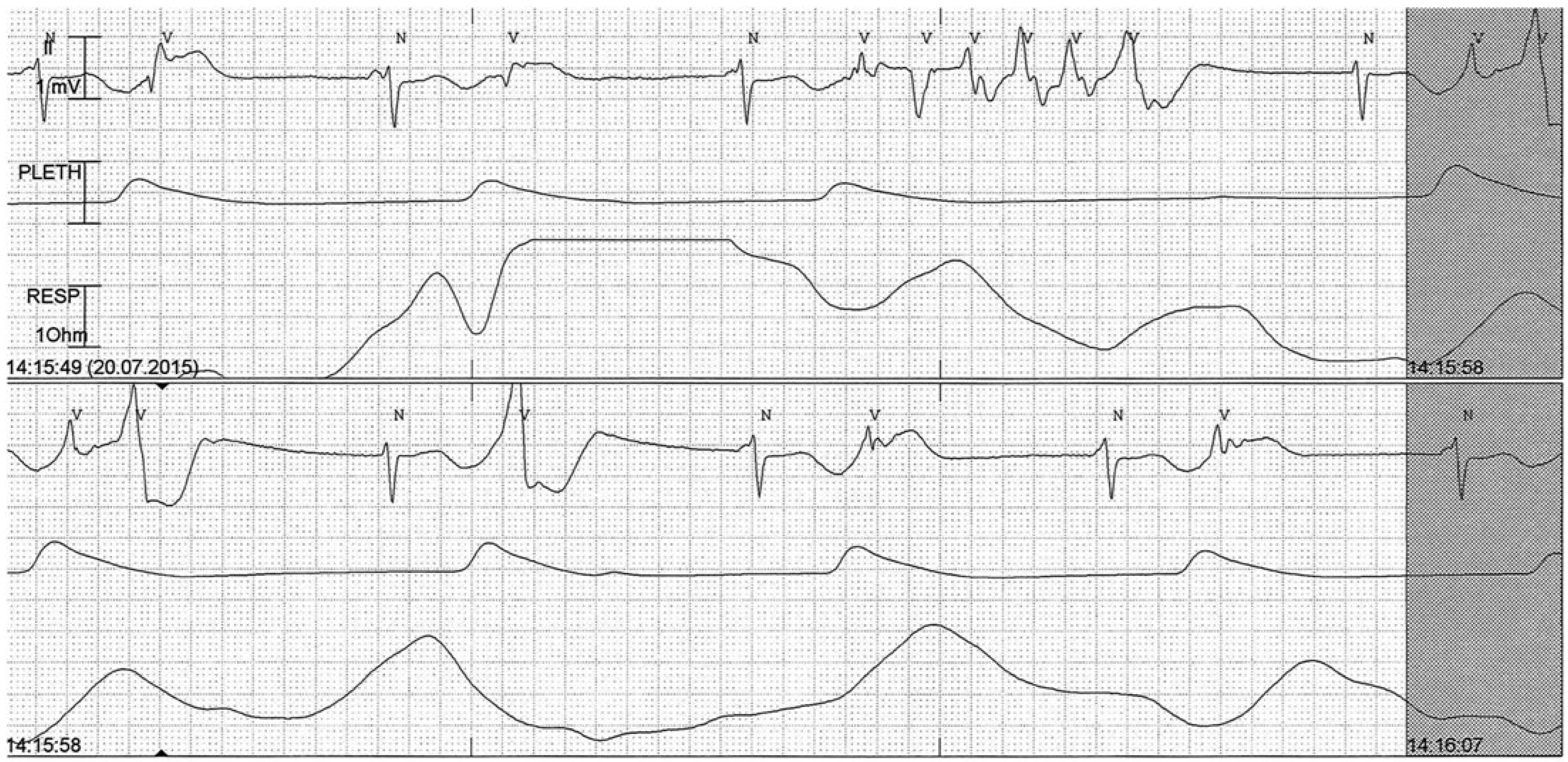
Sinus arrest
Junctional rhythm
Idioventricular rhythm
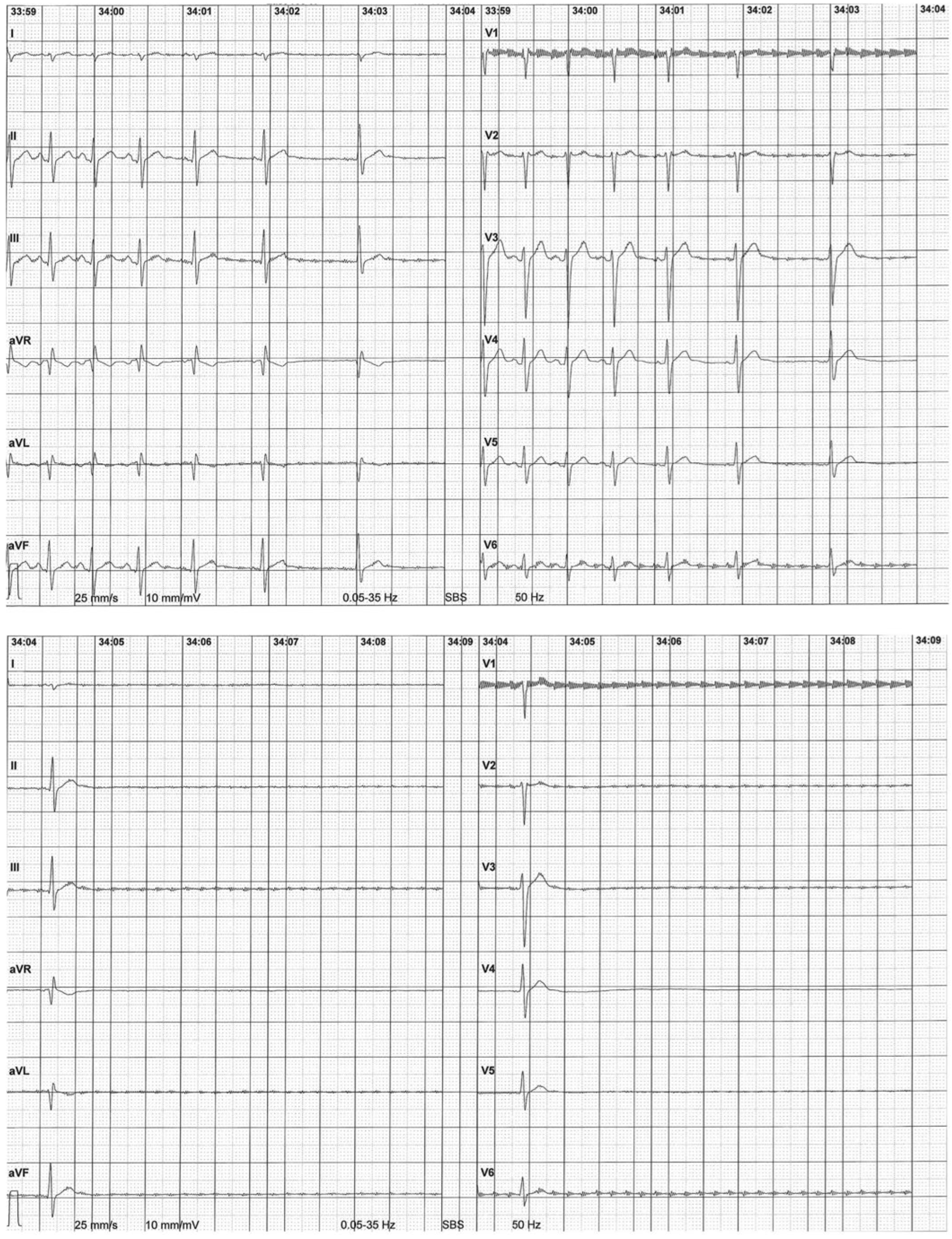
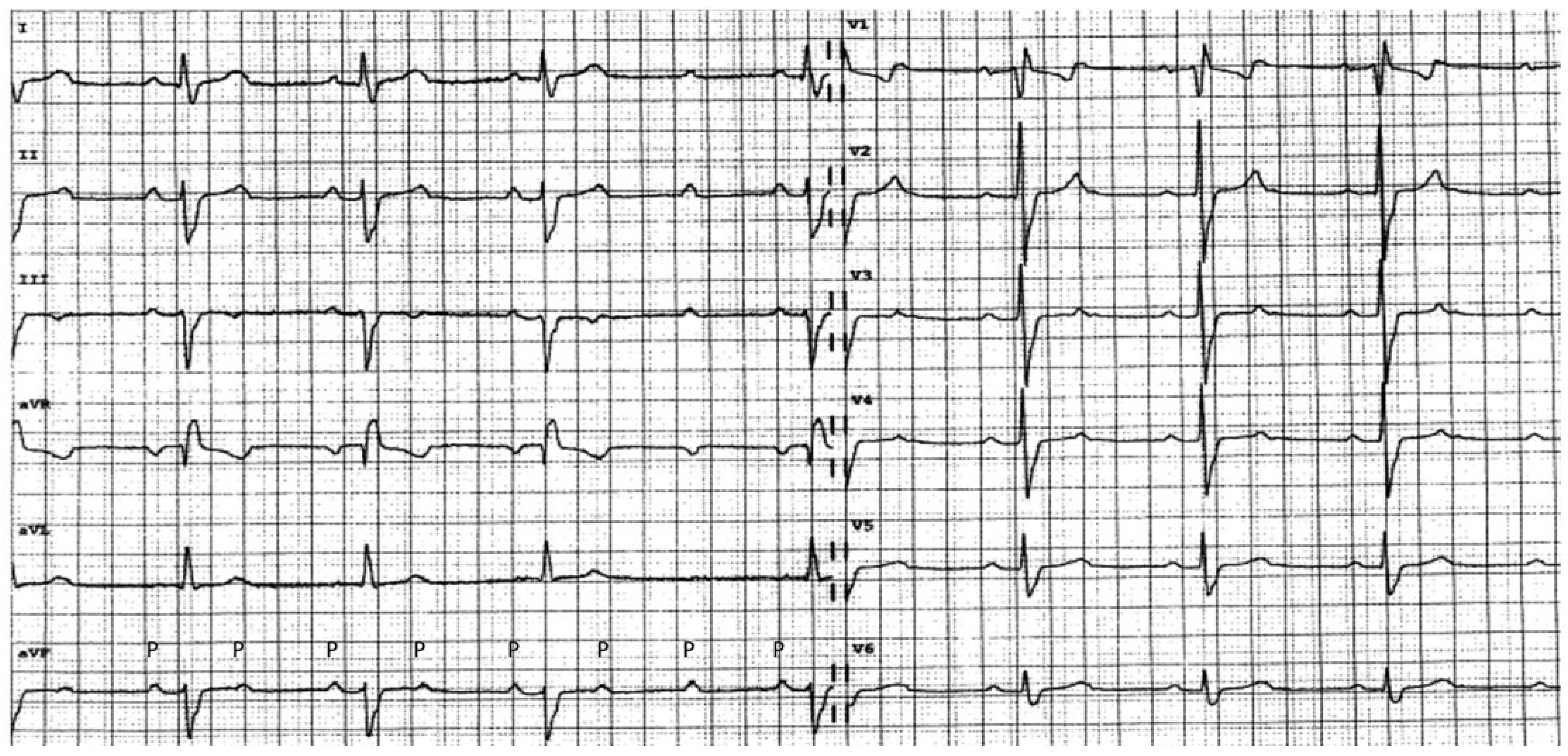
Atrioventricular blocks
Red flags for immediate referral
Indications for pacemaker therapy
Complications of pacemaker therapy
Recent advances in therapy
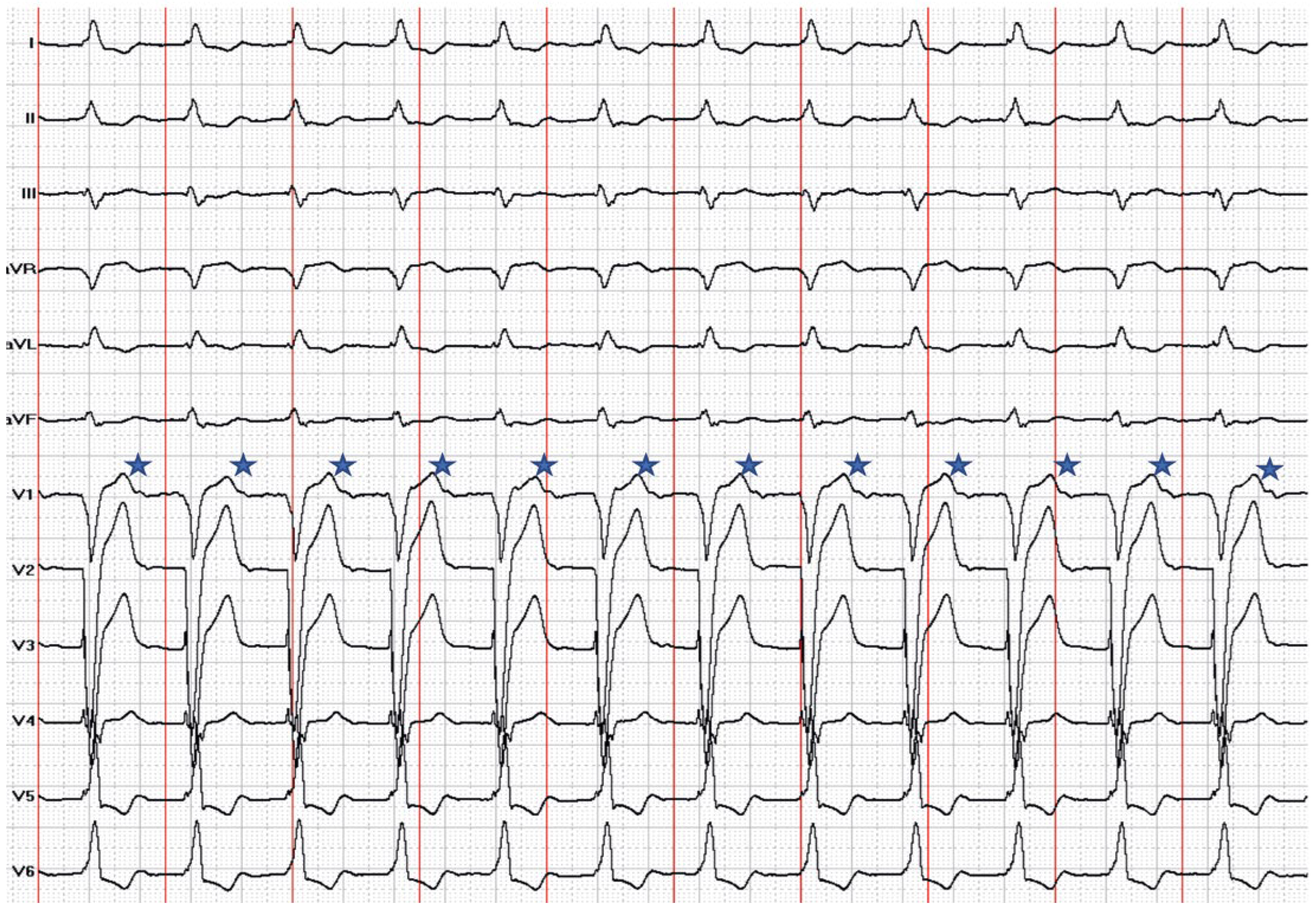
Leadless pacemakers
Battery-less pacemakers
His bundle pacing
Conflicts of Interest
References
- Semelka, M.; Gera, J.; Usman, S. Sick sinus syndrome: a review. Am Fam Physician. 2013, 87, 691–6. [Google Scholar] [PubMed]
- Brignole, M.; Auricchio, A.; Baron Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.A.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013, 34, 2281–329. [Google Scholar] [PubMed]
- Lamas, G.A.; Lee, K.L.; Sweeney, M.O.; Silverman, R.; Leon, A.; Yee, R.; et al. Ventricular pacing or dual chamber pacing for sinus node dysfunction. N Engl J Med. 2002, 346, 1854–62. [Google Scholar] [CrossRef] [PubMed]
- Nof, E.; Glikson, M.; Antzelevitch, C. Genetics and Sinus Node Dysfunction. J Atr Fibrillation. 2009, 1, 328–36. [Google Scholar]
- Huston, T.P.; Puffer, J.C.; Rodney, W.M. The athletic heart syndrome. N Engl J Med. 1985, 313, 24–32. [Google Scholar] [CrossRef]
- Shen, W.K.; Sheldon, R.S.; Benditt, D.G.; Cohen, M.I.; Forman, D.E.; Goldberger, Z.D.; et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2017, 70, 620–63. [Google Scholar] [CrossRef]
- Epstein, A.E.; DiMarco, J.P.; Ellenbogen, K.A.; Estes, N.A., 3rd; Freedman, R.A.; Gettes, L.S.; et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013, 127, e283–352. [Google Scholar]
- Israel, C.W.; Ekosso Ejangue, L. Problems, complications, and emergencies during pacemaker implantation. Importance of access. Herzschrittmacherther Elektrophysiol. 2015, 26, 309–19. [Google Scholar] [CrossRef]
- Lin, Y.S.; Hung, S.P.; Chen, P.R.; Yang, C.H.; Wo, H.T.; Chang, P.C.; et al. Risk factors influencing complications of cardiac implantable electronic device implantation: infection, pneumothorax and heart perforation: a nationwide population based cohort study. Medicine (Baltimore). 2014, 93, e213. [Google Scholar] [CrossRef]
- Roberts, P.R.; Clementy, N.; Al Samadi, F.; Garweg, C.; Martinez Sande, J.L.; Iacopino, S.; et al. A leadless pacemaker in the real world setting: The Micra Transcatheter Pacing System Post Approval Registry. Heart rhythm. 2017, 14, 1375–9. [Google Scholar] [CrossRef]
- Duray, G.Z.; Ritter, P.; El Chami, M.; Narasimhan, C.; Omar, R.; Tolosana, J.M.; et al. Long term performance of a transcatheter pacing system: 12 Month results from the Micra Transcatheter Pacing Study. Heart rhythm. 2017, 14, 702–9. [Google Scholar] [CrossRef]
- Chinitz, L.; Ritter, P.; Khelae, S.K.; Iacopino, S.; Garweg, C.; Grazia Bongiorni, M.; et al. Accelerometer based atrioventricular synchronous pacing with a ventricular leadless pacemaker: Results from the Micra atrioventricular feasibility studies. Heart rhythm. 2018.
- Dagdeviren, C.; Yang, B.D.; Su, Y.; Tran, P.L.; Joe, P.; Anderson, E.; et al. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc Natl Acad Sci U S A. 2014, 111, 1927–32. [Google Scholar] [CrossRef] [PubMed]
- Zurbuchen, A.; Haeberlin, A.; Bereuter, L.; Wagner, J.; Pfenniger, A.; Omari, S.; et al. The Swiss approach for a heartbeat driven leadand batteryless pacemaker. Heart rhythm. 2017, 14, 294–9. [Google Scholar] [CrossRef] [PubMed]
- Pfenniger, A.; Vogel, R.; Koch, V.M.; Jonsson, M. Performance analysis of a miniature turbine generator for intracorporeal energy harvesting. Artif Organs. 2014, 38, E68–81. [Google Scholar] [CrossRef]
- Bereuter, L.; Williner, S.; Pianezzi, F.; Bissig, B.; Buecheler, S.; Burger, J.; et al. Energy Harvesting by Subcutaneous Solar Cells: A Long Term Study on Achievable Energy Output. Ann Biomed Eng. 2017, 45, 1172–80. [Google Scholar] [CrossRef]
- Protze, S.I.; Liu, J.; Nussinovitch, U.; Ohana, L.; Backx, P.H.; Gepstein, L.; et al. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat Biotechnol. 2017, 35, 56–68. [Google Scholar] [CrossRef]
- Wilkoff, B.L.; Cook, J.R.; Epstein, A.E.; Greene, H.L.; Hallstrom, A.P.; Hsia, H.; et al. Dual chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002, 288, 3115–23. [Google Scholar]
- Sweeney, M.O.; Hellkamp, A.S.; Ellenbogen, K.A.; Greenspon, A.J.; Freedman, R.A.; Lee, K.L.; et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003, 107, 2932–7. [Google Scholar] [CrossRef]
- Sweeney, M.O.; Hellkamp, A.S. Heart failure during cardiac pacing. Circulation. 2006, 113, 2082–8. [Google Scholar]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002, 346, 1845–53. [Google Scholar]
- Cleland, J.G.; Daubert, J.C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005, 352, 1539–49. [Google Scholar] [CrossRef]
- Deshmukh, P.; Casavant, D.A.; Romanyshyn, M.; Anderson, K. Permanent, direct His bundle pacing: a novel approach to cardiac pacing in patients with normal His Purkinje activation. Circulation. 2000, 101, 869–77. [Google Scholar] [CrossRef] [PubMed]
- Kronborg, M.B.; Mortensen, P.T.; Poulsen, S.H.; Gerdes, J.C.; Jensen, H.K.; Nielsen, J.C. His or para His pacing preserves left ventricular function in atrioventricular block: a double blind, randomized, crossover study. Europace: European pacing, arrhythmias, and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2014, 16, 1189–96. [Google Scholar] [PubMed]
- Vijayaraman, P.; Naperkowski, A.; Ellenbogen, K.A.; Dandamudi, G. Electrophysiologic Insights Into Site of Atrioventricular Block: Lessons From Permanent His Bundle Pacing. JACC Clin Electrophysiol. 2015, 1, 571–81. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, D.L.; Crespo, E.M.; Arkhipova Jenkins, I.; Lobel, R.; Winget, J.; Koehler, J.; et al. His bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Heart rhythm. 2015, 12, 1548–57. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Subzposh, F.A.; Beer, D.; Durr, B.; Naperkowski, A.; Sun, H.; et al. Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing. Journal of the American College of Cardiology. 2018, 71, 2319–30. [Google Scholar] [CrossRef]

| Intrinsic causes | Sinus node dysfunction | AV node dysfunction |
| Ion channel dysfunction | + | |
| Ischaemic fibrosis | + | + |
| Heart failure | + | + |
| Infiltrative disease (amyloid heart disease, haemochromatosis, sarcoidosis) | + | + |
| Aging-related fibrosis of the sinoatrial node | + | + |
| Congenital | + | |
| Post-radiation fibrosis | + | |
| Inflammatory conditions (Chagas, Lyme disease, myocarditis, bacterial endocarditis, etc.) | + | |
| Autonomic dysfunction | + | + |
| Extrinsic causes | ||
| Drugs | + | + |
| Obstructive sleep apnoea | + | + |
| Intoxication | + | + |
| Hypothyroidism | + | + |
| Electrolyte abnormalities (e.g. hyperkalaemia hypocalcaemia) , | + | + |
| Neurally mediated conditions | + | + |
| Heart surgery (heart transplantation, valve surgery) | + | + |
| Interventions (TAVI, RF ablation, TASH) | + | |
| Intracranial hypertension | + | + |
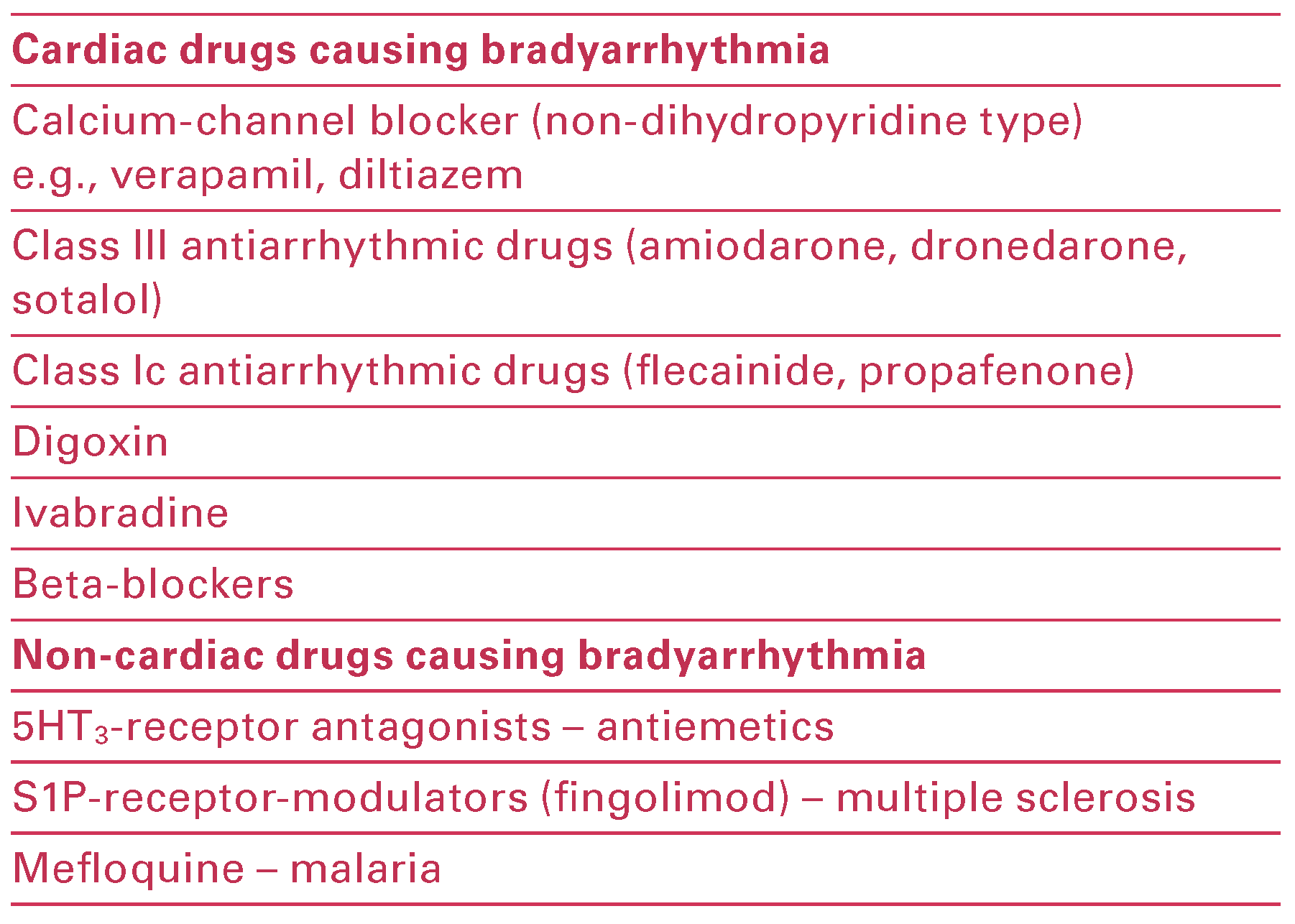 |
 |
© 2018 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Weberndörfer, V.; Russi, I.; Kobza, R. Bradyarrhythmias. Cardiovasc. Med. 2018, 21, 218. https://doi.org/10.4414/cvm.2018.00580
Weberndörfer V, Russi I, Kobza R. Bradyarrhythmias. Cardiovascular Medicine. 2018; 21(9):218. https://doi.org/10.4414/cvm.2018.00580
Chicago/Turabian StyleWeberndörfer, Vanessa, Ian Russi, and Richard Kobza. 2018. "Bradyarrhythmias" Cardiovascular Medicine 21, no. 9: 218. https://doi.org/10.4414/cvm.2018.00580
APA StyleWeberndörfer, V., Russi, I., & Kobza, R. (2018). Bradyarrhythmias. Cardiovascular Medicine, 21(9), 218. https://doi.org/10.4414/cvm.2018.00580




