Kardiomyopathie bei Morbus Fabry
Abstract
Einleitung
Was ist Morbus Fabry?
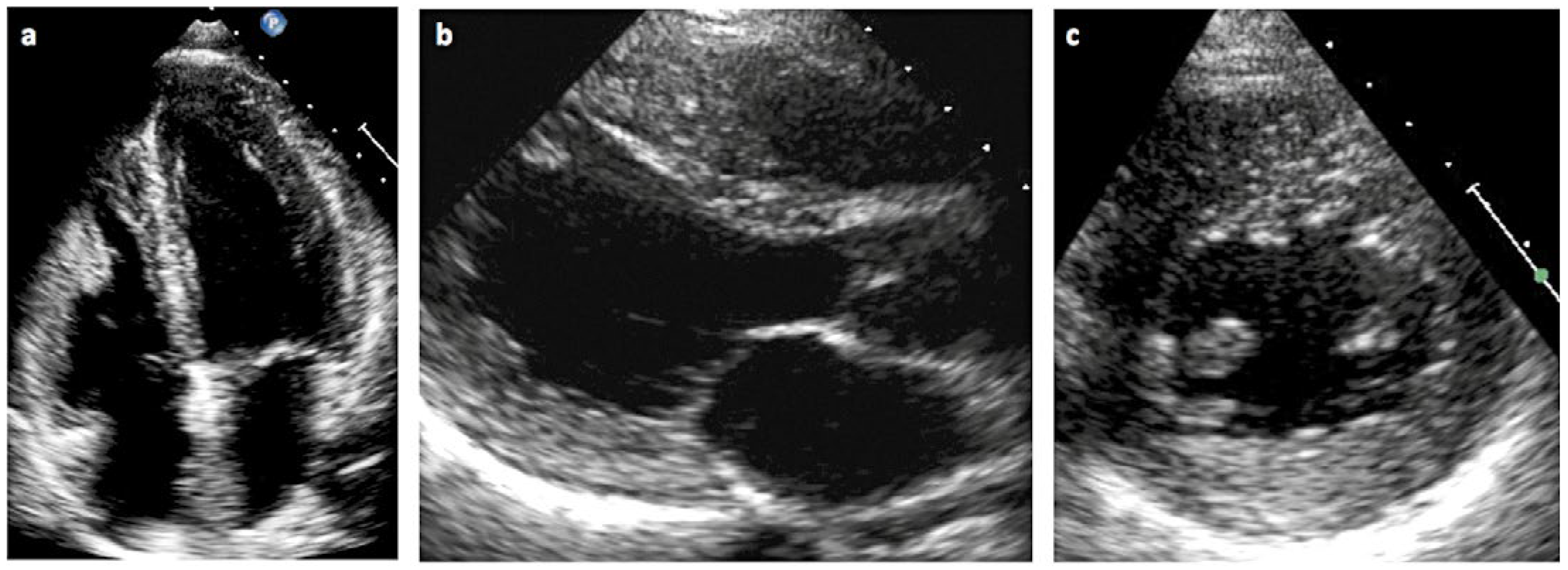
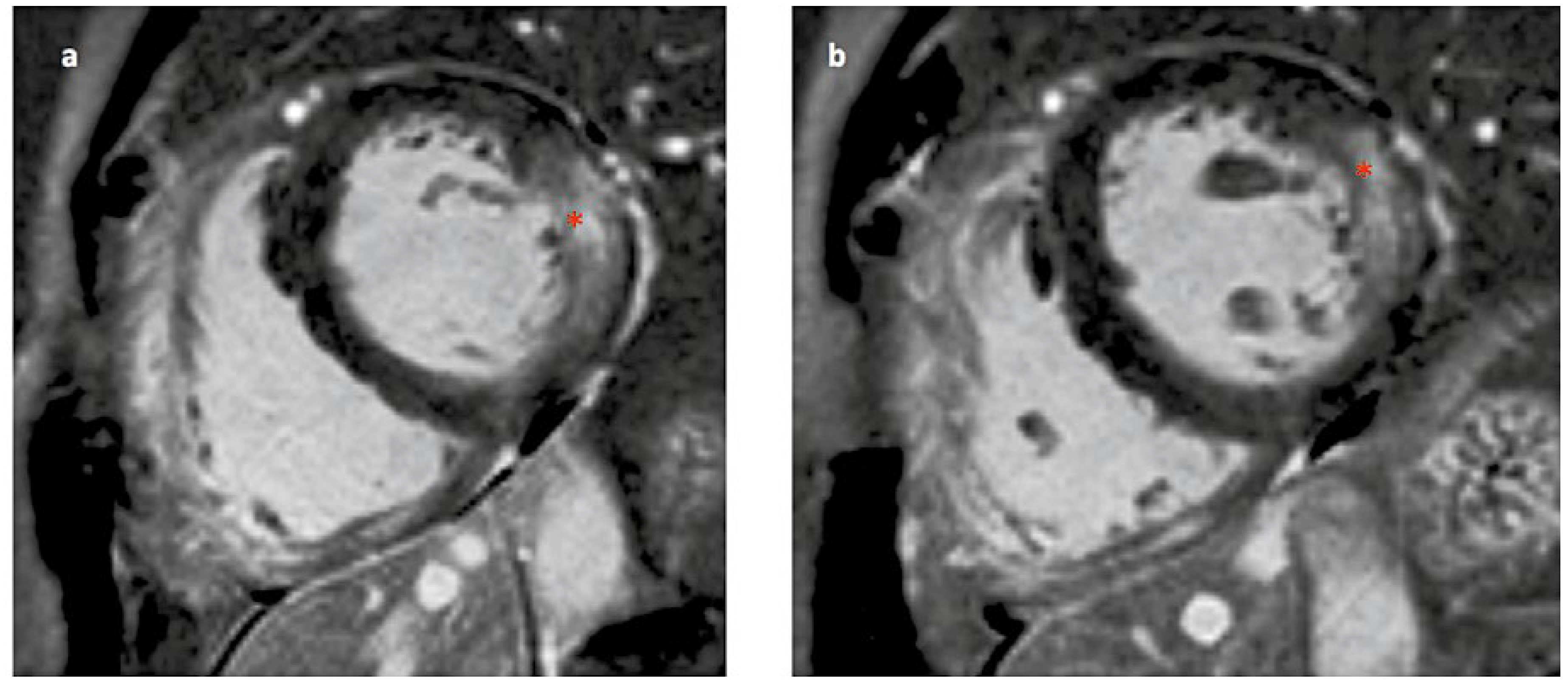
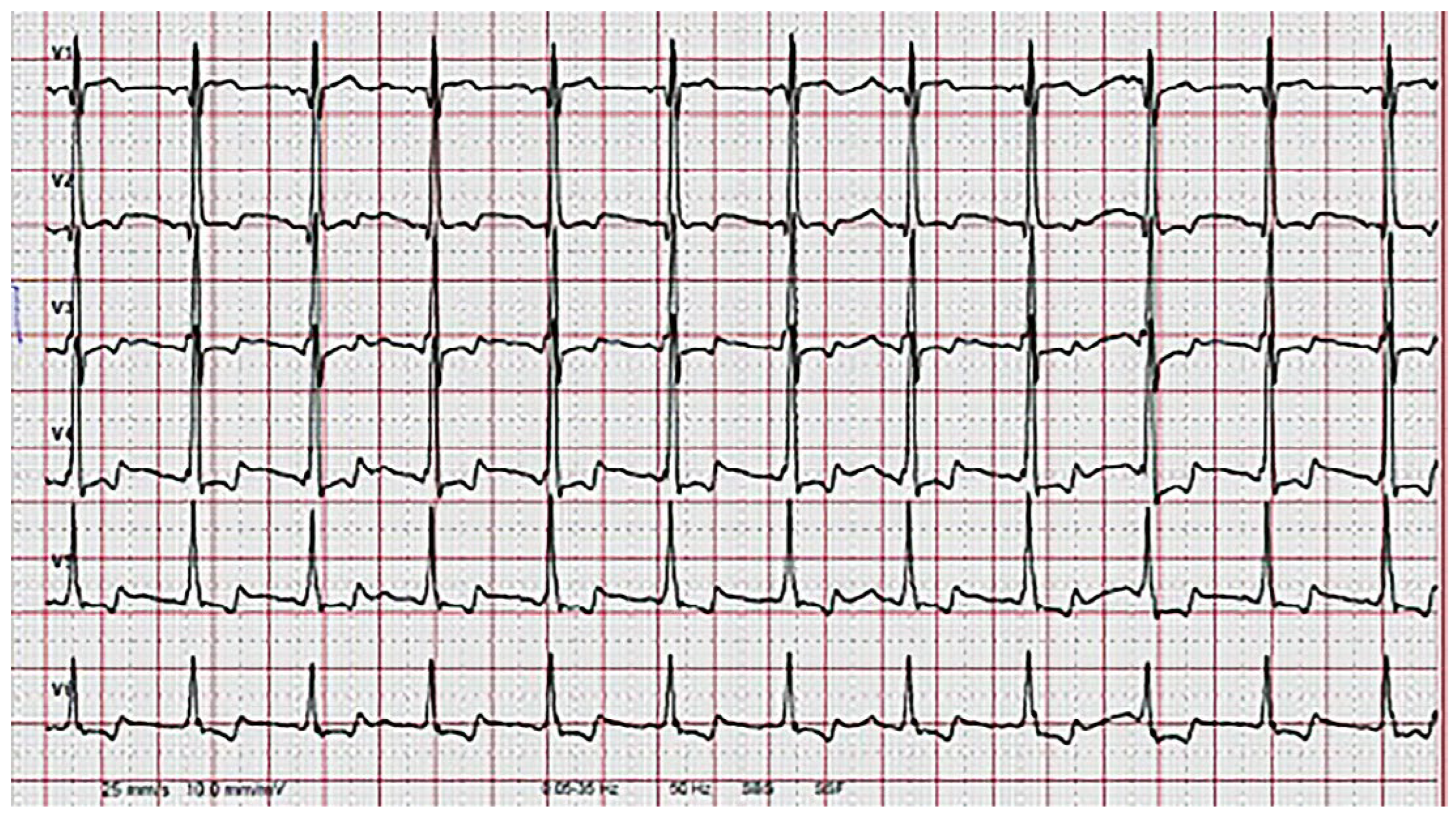
Wie manifestiert sich die Kardiomyopathie beim Morbus Fabry?
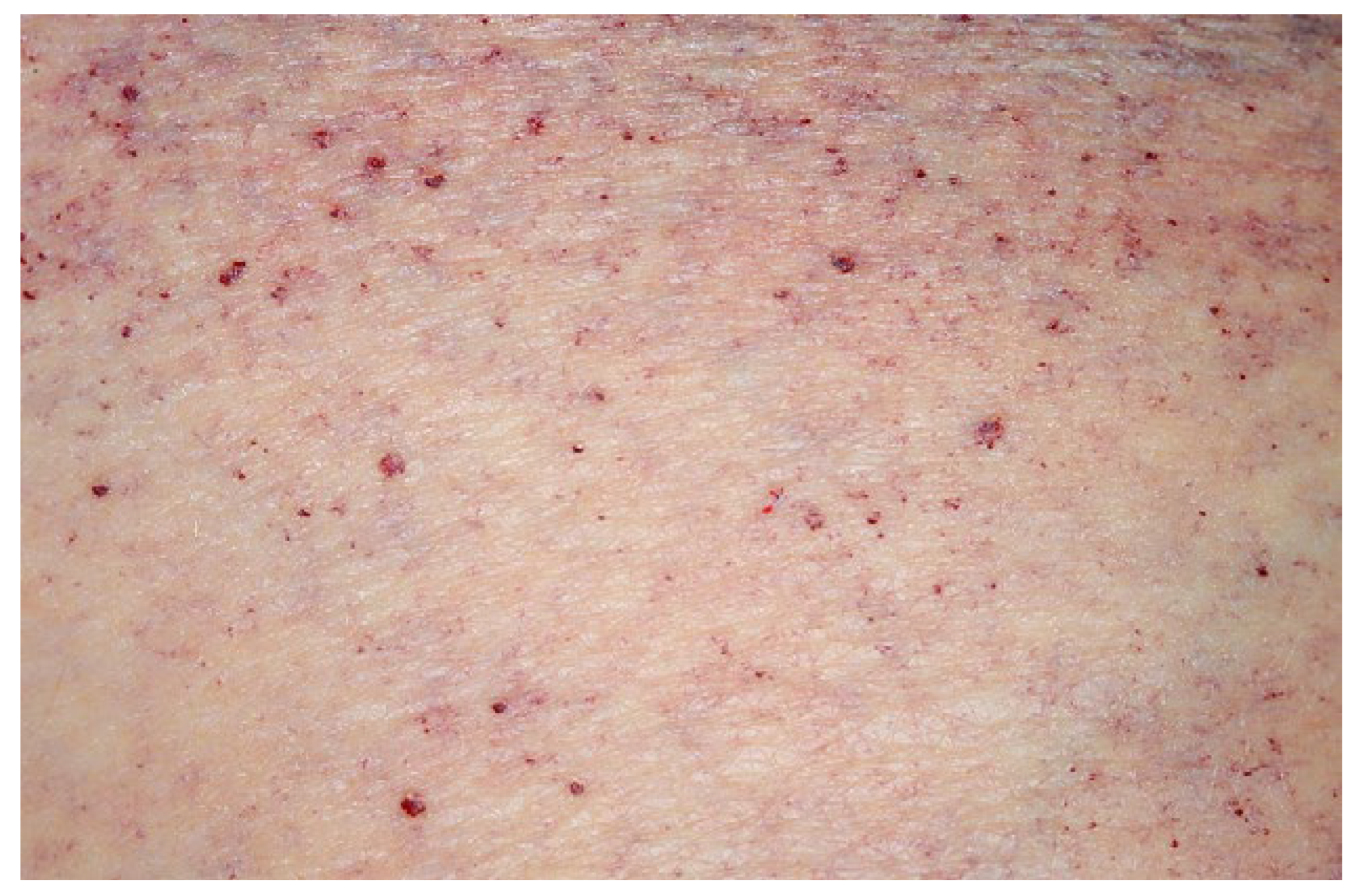
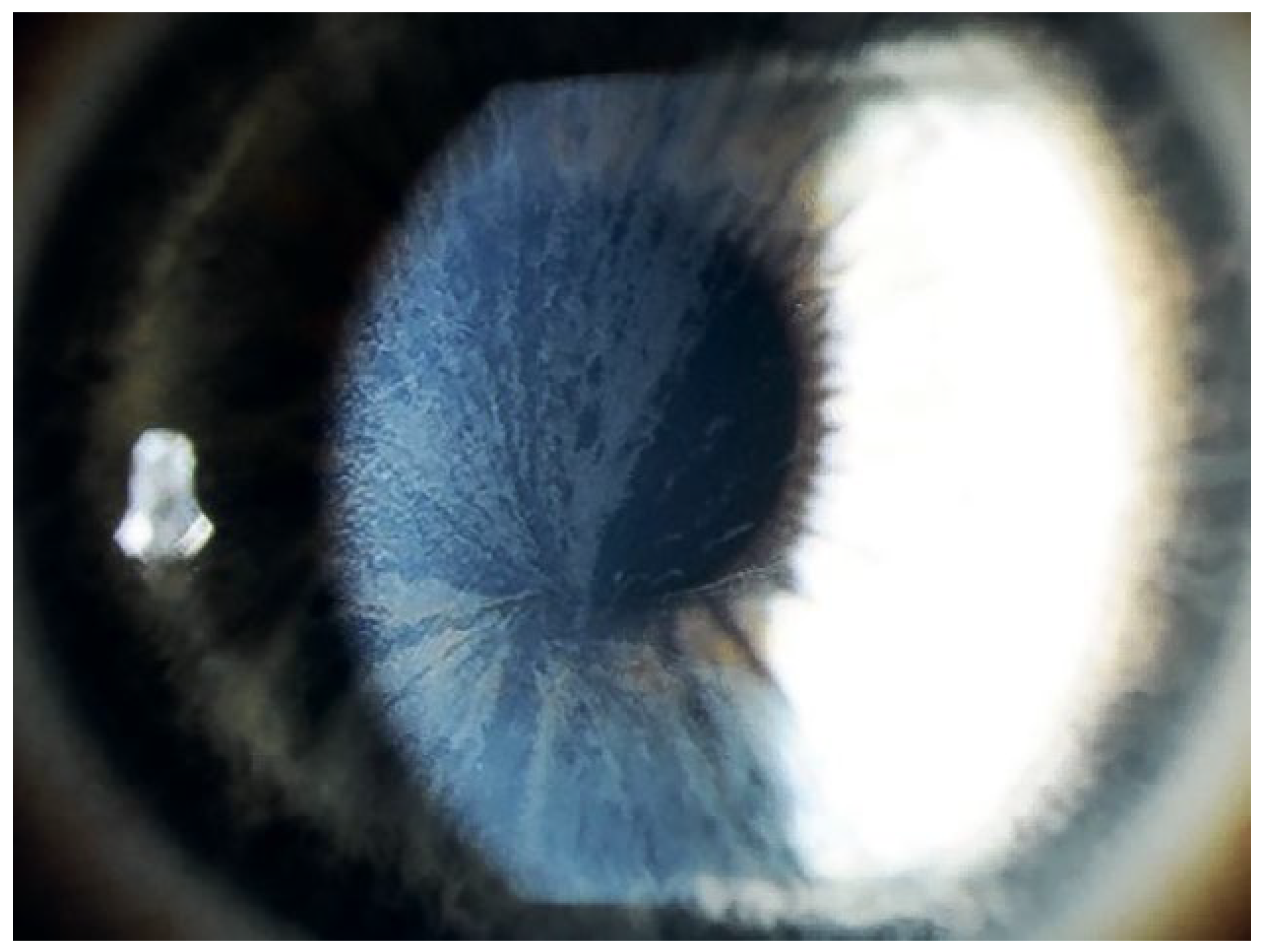
Welche anderen Organe sind betroffen?
Wie diagnostiziert man Morbus Fabry?
Wie therapiert man Morbus Fabry?
Take-Home-Message
- -
- Charakteristische EKG-Veränderungen und/oder echokardiografische veränderungen im Sinne einer linksventrikulären Hypertrophie können Manifestationen eines Morbus Fabry sein.
- -
- Zur Diagnose eignet sich bei Männern die Bestimmung der Alpha-Galactosidase-A-Aktivität. Eine normale Alpha-Galaktosidase-A-Aktivität schliesst hingegen bei Frauen einen Morbus Fabry nicht aus. Bei Frauen wird deshalb eine Gensequenzierung des GLA-Gens durchgeführt.
- -
- Eine frühzeitige kausale Enzymersatztherapie scheint essentiell für denTherapieerfolg zu sein.
Acknowledgments
References
- Brady, R.O.; Gal, A.E.; Bradley, R.M.; Martensson, E.; Warshaw, A.L.; Laster, L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967, 276, 1163–1167. [Google Scholar] [CrossRef]
- Desnick, R.J.; Ioannou, Y.A.; Eng, C.M. Disease. In The Online Metabolic and Molecular Bases of Inherited Disease; Beaudet, A.L., Vogelstein, B., Kinzler, K.W., Antonarakis, S.E., Ballabio, A., Gibson, K.M., et al., Eds.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2014. [Google Scholar]
- Shu, L.; Shayman, J.A. Caveolin-associated accumulation of globotriaosylceramide in the vascular endothelium of alpha-galactosidase A null mice. J Biol Chem. 2007, 282, 20960–20967. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, J.A.; Na, H.Y.; Cho, S.E.; Park, S.; Jung, S.C.; et al. Globotriaosylceramide induces lysosomal degradation of endothelial KCa3.1 in fabry disease. Arterioscler Thromb Vasc Biol. 2014, 34, 81–89. [Google Scholar] [CrossRef]
- Namdar, M.; Gebhard, C.; Studiger, R.; Shi, Y.; Mocharla, P.; Schmied, C.; et al. Globotriaosylsphingosine accumulation and not alpha-galactosidase-A deficiency causes endothelial dysfunction in Fabry disease. PLoS ONE 2012, 7, e36373. [Google Scholar] [CrossRef]
- Askari, H.; Kaneski, C.R.; Semino-Mora, C.; Desai, P.; Ang, A.; Kleiner, D.E.; et al. Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Arch. 2007, 451, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Clarke, J.T.; Giugliani, R.; Elliott, P.; Linhart, A.; Beck, M.; et al. Natural course of Fabry disease: changing pattern of causes of death in FOS - Fabry Outcome Survey. J Med Genet. 2009, 46, 548–552. [Google Scholar] [CrossRef] [PubMed]
- von Scheidt, W.; Eng, C.M.; Fitzmaurice, T.F.; Erdmann, E.; Hubner, G.; Olsen, E.G.; et al. An atypical variant of Fabry’s disease with manifestations confined to the myocardium. N Engl J Med. 1991, 324, 395–399. [Google Scholar] [CrossRef]
- Shabbeer, J.; Yasuda, M.; Benson, S.D.; Desnick, R.J. Fabry disease: identification of 50 novel alpha-galactosidase A mutations causing the classic phenotype and three-dimensional structural analysis of 29 missense mutations. Hum Genomics. 2006, 2, 297–309. [Google Scholar] [CrossRef]
- Nowak, A.; Mechtler, T.P.; Hornemann, T.; Gawinecka, J.; Theswet, E.; Hilz, M.J.; et al. Genotype, phenotype and disease severity reflected by serum LysoGb3 levels in patients with Fabry disease. Molecular genetics and metabolism. 2017. [Google Scholar] [CrossRef]
- Eng, C.M.; Fletcher, J.; Wilcox, W.R.; Waldek, S.; Scott, C.R.; Sillence, D.O.; et al. Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. Journal of inherited metabolic disease. 2007, 30, 184–192. [Google Scholar] [CrossRef]
- Desnick, R.J.; Brady, R.; Barranger, J.; Collins, A.J.; Germain, D.P.; Goldman, M.; et al. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003, 138, 338–346. [Google Scholar] [CrossRef] [PubMed]
- MacDermot, K.D.; Holmes, A.; Miners, A.H. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet. 2001, 38, 750–760. [Google Scholar] [CrossRef]
- Linhart, A.; Kampmann, C.; Zamorano, J.L.; Sunder-Plassmann, G.; Beck, M.; Mehta, A.; et al. Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry outcome survey. Eur Heart J. 2007, 28, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- MacDermot, K.D.; Holmes, A.; Miners, A.H. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001, 38, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Bach, G.; Rosenmann, E.; Karni, A.; Cohen, T. Pseudodeficiency of alpha-galactosidase A. Clin Genet. 1982, 21, 59–64. [Google Scholar] [CrossRef]
- Romeo, G.; D’Urso, M.; Pisacane, A.; Blum, E.; De Falco, A.; Ruffilli, A. Residual activity of alpha-galactosidase A in Fabry’s disease. Biochem Genet. 1975, 13, 615–628. [Google Scholar] [CrossRef]
- Putko, B.N.; Wen, K.; Thompson, R.B.; Mullen, J.; Shanks, M.; Yogasundaram, H.; et al. Anderson-Fabry cardiomyopathy: prevalence, pathophysiology, diagnosis and treatment. Heart Fail Rev. 2015, 20, 179–191. [Google Scholar] [CrossRef]
- European medicines agency - Science medicines health: Fabrazyme - agalsidase beta. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000370/human_med_000784.jsp&mid=WC0b01ac058001d124.
- European medicines agency - Science medicines health: Replagal - Agalsidase alfa. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000369/human_med_001029.jsp&mid=WC0b01ac058001d124.
- Germain, D.P.; Hughes, D.A.; Nicholls, K.; Bichet, D.G.; Giugliani, R.; Wilcox, W.R.; et al. Treatment of Fabry’s Disease with the Pharmacologic Chaperone Migalastat. N Engl J Med. 2016, 375, 545–555. [Google Scholar] [CrossRef]
- Huang, J.; Khan, A.; Au, B.C.; Barber, D.L.; Lopez-Vasquez, L.; Prokopishyn, N.L.; et al. Lentivector Iterations and Pre-Clinical Scale-Up/Toxicity Testing: Targeting Mobilized CD34(+) Cells for Correction of Fabry Disease. Mol Ther Methods Clin Dev. 2017, 5, 241–258. [Google Scholar] [CrossRef]
- Guerard, N.; Morand, O.; Dingemanse, J. Lucerastat, an iminosugar with potential as substrate reduction therapy for glycolipid storage disorders: safety, tolerability, and pharmacokinetics in healthy subjects. Orphanet journal of rare diseases. 2017, 12, 9. [Google Scholar] [CrossRef]
- Spada, M.; Pagliardini, S.; Yasuda, M.; Tukel, T.; Thiagarajan, G.; Sakuraba, H.; et al. High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet. 2006, 79, 31–40. [Google Scholar] [CrossRef]
- Eng, C.M.; Desnick, R.J. Molecular basis of Fabry disease: mutations and polymorphisms in the human alpha-galactosidase A gene. Hum Mutat. 1994, 3, 103–111. [Google Scholar] [CrossRef]
- Vedder, A.C.; Strijland, A.; vd Bergh Weerman, M.A.; Florquin, S.; Aerts, J.M.; Hollak, C.E. Manifestations of Fabry disease in placental tissue. Journal of inherited metabolic disease. 2006, 29, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Redonnet-Vernhet, I.; Ploos van Amstel, J.K.; Jansen, R.P.; Wevers, R.A.; Salvayre, R.; Levade, T. Uneven X inactivation in a female monozygotic twin pair with Fabry disease and discordant expression of a novel mutation in the alpha-galactosidase A gene. J Med Genet. 1996, 33, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Romeo, G.; Migeon, B.R. Genetic inactivation of the alpha-galactosidase locus in carriers of Fabry’s disease. Science. 1970, 170, 180–181. [Google Scholar] [CrossRef]
- Echevarria, L.; Benistan, K.; Toussaint, A.; Dubourg, O.; Hagege, A.A.; Eladari, D.; et al. X chromosome inactivation in female patients with Fabry disease. Clin Genet. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ferrans, V.J.; Hibbs, R.G.; Burda, C.D. The heart in Fabry’s disease. A histochemical and electron microscopic study. Am J Cardiol. 1969, 24, 95–110. [Google Scholar] [CrossRef]
- Strotmann, J.; Weidemann, F.; Breunig, F.; Knoll, A.; Wanner, C.; Ertl, G. Morbus Fabry of the heart. Why should cardiologists care? Z Kardiol. 2005, 94, 557–563. [Google Scholar] [CrossRef]
- Elleder, M.; Bradova, V.; Smid, F.; Budesinsky, M.; Harzer, K.; Kustermann-Kuhn, B.; et al. Cardiocyte storage and hypertrophy as a sole manifestation of Fabry’s disease. Report on a case simulating hypertrophic non-obstructive cardiomyopathy. Virchows Arch A Pathol Anat Histopathol. 1990, 417, 449–455. [Google Scholar] [CrossRef]
- Wu, J.C.; Ho, C.Y.; Skali, H.; Abichandani, R.; Wilcox, W.R.; Banikazemi, M.; et al. Cardiovascular manifestations of Fabry disease: relationships between left ventricular hypertrophy, disease severity, and alpha-galactosidase A activity. Eur Heart J. 2010, 31, 1088–1097. [Google Scholar] [CrossRef]
- Aerts, J.M.; Groener, J.E.; Kuiper, S.; Donker-Koopman, W.E.; Strijland, A.; Ottenhoff, R.; et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. 2008, 105, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Seydelmann, N.; Wanner, C.; Stork, S.; Ertl, G.; Weidemann, F. Fabry disease and the heart. Best Pract Res Clin Endocrinol Metab. 2015, 29, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Brakch, N.; Dormond, O.; Bekri, S.; Golshayan, D.; Correvon, M.; Mazzolai, L.; et al. Evidence for a role of sphingosine-1 phosphate in cardiovascular remodelling in Fabry disease. Eur Heart J. 2010, 31, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Linhart, A.; Palecek, T.; Bultas, J.; Ferguson, J.J.; Hrudova, J.; Karetova, D.; et al. New insights in cardiac structural changes in patients with Fabry’s disease. Am Heart J. 2000, 139, 1101–1108. [Google Scholar] [CrossRef]
- Nakao, S.; Takenaka, T.; Maeda, M.; Kodama, C.; Tanaka, A.; Tahara, M.; et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med. 1995, 333, 288–293. [Google Scholar] [CrossRef]
- Weidemann, F.; Strotmann, J.M.; Niemann, M.; Herrmann, S.; Wilke, M.; Beer, M.; et al. Heart valve involvement in Fabry cardiomyopathy. Ultrasound Med Biol. 2009, 35, 730–735. [Google Scholar] [CrossRef]
- Weidemann, F.; Breunig, F.; Beer, M.; Sandstede, J.; Stork, S.; Voelker, W.; et al. The variation of morphological and functional cardiac manifestation in Fabry disease: potential implications for the time course of the disease. Eur Heart J. 2005, 26, 1221–1227. [Google Scholar] [CrossRef]
- Shah, J.S.; Hughes, D.A.; Sachdev, B.; Tome, M.; Ward, D.; Lee, P.; et al. Prevalence and clinical significance of cardiac arrhythmia in Anderson-Fabry disease. Am J Cardiol. 2005, 96, 842–846. [Google Scholar] [CrossRef]
- Namdar, M.; Steffel, J.; Vidovic, M.; Brunckhorst, C.B.; Holzmeister, J.; Luscher, T.F.; et al. Electrocardiographic changes in early recognition of Fabry disease. Heart. 2011, 97, 485–490. [Google Scholar] [CrossRef]
- Namdar, M. Electrocardiographic Changes and Arrhythmia in Fabry Disease. Front Cardiovasc Med. 2016, 3, 7. [Google Scholar] [CrossRef]
- Kint, J.A. Fabry’s disease: alpha-galactosidase deficiency. Science. 1970, 167, 1268–1269. [Google Scholar] [CrossRef]
- Mayes, J.S.; Scheerer, J.B.; Sifers, R.N.; Donaldson, M.L. Differential assay for lysosomal alpha-galactosidases in human tissues and its application to Fabry’s disease. Clin Chim Acta. 1981, 112, 247–251. [Google Scholar] [CrossRef]
- Desnick, R.J.; Allen, K.Y.; Desnick, S.J.; Raman, M.K.; Bernlohr, R.W.; Krivit, W. Fabry’s disease: enzymatic diagnosis of hemizygotes and heterozygotes. Alpha-galactosidase activities in plasma, serum, urine, and leukocytes. J Lab Clin Med. 1973, 81, 157–171. [Google Scholar] [PubMed]
- Motabar, O.; Liu, K.; Southall, N.; Marugan, J.J.; Goldin, E.; Sidransky, E.; et al. High throughput screening for inhibitors of alpha-galactosidase. Curr Chem Genomics. 2010, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.L.; Convit, J.; Velazquez-Avila, G. Fabry’s disease: normal alpha-galactosidase activity and urinary-sediment glycosphingolipid levels in two obligate heterozygotes. Br J Dermatol. 1973, 89, 149–157. [Google Scholar] [CrossRef]
- Vedder, A.C.; Linthorst, G.E.; Houge, G.; Groener, J.E.; Ormel, E.E.; Bouma, B.J.; et al. Treatment of Fabry disease: outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg. PLoS ONE 2007, 2, e598. [Google Scholar] [CrossRef] [PubMed]
- Biegstraaten, M.; Arngrimsson, R.; Barbey, F.; Boks, L.; Cecchi, F.; Deegan, P.B.; et al. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: the European Fabry Working Group consensus document. Orphanet J Rare Dis. 2015, 10, 36. [Google Scholar] [CrossRef]
- Eng, C.M.; Guffon, N.; Wilcox, W.R.; Germain, D.P.; Lee, P.; Waldek, S.; et al. Safety and efficacy of recombinant human alpha-galactosidase A--replacement therapy in Fabry’s disease. N Engl J Med. 2001, 345, 9–16. [Google Scholar] [CrossRef]
- Hughes, D.A.; Elliott, P.M.; Shah, J.; Zuckerman, J.; Coghlan, G.; Brookes, J.; et al. Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: a randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart. 2008, 94, 153–158. [Google Scholar] [CrossRef]
- Beer, M.; Weidemann, F.; Breunig, F.; Knoll, A.; Koeppe, S.; Machann, W.; et al. Impact of enzyme replacement therapy on cardiac morphology and function and late enhancement in Fabry’s cardiomyopathy. Am J Cardiol. 2006, 97, 1515–1518. [Google Scholar] [CrossRef]
- Müller, S. Diastolische Dysfunktion bei Morbus Fabry Patienten: Verlauf der Kardiomyopathie unter Enzymersatztherapie. 2017. [Google Scholar]
- Madsen, C.V.; Bundgaard, H.; Rasmussen, A.K.; Sorensen, S.S.; Petersen, J.H.; Kober, L.; et al. Echocardiographic and clinical findings in patients with Fabry disease during long-term enzyme replacement therapy: a nationwide Danish cohort study. Scand Cardiovasc J. 2017, 1–10. [Google Scholar] [CrossRef]
- Nowak, A.; Koch, G.; Huynh-Do, U.; Siegenthaler, M.; Marti, H.P.; Pfister, M. Disease Progression Modeling to Evaluate the Effects of Enzyme Replacement Therapy on Kidney Function in Adult Patients with the Classic Phenotype of Fabry Disease. Kidney Blood Press Res. 2017, 42, 1–15. [Google Scholar] [CrossRef]
- Siegenthaler, M.; Huynh-Do, U.; Krayenbuehl, P.; Pollock, E.; Widmer, U.; Debaix, H.; et al. Impact of cardio-renal syndrome on adverse outcomes in patients with Fabry disease in a long-term follow-up. Int J Cardiol. 2017. [Google Scholar] [CrossRef]
- Lombardo, M.; Alli, C.; Broccolino, M.; Ferrari, S.; Montemurro, L.; Zaini, G.; et al. Long-term effects of angiotensin-converting enzyme inhibitors and calcium antagonists on the right and left ventricles in essential hypertension. Am Heart J. 1997, 134, 557–564. [Google Scholar] [CrossRef]
- Stenson, P.D.; Ball, E.V.; Mort, M.; Phillips, A.D.; Shiel, J.A.; Thomas, N.S.; et al. Human Genetic Mutation Datobase (HGMD): 2003 update. Hum Mutat. 2003, 21, 577–581. [Google Scholar] [CrossRef]

© 2018 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Müller, S.; Tanner, F.C.; Gruner, C.; Ruschitzka, F.; Flammer, A.; Nowak, A. Kardiomyopathie bei Morbus Fabry. Cardiovasc. Med. 2018, 21, 212. https://doi.org/10.4414/cvm.2018.00578
Müller S, Tanner FC, Gruner C, Ruschitzka F, Flammer A, Nowak A. Kardiomyopathie bei Morbus Fabry. Cardiovascular Medicine. 2018; 21(9):212. https://doi.org/10.4414/cvm.2018.00578
Chicago/Turabian StyleMüller, Simone, Felix C. Tanner, Christiane Gruner, Frank Ruschitzka, Andreas Flammer, and Albina Nowak. 2018. "Kardiomyopathie bei Morbus Fabry" Cardiovascular Medicine 21, no. 9: 212. https://doi.org/10.4414/cvm.2018.00578
APA StyleMüller, S., Tanner, F. C., Gruner, C., Ruschitzka, F., Flammer, A., & Nowak, A. (2018). Kardiomyopathie bei Morbus Fabry. Cardiovascular Medicine, 21(9), 212. https://doi.org/10.4414/cvm.2018.00578



