Chagas Heart Disease
Abstract
Introduction
Case description
Patient history
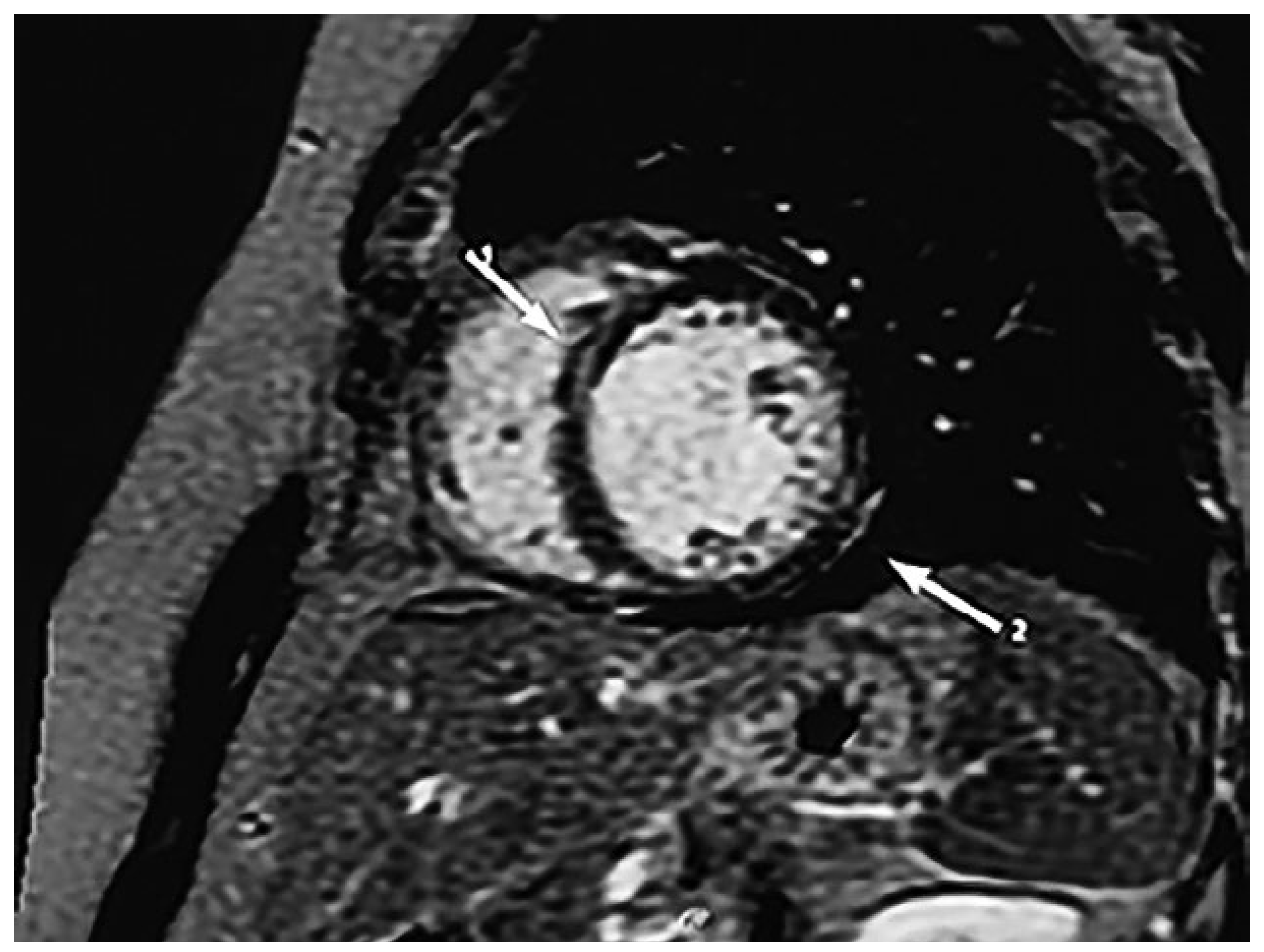
Diagnostic assessment
Treatment
Follow-up and outcome
Discussion
The acute, indeterminate and chronic infection:
Diagnostic evaluation of Chagas disease
Antitrypanosomal treatment
Treatment of cardiac symptoms
Management of ventricular dysfunction and heart failure
Management of bradyarrhythmias
Management of ventricular arrhythmias
Management of chest pain
Management of thromboembolism
Prognostic markers and risk stratification in chronic Chagas heart disease
- Dilated cardiomyopathy is often idiopathic; however searching for specific causes is crucial as therapy may differ from simple heart failure treatment
- Clinicians should be aware of Chagas disease, which has become more relevant due to migration and travel, as a cause for dilated cardiomyopathy in Europe.
- The diagnosis of the chronic disease is established via a compatible clinical presentation (cardiac, digestive or cardio-digestive) and detection of antibodies against T. cruzi antigens with least two different serological tests.
- Acute Chagas disease must be treated with antiparasitic medication. Antiparasitic treatment in chronic Chagas disease depends on the patient’s age and how advanced the disease is.
Author Contributions
Informed Consent Statement
Disclosure Statement
References
- Rassi, A., Jr.; Rassi, A.; Little, W.C. Chagas’ heart disease. Clin Cardiol 2000, 23, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Ries, J.; Komarek, A.; Gottschalk, J.; Brand, B.; Amsler, L.; Jutzi, M.; et al. A Case of Possible Chagas Transmission by Blood Transfusion in Switzerland. Transfus Med Hemother 2016, 43, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Guillermo, M. Chagas cardiomyopathy. E-Journal of Cardiology Practice 2016, 14, N°31. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-14/Chagas-cardiomyopathy (accessed on 13 May 2018).
- Jackson, Y.; Chappuis, F. Chagas disease in Switzerland: History and challenges. Euro Surveill 2011, 16, 19963. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.P.; Badano, L.P.; Marin-Neto, J.A.; Edvardsen, T.; Fernández-Golfín, C.; Bicciarelli-Ducci, C.; et al. Multimodality imaging evaluation of Chagas disease: An expert consensus of Brazilian Cardiovascular Imaging Department (DIC) and the European Association of Cardiovascular Imaging (EACVI). Eur Heart J Cardiovasc Imaging 2018, 19, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Viotti, R.; Vigliano, C.; Lococo, B.; Bertocchi, G.; Petti, M.; Alvarez, M.G.; et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: A nonrandomized trial. Ann Intern Med 2006, 144, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A., Jr.; Rosas, F.; et al. BENEFIT Investigators. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N Engl J Med 2015, 373, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.P.; Marin-Neto, J.A.; Paola, A.A.; Vilas-Boas, F.; Oliveira, G.M.; Bacal, F.; et al. Sociedade Brasileira de Cardiologia. I Diretriz Latino Americana para o Diagnóstico e Tratamento da Cardiopatia Chagásica. Diretriz Latino-Americana para o Diagnóstico e Tratamento da Cardiopatia Chagásica [Latin American guidelines for the diagnosis and treatment of Chagas cardiomyopathy]. Arq Bras Cardiol 2011, 97 (Suppl. 3), 1–48, Article in Protugese. [Google Scholar] [CrossRef] [PubMed]
- Muratore, C.A.; Baranchuk, A. Current and emerging therapeutic options for the treatment of chronic chagasic cardiomyopathy. Vasc Health Risk Manag 2010, 6, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Alves Fagundes, A.; Latado Braga, A.; Pereira Magalhaes, L. Impacto da terapia de ressincronizacao cardíaca na cardiopatía chagásica. Rev Latino Americana MCP y Arritmias 2009, 22, 243. [Google Scholar]
- Bocchi, E.A. Heart transplants for patients with Chagas’ heart disease. Sao Paulo Med J 1995, 113, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Keusch, S.; Stämpfli, S.F.; Hufschmid, U.; Beer, J.H.; Friedli, F.C. Blocking bug - complete atrioventricular block in chronic Chagas disease. Cardiovasc Med 2012, 15, 362–364. [Google Scholar] [CrossRef]
- Haedo, A.H.; Chiale, P.A.; Bandieri, J.D.; Lázzari, J.O.; Elizari, M.V.; Rosenbaum, M.B. Comparative antiarrhythmic efficacy of verapamil, 17-monochloracetylajmaline, mexiletine and amiodarone in patients with severe chagasic myocarditis: Relation with the underlying arrhythmogenic mechanisms. J Am Coll Cardiol 1986, 7, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Posse, R.; Sgammini, H.; Núñez Burgos, J.; Chiale, P.A.; Pastori, J.D.; et al. Estudio clínico multicéntrico comparativeo de la flecainida y la amiodarona en el tratamiento de las arritmias ventriculares asociadas a la cardiopatía chagásica cronica [Comparative multicenter clinical study of flecainide and amiodarone in the treatment of ventricular arrhythmias associated with chronic Chagas cardiopathy]. Arch Inst Cardiol Mex 1987, 57, 325–330, Article in Spanish. [Google Scholar] [PubMed]
- Gascón, J.; Albajar, P.; Cañas, E.; Flores, M.; Gómez i Prat, J.; Herrera, R.N.; et al. Diagnóstico, manejo y tratamiento de la cardiopatía chagásica crónica en áreas donde la infección por Trypanosoma cruzi no es endémica [Diagnosis, management and treatment of chronic Chagas’ heart disease in areas where Trypanosoma cruzi infection is not endemic]. Rev Esp Cardiol 2007, 60, 285–293, Article in Spanish. [Google Scholar] [PubMed]
- Rassi, A., Jr.; Rassi, A.; Little, W.C.; Xavier, S.S.; Rassi, S.G.; Rassi, A.G.; et al. Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med 2006, 355, 799–808. [Google Scholar] [CrossRef] [PubMed]
| Reference value | Patient’s value | |
|---|---|---|
| Haematology | ||
| Haemoglobin (g/l) | 120–160 | 145 |
| Mean corpuscular volume (fl) | 85–101 | 87 |
| Mean corpuscular haemoglobin (pg) | 28–33 | 30 |
| Mean corpuscular haemoglobin concentration (g/l) | 300–360 | 338 |
| Leucocyte count (G/l) | 3.5–10.0 | 5.8 |
| Platelet count (G/l) | 140–360 | 189 |
| Erythrocyte sedimation rate (mm/h) | <10 | 8 |
| Chemistry studies | ||
| Sodium (mmol/l) | 136–145 | 138 |
| Potassium (mmol/l) | 3.6–5.1 | 4.1 |
| Creatinine (µmol/l) | 49–90 | 70 |
| Urea (mmol/l) | 2.5–6.7 | 3.1 |
| C-reactive protein (mg/l) | <5.1 | 11.3+ |
| Troponin I high-sensitive (ng/l) | <26.3 | <10 |
| Creatinine kinase (U/l)) | 29–168 | 78 |
| Brain natriuretic peptide (ng/l) | <111 | 815+ |
| Alanine aminotransferase (U/l) | <35 | 44+ |
| Aspartate aminotransferase (U/l) | 5–31 | 33+ |
| Glycated haemoglobin (%) | <6% | 5.2 |
| Thyroid stimulating hormone (mU/l) | 0.35–4.94 | 11.60+ |
| Free tri-iodothyronine (fT3) (pmol/l) | 2.6–5.7 | 3.8 |
| Free thyroxine (fT4) (pmol/l) | 9–19 | 12 |
| Ferritin (μg/l) | 50–200 | 60 |
| Angiotensin converting enzyme (U/l) | 20–70 | 68 |
| Antinuclear antibodies (ANA) | <80 | <80 |
| p-ANCA (antineutrophil cytoplasmic antibodies) | <20 | <20 |
| c-ANCA | <20 | <20 |
| Serology | ||
| Anti-HBs (hepatitis B) | Negative | Negative |
| Anti-HBc (hepatitis B) | Negative | Negative |
| Anti-HCV (hepatitis C) | Negative | Negative |
| Anti-HIV 1 + 2 / p24-antigene | Negative | Negative |
| Anti-Treponema IgG | Negative | Negative |
| Anti-Treponema IgM | Negative | Negative |
| Anti-Trypanosoma (Chagas) ELISA | <0.30 | 1.66+ |
| Anti-Trypanosoma (Chagas) IFAT | <160 | 1280+ |
| Predictors of mortality | Points |
|---|---|
| New York Heart Association functional class III or IV | 5 |
| Cardiomegaly on chest radiography | 5 |
| Segmental or global wall motion abnormality | 3 |
| Non-sustained ventricular tachycardia on 24-hour ECG monitoring | 2 |
| Male sex | 2 |
| Maximum total | 17 |
© 2018 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Schild, D.; Fankhauser, R.; Arenja, N.; Novak, J. Chagas Heart Disease. Cardiovasc. Med. 2018, 21, 316. https://doi.org/10.4414/cvm.2018.02002
Schild D, Fankhauser R, Arenja N, Novak J. Chagas Heart Disease. Cardiovascular Medicine. 2018; 21(12):316. https://doi.org/10.4414/cvm.2018.02002
Chicago/Turabian StyleSchild, Deborah, Regula Fankhauser, Nisha Arenja, and Jan Novak. 2018. "Chagas Heart Disease" Cardiovascular Medicine 21, no. 12: 316. https://doi.org/10.4414/cvm.2018.02002
APA StyleSchild, D., Fankhauser, R., Arenja, N., & Novak, J. (2018). Chagas Heart Disease. Cardiovascular Medicine, 21(12), 316. https://doi.org/10.4414/cvm.2018.02002




