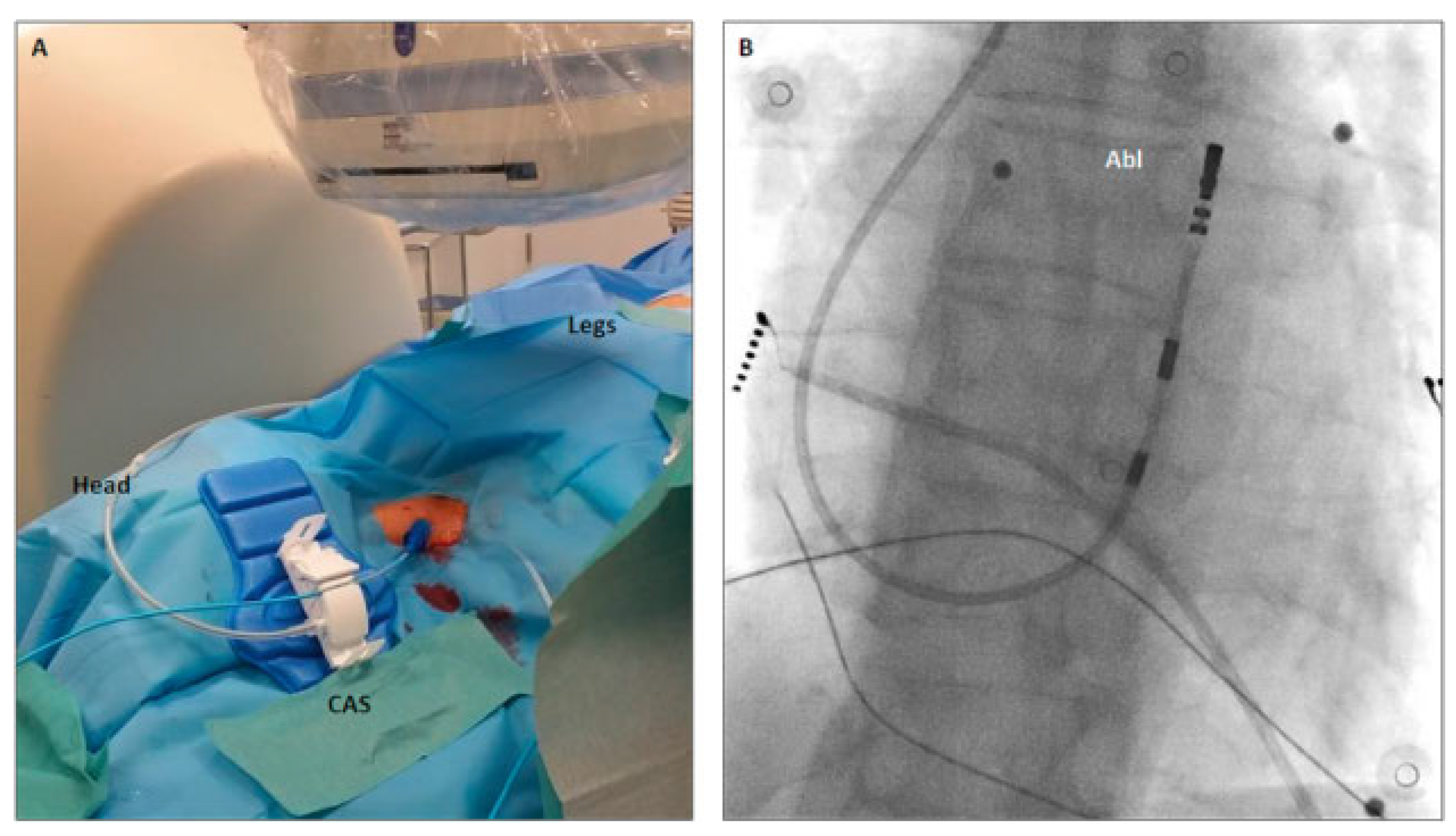
A 51-year-old female was referred for radiofrequency ablation of symptomatic frequent premature ventricular contraction (PVCs) originating from the right ventricular outflow tract (RVOT) (PVC burden 20%). Because of a congenital interruption of the inferior vena cava (IVC) between the renal and hepatic veins, the right jugular vein was chosen for access to the right ventricle via the superior vena cava. For optimal steerability of the catheter, we used the Niobe remote magnetic navigation system (Stereotaxis Inc, St Louis, MO, USA). The right jugular vein was punctured under echocardiographic sonographic guidance and a 3.5 mm open irrigated-tip ablation catheter (Navistar RMT TC, Biosense Webster) was advanced into the right atrium over an 8 Fr introducer. For remote advancement of the catheter via the tricuspid valve into the right ventricle, the catheter advancer system (Cardiodrive unit, Stereotaxis Inc, USA) was placed alongside the head of the patient (
Figure 1A). A fast anatomical map was created by remote navigation with the CARTO 3 RMT system (Biosense Webster, Diamond Bar, CA, USA). Despite isoproterenol infusion, only very few PVCs were documented at the beginning of the intervention. Therefore, pace mapping was performed to localise the origin of the extrasystoles. A pace map with 12 out of 12 matching electrocardiogram leads (
Figure 2A,B) was found in the anterolateral segment of the RVOT. Ablation was performed for 618 seconds and with a power of 35 watts (
Figure 2C). Total procedure duration was 111 minutes with a fluoroscopy time of 0.8 minutes. After a waiting period of 20 minutes and isoproterenol infusion, no PVCs could be documented. Twenty-four-hour Holter monitoring 1 month after the ablation showed no recurrence of the PVCs.
Discussion
Vascular Access via the Internal Jugular Vein
In patients without congenital malformations, the RVOT is usually accessed via the femoral vein and the IVC. In our patient without an IVC connection between the renal and hepatic vein, this standard approach was precluded. Jugular vein access was favoured over a femoral vein puncture and then access to the right atrium via the azygos vein because the latter may be more challenging for anatomical reasons. To enable optimal performance, we used the right internal jugular vein and not the subclavian vein to allow straight access of the catheter via the catheter advancer system and the 8 Fr introducer (
Figure 1A) without additional bending of the catheter. Puncture of the vein, fixation of the catheter advancer system and of the catheter (
Figure 1A) could safely be performed.
Remote Magnetic Navigation
Electroanatomical mapping and ablation of PVCs originating from the RVOT with conventional ablation catheters via the internal jugular vein may be challenging because of the unfamiliar orientation for the operator. Remote magnetic navigation allows performing the procedure from the control room with maximal flexibility of catheter manoeuvrability [
1]. The Niobe system also enables positioning of the catheter in small increments and high position stability due to its constant magnetic field [
2,
3]. Owing to the lower catheter contact force of remote magnetic navigation in comparison with conventional ablation, we increased the ablation power by 5 watt. Because of the floppy nature of the catheter, we observed, on both fluoroscopy and the Carto3 system, striking catheter movement with breathing. This pattern is reflected by the colour-coded energy distribution of the Niobe software on the static Carto3 map (
Figure 2C). However, this did not have an impact on either the outcome or safety of the intervention.
Conclusion
We showed that access to the right cardiac chambers via the internal jugular vein with use of remote magnetic navigation is feasible in patients without the possibility for standard femoral access through the IVC as a result of venous malformation
Disclosure Statement
No financial support and no other potential conflict of interest relevant to this article was reported.