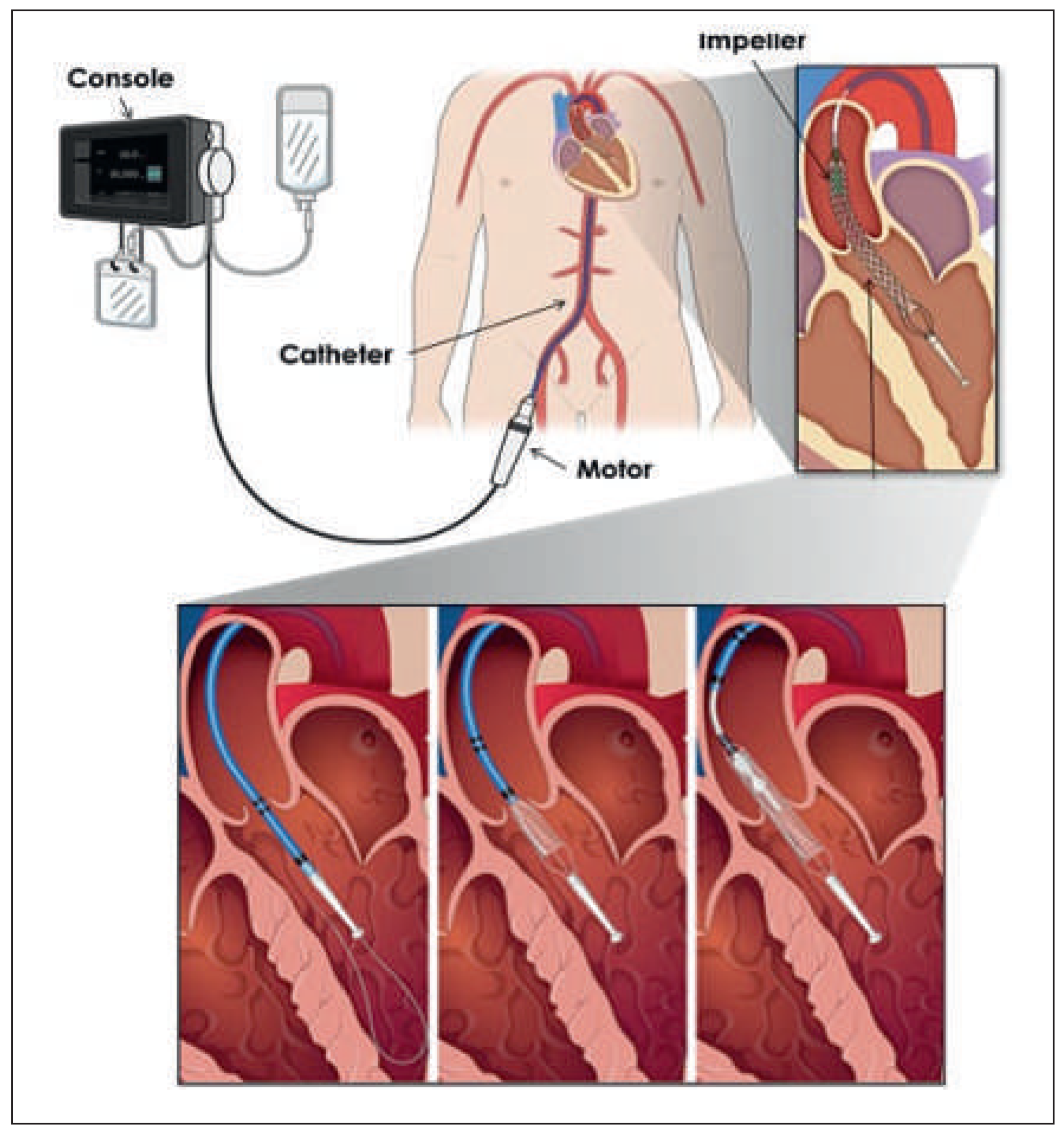
Introduction
Several percutaneous ventricular assist devices (pVADs) have become clinically available for cardiac support either in cardiogenic shock or, prophylactically, for percutaneous coronay interventions (PCIs) in patients at high-risk of haemodynamic deterioration [1]. To date, the most used pVAD in Switzerland is the Impella LP 2.5 (Abiomed – Impella Cardiosystems AG; Aachen, Germany) [2,3]. The benefit of pVADs in cardiogenic shock, particularly interms of 30-day mortality, is uncertain [4]. The new HeartMate PHP is a nonpulsatile axial flow pump (St Jude Medical) that works on the principle of an Archimedes screw, driving blood from the left ventricle into the ascending aorta. The deployable ventriculoaortal conduit with a large impeller reaches a theoretical output of up to 4–5 l/min with typically 17 000–20 000 rotations per minute (rpm) (
Figure 1).
We report the first clinical use of this device in Switzerland in March 2016, in a patient undergoing a high-risk PCI.
Case report
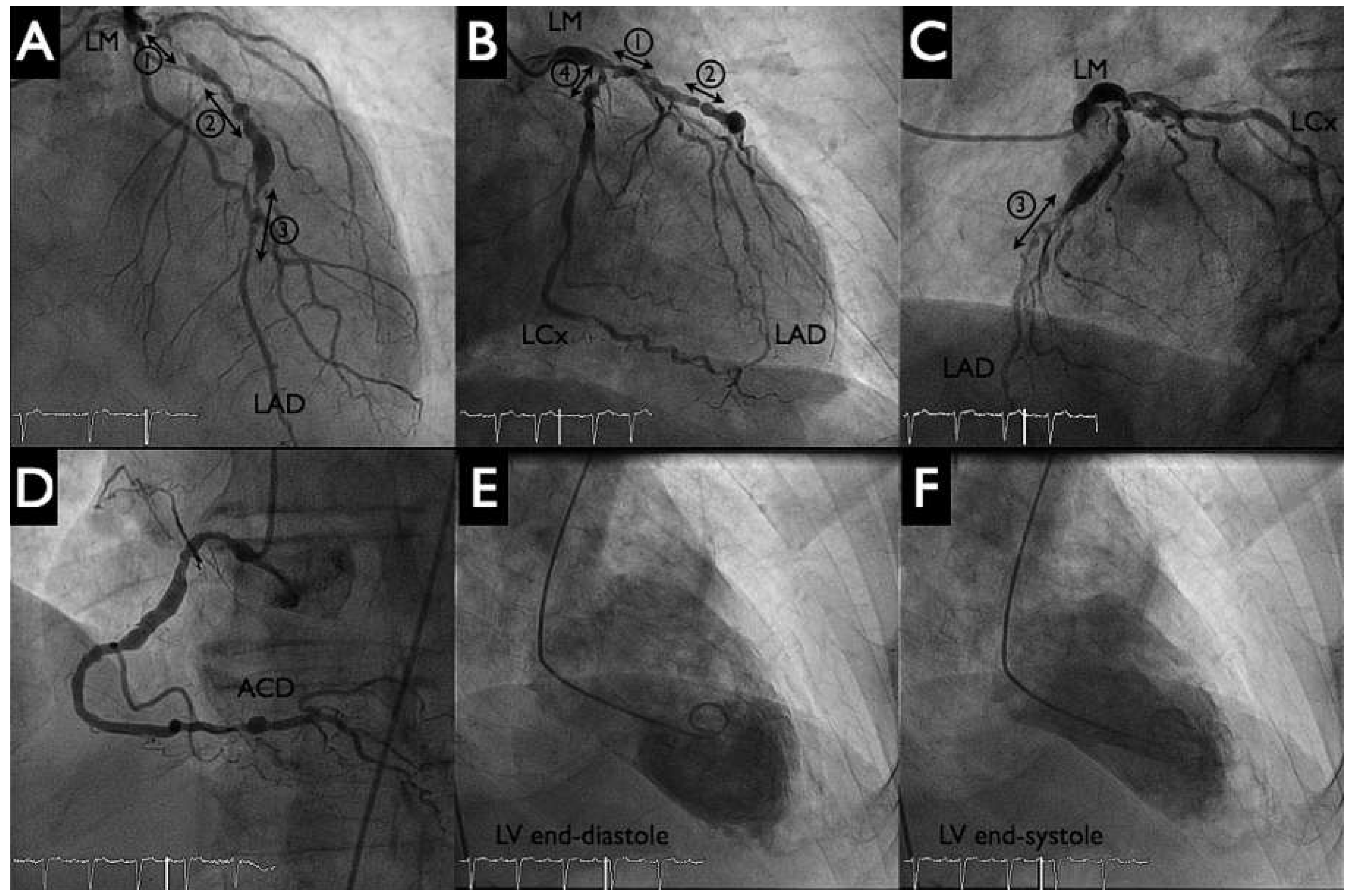
An otherwise active and independent 86-year-old male with a history of poorly controlled hypertension consulted for progressive shortness of breath and typical angina Canadian Cardiovascular Society (CCS) class III. His ECG showed first degree atrioventricular block, a left anterior hemiblock and Q waves from V1–V4. There was left ventricular hypertrophy with severe systolic dysfunction (left ventricular ejection fratction [LVEF] 30%) and anterior wall hypokinesis, but no significant valvular disease on echocardiogram (left ventricular angiogram,
Figure 2 panels E, F). The coronary angiogram revealed severe three-vessel coronary artery disease (
Figure 2 panels A–D). There was a significant stenosis of the distal left main (Medina 1.1.1). The left anterior descending artery was suboccluded in the proximal and distal portion with numerous calcified and aneurysmal lesions. The proximal, middle and distal circumflex artery, as well as the mid portion of the right coronary artery, had significant stenosis.
The patient opted for an invasive approach after he had been thoroughly informed about the different treatment possibilities, including conservative medical management. Given the severity of the coronary artery disease, the procedure was deemed high risk and pVAD support was planned.
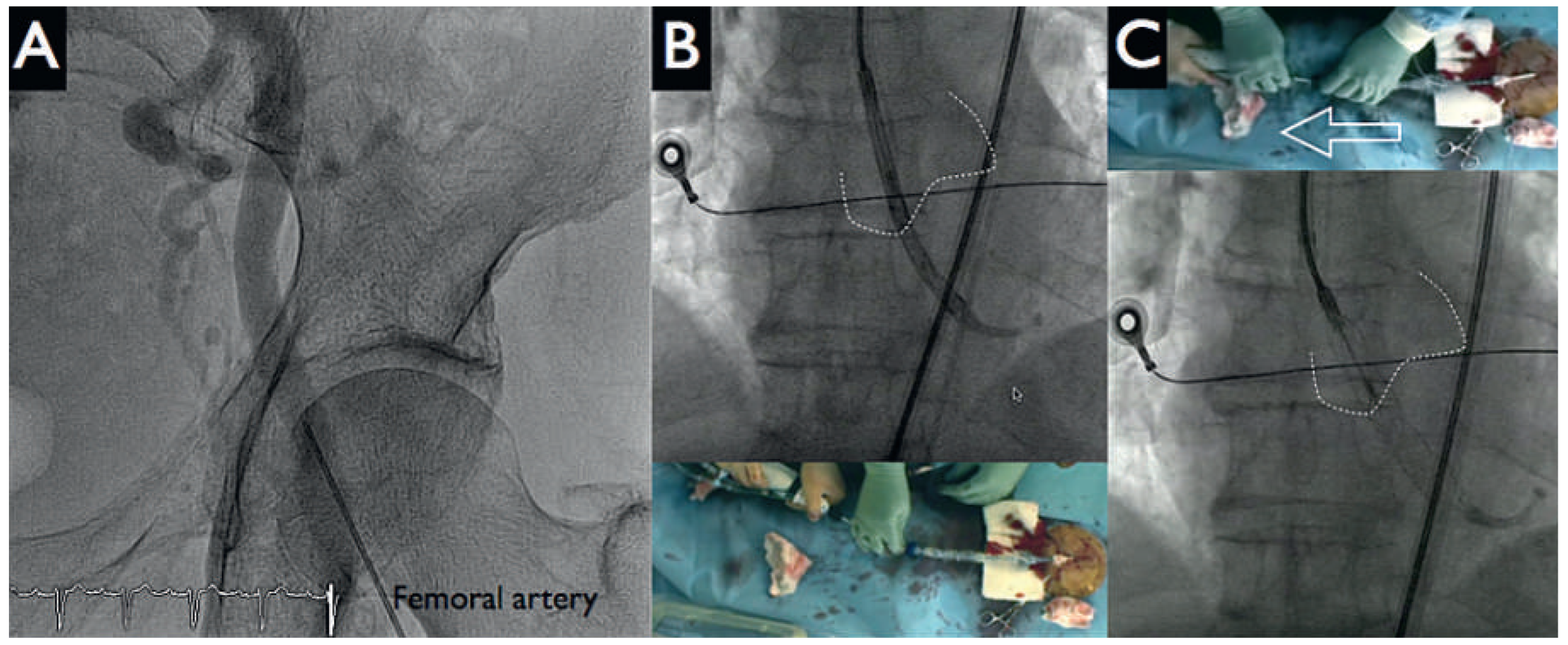
After the patient had given written informed consent, the decision to perform a HeartMate-PHP-assisted PCI was made. Both groins were used for vascular access. Following local anaesthesia, puncture of the left femoral artery under fluoroscopy (
Figure 3 panel A) and preclosure of the left femoral with two Perclose devices were performed. Then, a 14 French (Fr) sheath was placed into the descending aorta. Systemic anticoagulation was achieved with administration of unfractionated heparin (70 U/kg); the patient had been pretreated with acetylsalicylic acid 100 mg/day. A 5 Fr JR4 catheter was positioned in the left ventricle and then exchanged over a 0.018 inch guidewire for the HeartMate-PHP catheter. The correct position of the device was verified by fluoroscopy by using a 5 Fr pigtail catheter from the right femoral artery as a marker of the aortic valve and the conduit was unsheathed up to its final 24 Fr size (
Figure 3 panels B, C). Circulatory support was initiated at the minimum pump speed (approximately 1 l/min) and maintained throughout the intervention. Activated clotting time was kept at 250 s.
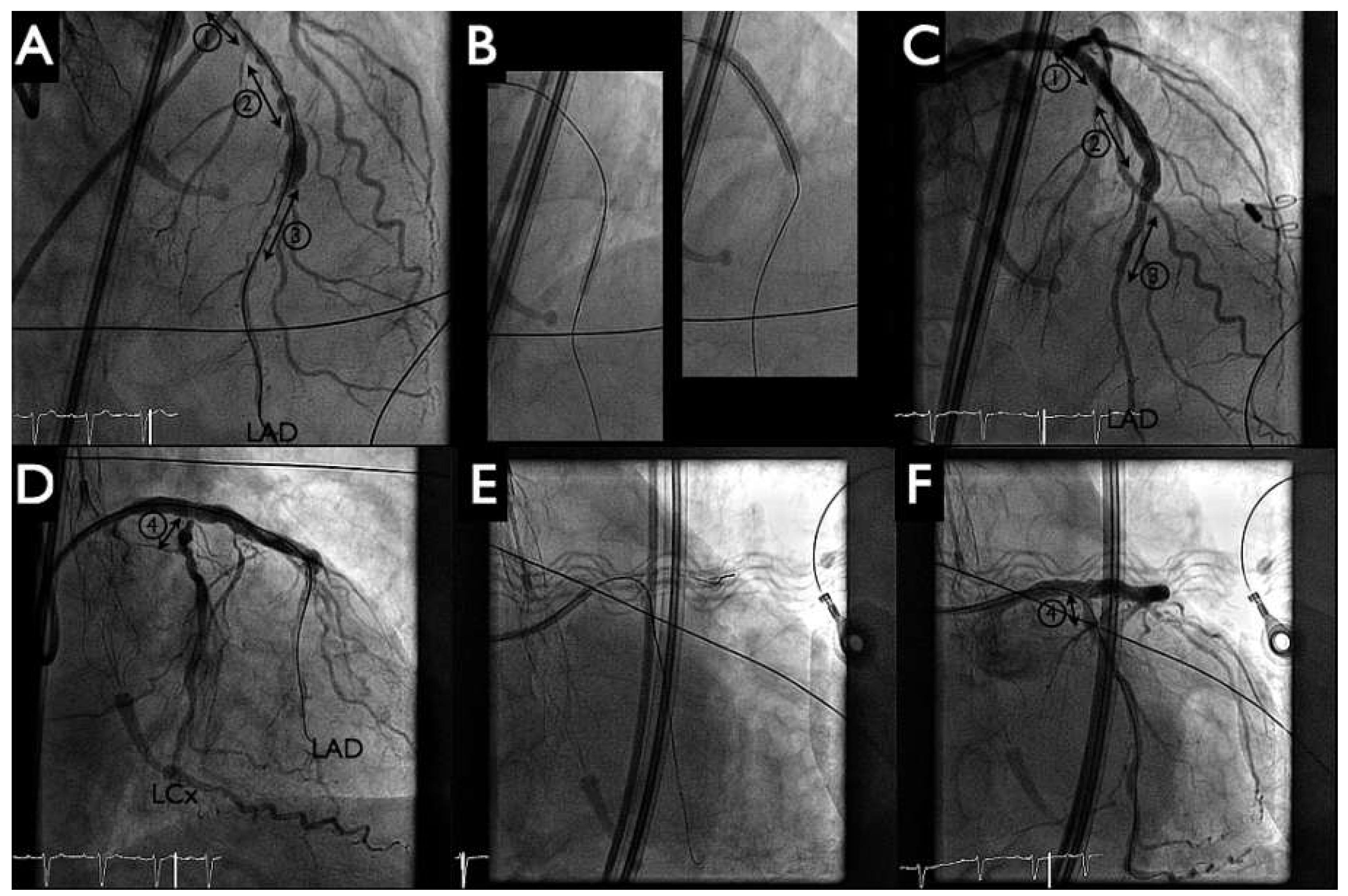
Following this, the pigtail catheter was exchanged for a 6 Fr Q4 guiding catheter with PCI of the distal left anterior descending artery (LAD) (SYNERGY II 2.25–20 mm, Boston Scientific,
Figure 4 panel B left) and proximal LAD to distal left main artery (SYNERGY II 4.00–38 mm, Boston Scientific,
Figure 4 panel B right) with T-stenting into the left circumflex artery (SYNERGY II 2.75–12 mm, Boston Scientific,
Figure 4 panels B, E). Postdilatation with kissing balloon inflation (4.00–15 mm in LAD, 2.75–12 mm in the left circumflex artery) demonstrated a good final angiographic result (
Figure 4 panels C, F). During the intervention no significant haemodynamic changes were noted, and the patient tolerated the balloon inflations well. The HeartMate-PHP was then gently removed into the descending aorta. The pump was stopped and the conduit could be easily resheathed into the catheter (
Figure 5 panels A, B). Haemostasis was achieved with tightening of the two Perclose (
Figure 5 panel C) and the patient was given a loading dose of 60 mg prasugrel.
Discussion
This first case shows the feasibility of implantating a new pVAD for partial circulatory support during a high-risk percutaneous coronary intervention. The current evidence for the use of HeartMate PHP is very limited. The first-in-human study in 10 patients with complex coronary disease and reduced left ventricular function showed good support during procedures and no adverse events at 30 days [5]. The Shield I trial, which contributed to the CE approval, tested the device in 30 patients undergoing PCI. None had device-related cardiac death, myocardial infarction or intraprocedural hypotension, and the overall incidence of complications related to HeartMate PHP was low [6]. The Shield II trial will aim to randomise 425 patients to either HearMate PHP or Impella LP 2.5 for high-risk PCI [7]. Potential complications in cases of prolonged use are haemolysis, limb ischaemia, vascular injury and bleeding. The mid-term and long-term safety of the device in terms of effects on the aortic valve remains unknown. Classic contraindications are mechanical aortic valve, left ventricular thrombus and aortic dissection; aortic stenosis and regurgitation are relative contraindications. One inconvenience of the Heart-Mate PHP device noted by the operators was the noise from the motor of the impeller.
The Impella CP® (also known as cVAD in Europe) (Abiomed – Impella Cardiosystems AG; Aachen, Germany) is another axial pump that achieves higher flows than the Impella LP 2.5 while using the same 13 Fr insertion sheath. The flow reaches 3.5 l/min at the cost of higher (up to 46 000) rpm. Presumed advantages of the Heart- Mate PHP over both Impella devices include a higher antegrade flow mimicking physiological cardiac output, and possibly lower risk of hemolysis given the lower rpm. However, the higher 24 Fr profile could translate into more valvular complications. There is no available data directly comparing Impella CP® to HeartMate PHP.
Venoarterial extracorporeal life support (VA-ECLS) may also deliver the necessary support to both heart and lungs during high-risk PCI. The system is a modified cardiopulmonary bypass, providing higher nonpulsatile flows (up to 6 l/min) at lower rpm (up to 4500) and full lung support (oxygenation and CO2 removal) in cases of concomitant respiratory failure. The systemic blood is drained via a 21–29 Fr femoral venous cannula placed in the inferior vena cava. It then passes through a centrifugal pump, an oxygenator and heat exchanger membrane, to return to the patient via a 17–21 Fr femoral arterial cannula. The high-profile cannulas increase the risk of bleeding and ischaemia, and if the system is kept in place for several hours, a distal reperfusion cannula in the superior femoral artery is mandatory. The perfusion is retrograde and there is a concern of increased left ventricular afterload and stress, and thus myocardial oxygen demand. Finally, the cannula implantation and removal is more cumbersome and the procedure usually requires the involvement of a highly trained multidisciplinary team, often including perfusionists. Although it does have a crucial place in high-risk transcatheter aortic valve implantation, its place in high-risk PCI is likely to be limited to very specific cases, especially with the improvement of axial pumps.
Clinical evidence in the years to come should provide the necessary data to identify the needs, risks and benefits associated with these devices.