Introduction
Metabolic changes in the heart muscle are barely addressed during medical education. However, most cardiac diseases are associated with alterations in energy metabolism, which may contribute to cardiac dysfunction and provide targets for therapeutic interventions. My personal interest in energy metabolism was kindled more or less by chance during my first training position in cardiology at the Cardiology Division of the University Hospital in Zurich, when I was assigned by the head of cardiology, Professor Wilhelm Rutishauser, to an experimental study on myocardial high-energy phosphate content during coronary flow reduction. The present article summarises some lessons I learnt during my continuing research interest in myocardial energy metabolism since the early 1970s.
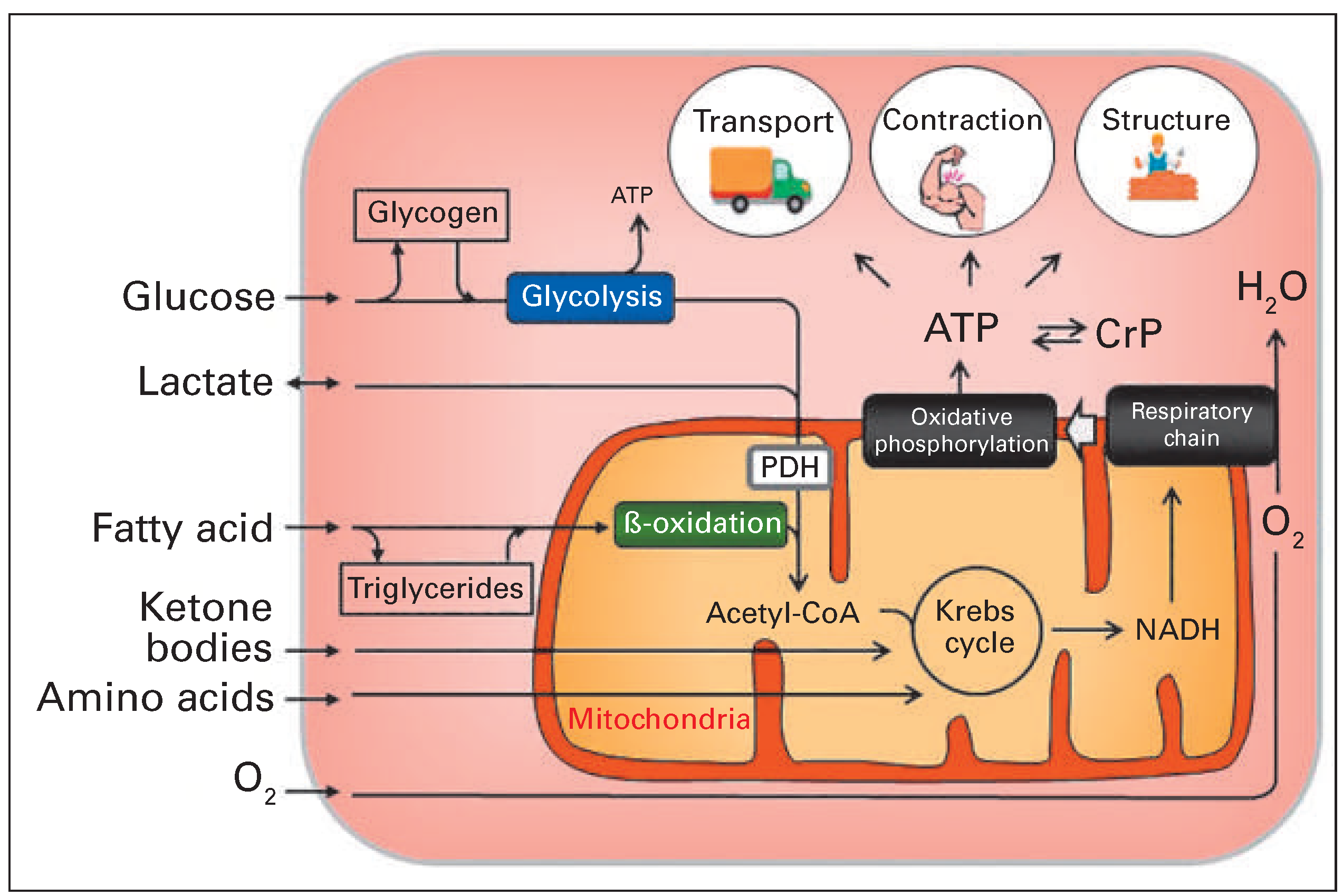
Cardiac function requires uninterrupted synthesis of adenosine triphosphate (ATP), of which more than 90% is derived in the mitochondria from oxidative degradation of metabolic substrates taken up by the cardiac myocytes [1,2]. The remainder originates mainly from substrate-level phosphorylation during glycolysis of glucose in the cytoplasmic compartment (
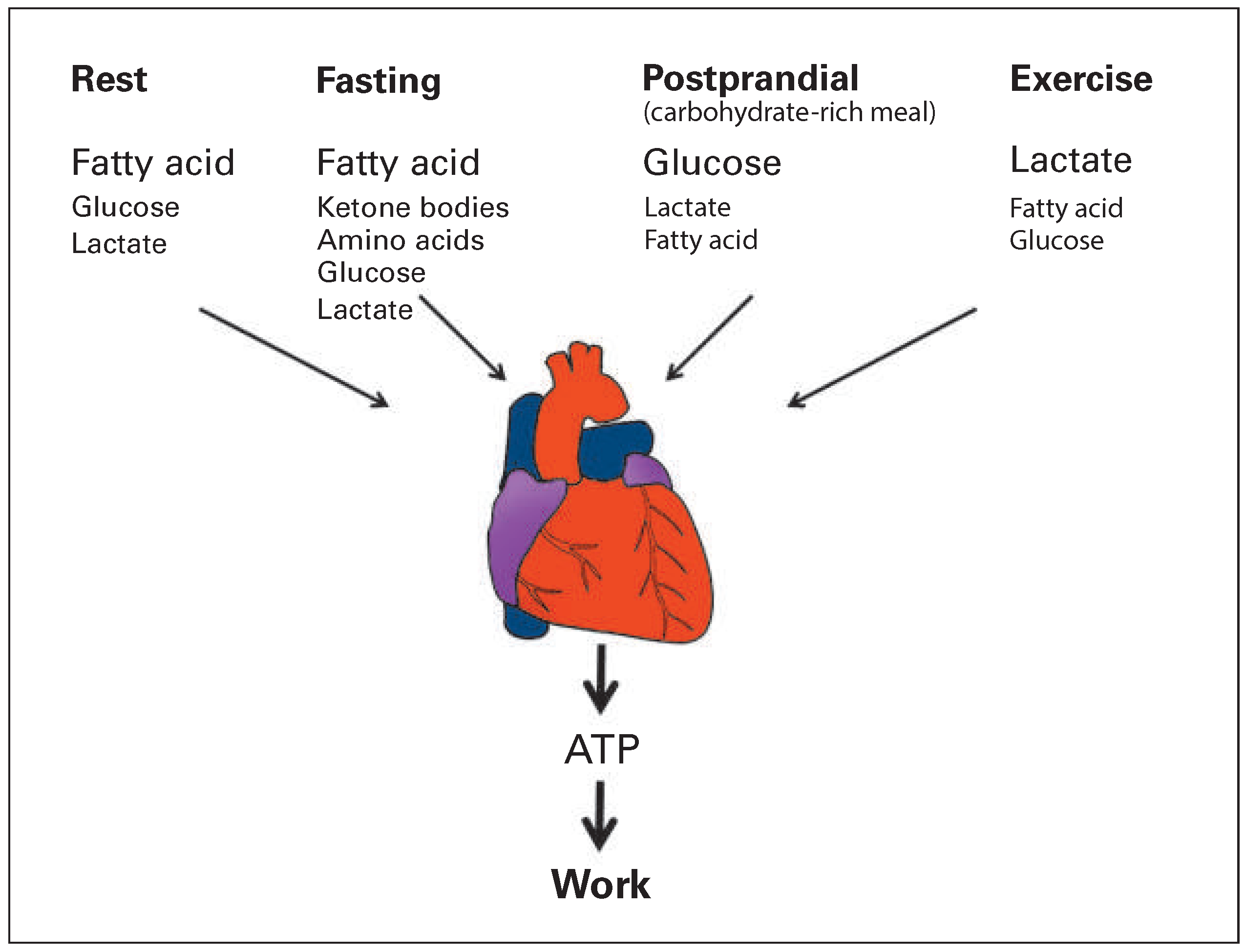
Figure 1). The term “substrate” denotes the “fuel” used by the cell for energy production. The healthy heart can utilise almost every metabolic substrate supplied by coronary circulation and can rapidly switch between substrates without affecting contractile performance [3]. Circulating substrates include glucose, lactate, pyruvate, fatty acid, ketone bodies and amino acids. The capacity of the myocardium to adapt energy metabolism to substrate availability with unaltered contractile function is often referred to as “metabolic flexibility”. Metabolic flexibility is a hallmark of healthy myocardium and is lost in cardiac disease conditions including ischaemic heart disease, heart failure, diabetes and obesity. Loss of metabolic flexibility may contribute to cardiac dysfunction [3–7]. In this review article I will focus on metabolic changes in two common disease conditions: (1) ischaemic heart disease, specifically myocardium that is reperfused after an ischaemic insult, and (2) heart failure. Known metabolic diseases such as diabetes and obesity are not addressed and the interested reader is referred to excellent review articles on these topics [2,8–10].
The challenge of assessing myocardial substrate metabolism in patients
Coronary sinus catheterisation, as used by Richard Bing, has two major limitations for the characterisation of myocardial metabolism in cardiac patients. First, the method allows measurement of global substrate extraction across the entire heart, but cardiac disease conditions, above all coronary artery disease, exhibit marked regional differences in myocardial metabolism. Second, the method does not provide information on the intracellular fate of extracted substrates. In fact, extracted fatty acid and glucose may not be channelled to energy metabolism, but be stored as triglycerides or glycogen, respectively, or enter other pathways required for cellular structure or function [7].
In the second half of the 1970s, positron emission tomography (PET), a new radionuclide imaging modality, emerged and held great promise for the assessment of substrate metabolism in different regions of the heart; in addition, it is noninvasive [16]. PET involves shortlived cyclotron-produced radionuclides such as carbon-11, nitrogen-13, and oxygen-15, with half-lives of 20.3, 10.0 and 2.1 minutes, respectively. These radionuclides offer at least three major advantages for the assessment of myocardial substrate metabolism: (1) they can be incorporated into biomolecules such as metabolic substrates without altering the molecular structure and, accordingly, the metabolic behaviour; (2) the content of the radionuclides can be quantified in each region of the heart with PET; and (3) the short halflives of the radionuclides allow repetition of the study after a short time interval [17,18]. Fluorine-18 is another positron-emitting radionuclide that has attracted a lot of interest in PET. Because of its somewhat longer halflife of 110 minutes, it allows transport of labelled compounds over some distance, obviating the need of an in-hospital cyclotron. Although fluorine is not a natural component of metabolic substrates, it can substitute for a hydroxyl group in a number of parent molecules. Fluorine-18-labelled deoxyglucose (18FDG) has proved useful for the delineation of regional glucose uptake in the myocardium [19].
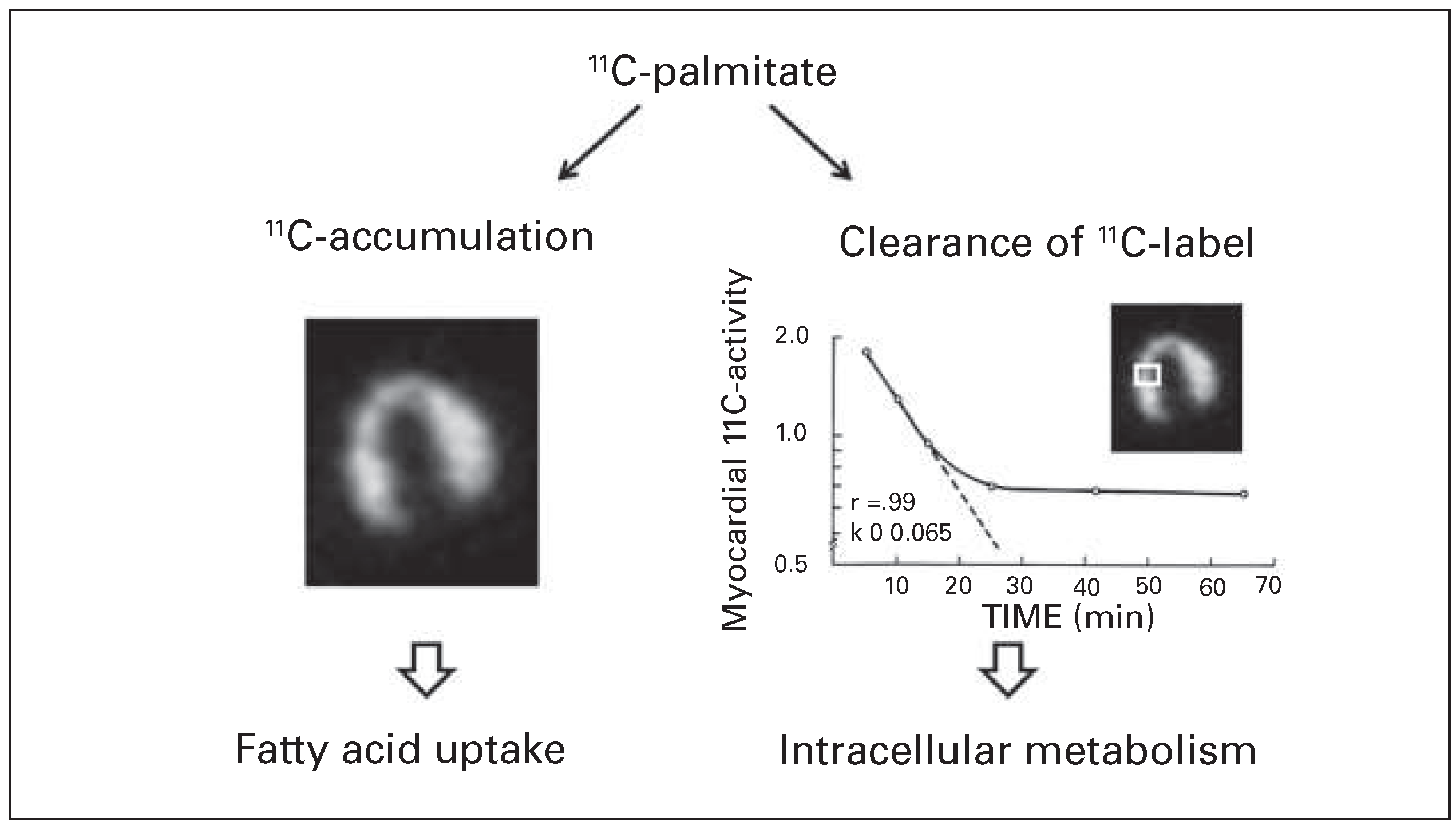
What kind of metabolic information is obtained from PET with labelled metabolic substrates? I had the opportunity to participate during a research fellowship at Washington University St. Louis in early experimental studies designed to define the potential of PET with 11C-labelled palmitic acid for the assessment of regional myocardial fatty acid metabolism. After intravenous injection of 11C-palmitate, metabolic information is derived (1) from accumulation 11C-radioactivity in different regions of the myocardium, and (2) from release of the 11C-label, and hence the clearance rate of radioactivity, from the myocardium. Accumulation of the label reflects myocardial fatty acid uptake [20]. Clearance of 11C-radioactivity provides information on the rate of intracellular fatty acid metabolism (
Figure 3) [21,22]. The clearance curve of radioactivity from 11C-palmitate exhibits a biexponential pattern composed of an early rapid component, corresponding to clearance of label from palmitate that is rapidly oxidised, and a late slow component, reflecting slow clearance of radioactivity from palmitate that is first incorporated into triglycerides and other lipid esters [21,23]. In ischaemic and hypoxic myocardium the early rapid clearance component of radioactivity from 11C-palmitate was slower, consistent with reduced fatty acid oxidation [21,22].
Although PET with labelled metabolic substrates allowed for the first time noninvasive assessment of regional substrate metabolism, quantification of the rate of substrate oxidation in absolute terms remained a major challenge because of the rather complex intracellular metabolism of extracted substrates, with channelling of the tracer to several intracellular pools with different turnover rates [23]. Therefore, strategies have been developed to monitor only a selected segment of metabolism of a given substrate [18]. The best-known example is measurement of myocardial glucose uptake with 18FDG.18FDG is a structurally modified glucose analogue molecule that is taken up by the myocardium similarly to naturally occurring glucose, but which is not further metabolised in the cardiomyocytes and allows quantification of regional myocardial glucose uptake by use of mathematical modelling [19].
An emerging approach for the assessment of myocardial substrate metabolism is magnetic resonance spectroscopy (MRS) of 13C-labelled molecules. MRS offers the unique opportunity not only to noninvasively detect the isotope in the myocardium, but also to identify in which molecules and in which position in the labelled molecules the isotope resides. Until recently, the application of carbon-13 MRS has been limited by the low abundance and low signal intensity of 13C-labelled compounds in the heart, even after administration of 13C-labelled substrates. More recently, enhancement of signal intensity by several magnitudes has been achieved by hyperpolarisation of 13C-labelled substrates [24]. After intravenous injection the method allows not only detection of uptake and global clearance of the 13C-label, but also tracking of the isotope in different downstream metabolites [25,26]. Initial preclinical studies with hyperpolarised 13C-pyruvate labelled in position 1 for the assessment of flux rate through the pyruvate dehydrogenase (PDH) reaction [24,27], or labelled in position 2 for the assessment of Krebs cycle activity [24,28], have provided the proof-of-principle. One major challenge of this approach is the rapid signal decay of hyperpolarised carbon-13 in the order of 1 minute. Although the method is at an early stage, it has the potential to enhance knowledge on regional myocardial substrate metabolism in both clinical and research settings.
At present, cardiac metabolic imaging is applied mainly in research on cardiac metabolism. Widespread clinical application of both PET and MRS is hampered by a number of limitations. First, the costs involved in these technologies are high. Second, alternative, less expensive imaging methods provide sufficient information for clinical decision making in most cases, including assessment of myocardial viability [29]. Finally, although the potential for detailed characterisation of cardiomyocyte dysfunction in cardiac disease is large, definition of the clinical impact of metabolic indices on patient management requires further research.
Loss of substrate flexibility in heart disease: does it matter for outcome?
Postischaemic reperfusion
Shortly after the introduction of reperfusion therapy of myocardial infarction into clinical practice in the 1980s, Schwaiger et al. from the University of California Los Angeles observed, when using PET with 11C-palmitate and 18FDG in a dog model, that the reperfused region seems to oxidise less fatty acid and take up more glucose [30,31]. Consistent with this in vivo observation, we observed by direct metabolic measurements in isolated perfused rat hearts subjected to 40 minutes of no-flow ischaemia, a reduction in fatty acid oxidation and a concomitant increase of glucose oxidation early during reperfusion [32]. This degree of ischaemic injury corresponds to evolving myocardial infarction. Rapid activation of glucose oxidation in advanced ischaemic injury was mediated by activation of the regulatory enzyme of glucose oxidation, PDH, by cytosolic calcium overload [33]. Concomitant reduction of fatty acid oxidation is most likely secondary, and reflects inhibition of this pathway by substrate competition during activation of glucose oxidation.
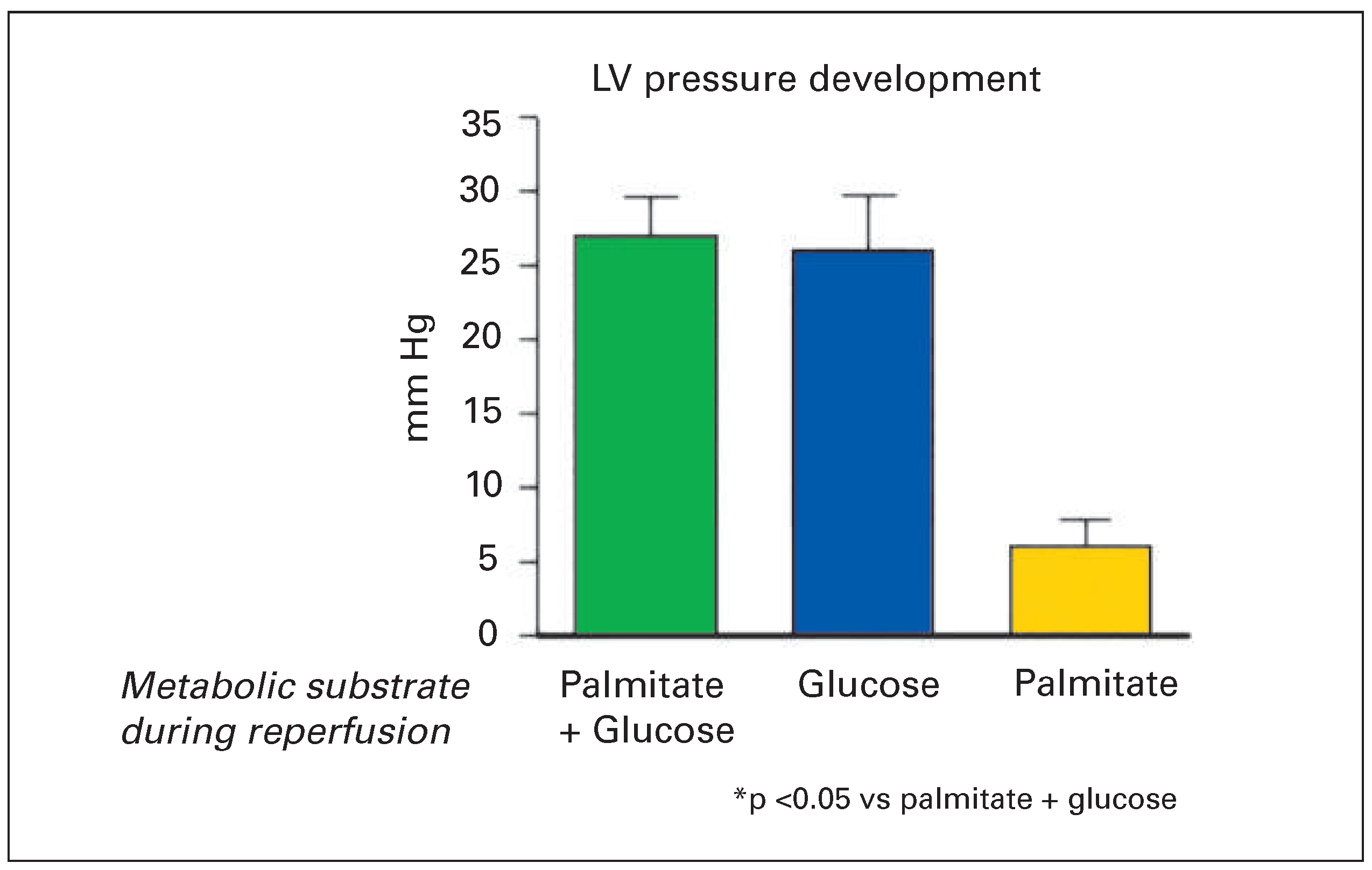
The crucial question from the clinical point of view is whether partial loss of metabolic flexibility during postischaemic reperfusion, with a shift from fatty acid to glucose oxidation, is an adaptive, favourable response, which prevents cardiomyocyte dysfunction and cell death or rather contributes to myocardial injury. Evidence from our group [32] and others [34,35] indicates that glucose as metabolic substrate is essential for recovery of cardiomyocytes early during reperfusion. If, after 40 minutes of no-flow ischaemia, isolated perfused hearts were reperfused with either fatty acid plus glucose or with glucose alone, recovery of contractile function was virtually identical (
Figure 4) [32]. However, if glucose was withdrawn from the perfusate at the moment of reperfusion and the hearts reperfused with fatty acid as a sole substrate, recovery of contractile function was much worse and more creatine kinase was released into the coronary effiuent as sign of increased irreversible injury [32]. Accordingly, unlike in healthy myocardium with preserved substrate flexibility, contractile function and survival of postischaemic myocardium is critically dependent on the presence of glucose. The precise mechanism of the protective effect of glucose during reperfusion is not entirely clarified. However, Jeremy et al. observed that inhibition of glycolysis during the first 30 minutes of reperfusion results in aggravation of intracellular calcium accumulation, triggering lethal contracture of the cardiomyocytes [34,36]. It is likely that ATP produced during glycolysis by substrate-level phosphorylation during the cytoplasmic compartment is essential early in postischaemic reperfusion to support the ion pumps necessary for restoration of normal ion homeostasis [37].
A clinically important message of the protective effect of glucose early during reperfusion is that myocardial salvage achieved by timely reperfusion can be further enhanced by an intervention applied during the early reperfusion period. However, the protective effect of glucose during postischaemic reperfusion observed in numerous experimental studies contrasts with the rather disappointing results of a considerable number of clinical studies. Known to many cardiologists is the infusion of glucose, insulin and potassium, which was postulated by Sodi-Pallares in 1962 [38]—well before the introduction of coronary reperfusion strategies—to favourably influence the course of myocardial infarction. However, even the subset of studies performed in the reperfusion era did not demonstrate an appreciable clinical benefit [39,40]. This also applies to non-metabolic therapies adjunctive to coronary reperfusion, including postconditioning and administration of ciclosporin among other interventions [41]. The numerous reasons for failure of translation of experimental findings into clinical practice have been extensively discussed recently [42–44]. One important factor is the short time interval of only a few minutes for interventions designed to prevent early reperfusion injury [39].
Heart failure
A shift from fatty acid to glucose oxidation has also been observed in experimental models of heart failure [3,45,46] and in patients with dilated cardiomyopathy examined by PET with 11C-palmitate and 11C-glucose [47]. Unlike during postischaemic reperfusion, in heart failure, reduction of fatty acid oxidation seems to be the primary event, with secondary activation of glucose oxidation as a result of substrate competition [45].
In advanced heart failure glucose oxidation also becomes reduced [48]. We have studied metabolic changes during progression from compensated hypertrophy to heart failure in a mouse model with targeted overexpression of angiotensinogen in the myocardium [49,50]. Because there is abundant angiotensin converting enzyme in the myocardium, the myocardial tissue is chronically exposed to increased levels of angiotensin II, which is known to contribute to maladaptive remodelling and progression into heart failure [51]. After 1 year, all mice exhibited left ventricular hypertrophy and approximately half of them presented signs of heart failure, associated with left ventricular dysfunction. Oxidation of fatty acid, measured during in vitro working heart perfusion, was markedly reduced in failing hearts, but not in non-failing hypertrophied hearts [50,52]. Conversely, glucose oxidation was slightly increased in failing hearts. Accordingly, the shift from fatty acid to glucose oxidation seems to occur during progression from compensated hypertrophy to heart failure. The mechanisms of reduction of fatty acid oxidation in heart failure are presently being elucidated. Available evidence suggests that reduced fatty acid oxidation in heart failure is mediated by reduction of expression of a number of regulatory enzymes of fatty acid oxidation [46,50,52]. This reduction is mediated, at least in part, by inhibition of the transcription factors peroxisome proliferator-activated receptor α (PPARα) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) [53,54]. Reduction of the fatty acid oxidation pathway is today considered a well established characteristic of heart failure.
At first glance, a shift from fatty acid to glucose oxidation seems reasonable in view of the lower oxygen costs of oxidation of glucose compared with oxidation of fatty acid. Stoichiometric calculation discloses that oxidation of fatty acid requires approximately 12% more oxygen for production of the same amount of ATP compared with oxidation of glucose [15]. Based on this consideration and observations of the previously discussed favourable effects of glucose oxidation in postischaemic myocardium, most metabolic interventions designed to prevent development and progression of heart failure have focused to date on stimulation of glucose oxidation [55]. This is mostly achieved by pharmacological inhibition of fatty acid oxidation. Although some improvement of symptoms and cardiac function has been observed in smaller clinical studies, overall success has remained limited so far. On the other hand, oxidation of one molecule of palmitate provides 130 molecules of ATP compared with 38 molecules of ATP per molecule of glucose. In fact, a number of observations indicate that enhancement of glucose oxidation observed during ex vivo perfusion of failing hearts does not fully compensate for loss of ATP synthesis capacity during downregulation of fatty acid oxidation [3,56,57]. Further inhibition of fatty acid oxidation, with the aim to enhance glucose oxidation, may therefore aggravate energy deficiency [3,6,58].
The concept that the failing heart is energy-starved is increasingly considered, as proposed several years ago by Stefan Neubauer in his review entitled The failing heart—an engine out of fuel [59]. In support of this concept, the myocardial CrP/ATP ratio is reduced in heart failure, both in experimental models and in patients examined by means of phosphorus-31 MRS [60,61]. However, reduction of fatty acid oxidation is most likely not the sole factor limiting ATP production in failing hearts. Other factors include dysfunction at the level of the respiratory chain, oxidative phosphorylation and ATP transfer to contractile elements [62].
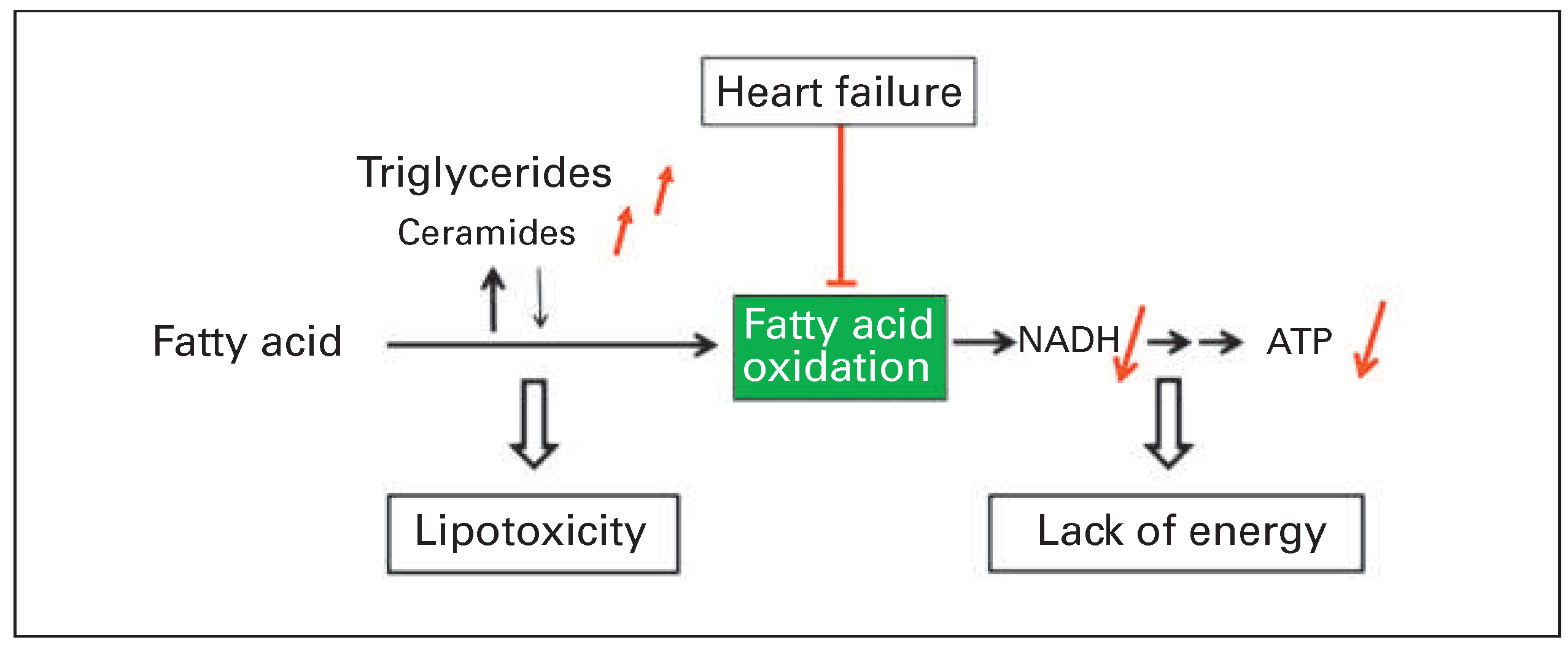
A second consequence of reduced fatty acid oxidation that may contribute to myocardial dysfunction is lipotoxicity (
Figure 5). If reduction of fatty acid oxidation is not associated with a balanced reduction of fatty acid uptake into the cardiomyocytes, accumulation of triglycerides and other lipid esters may occur [63,64]. In our mouse model with angiotensin II-mediated ventricular remodelling, accumulation of lipid droplets was observed in the myocardium of failing hearts during feeding with a high fat diet [52]. No lipid droplets were observed in mice without heart failure. This suggests that preserved fatty acid oxidation capacity allows coping with increased fat supply. Of note, triglycerides per se are not necessarily toxic and may even present a sink for excess fatty acid taken up by the myocardium. However, there was concomitant accumulation of another class of lipid esters, ceramides, which have been shown to promote cell death by apoptosis [52,65].
Taken together, loss of substrate flexibility with restriction of fatty acid oxidation in failing hearts seems to contribute to cardiac dysfunction. Today research on therapeutic interventions to optimise energy metabolism in heart failure is no longer focused on stimulation of glucose oxidation alone, but addresses a broader spectrum of targets including activation of fatty acid oxidation [5,58].
Conclusion
Understanding of changes in myocardial energy metabolism in heart disease is of clinical interest for at least two reasons. First, metabolic changes may be involved in the cellular mechanisms underlying cardiac dysfunction and allow identification of new therapeutic targets. Second, assessment of metabolism, by imaging modalities including PET and MRS, or by measurement of metabolic biomarkers in the blood, may allow better noninvasive characterisation of the type and degree of myocardial injury in patients [66–68].
Although there is growing knowledge of cellular mechanisms of cardiac disease, including energy metabolism, translation of laboratory findings into clinical medicine is more difficult than anticipated a few decades ago [69]. To pave the way for translational research, methodological issues in both experimental and clinical research on myocardial energy metabolism have recently been critically appreciated in an AHA Scientific Statement [70]. Moreover, current developments of noninvasive metabolic imaging methods, applicable to both animal models and patients, will facilitate translational research.
After all, many established therapeutic strategies in heart disease already act by improving myocardial metabolism. Andreas Grüntzig, after whom the lecture motivating this review article is named, developed the probably most effective metabolic intervention, namely restoration of oxygen supply for safeguarding oxidative metabolism.