Real-Time Contact Force Measurement for Catheter Ablation of Cardiac Arrhythmias
Abstract
Biophysics of lesion formation
Advances in ablation catheter technology
The role of catheter contact: a historical perspective
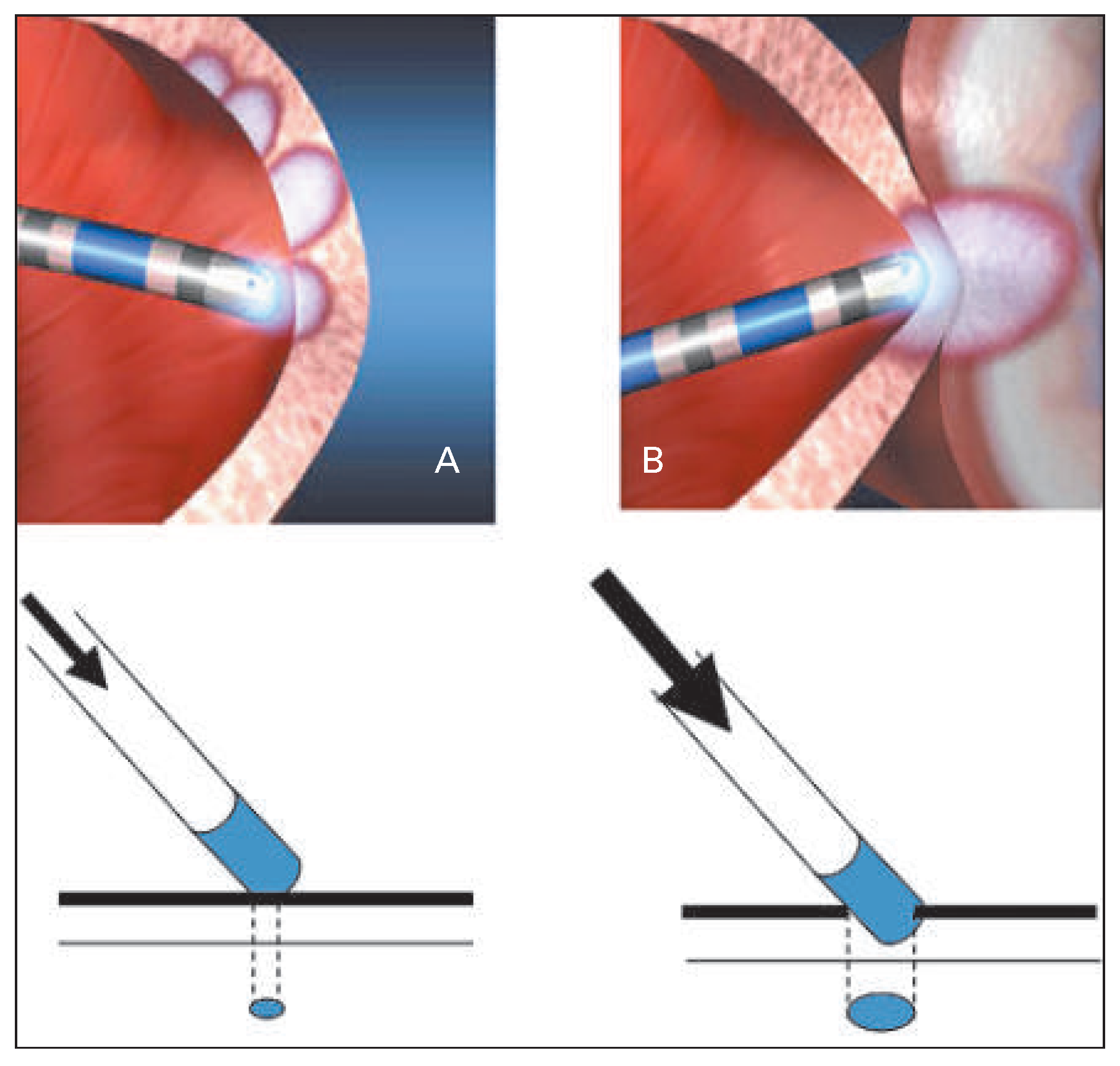
Available systems
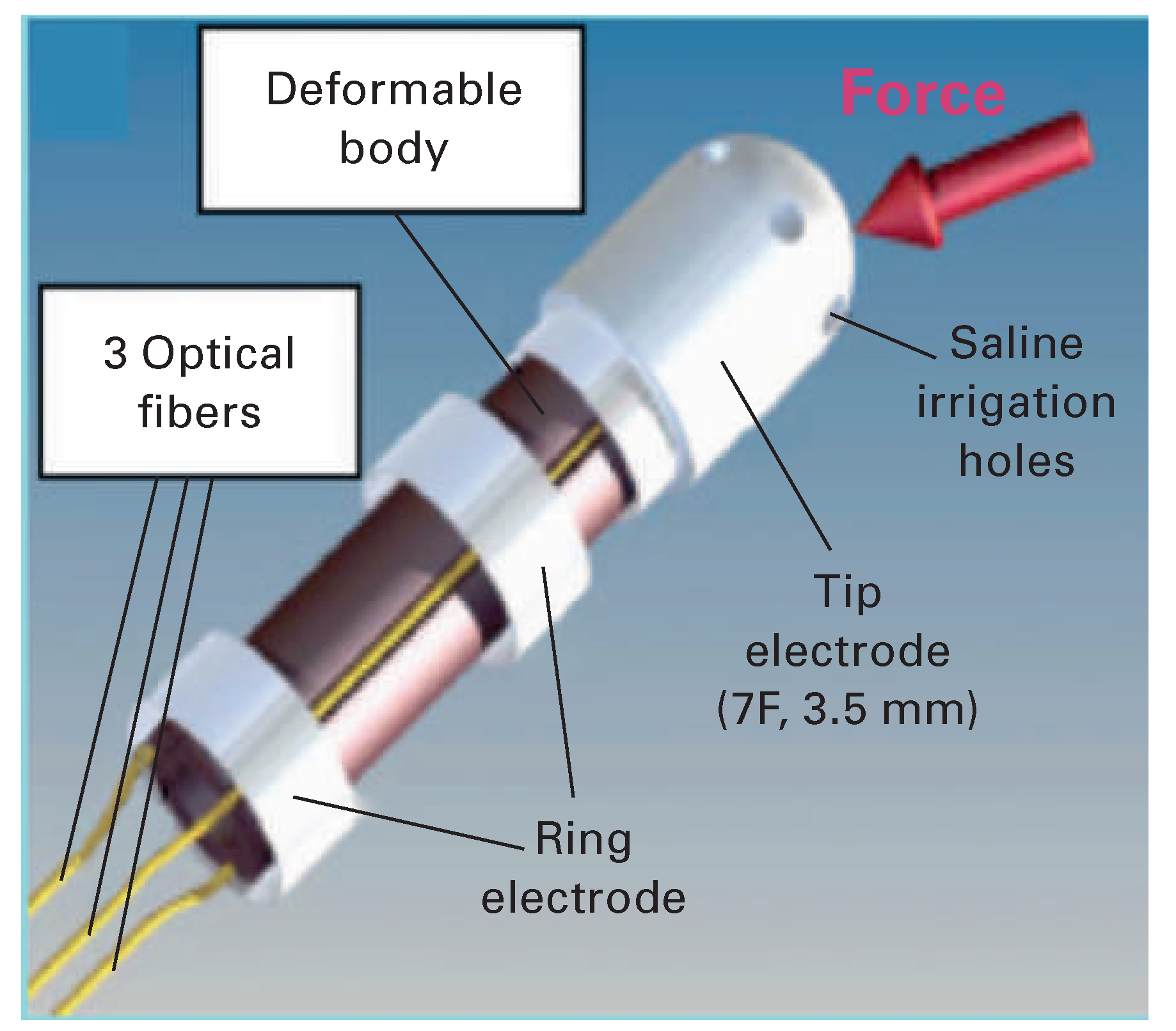
IntelliSense® Fine Force Technology and EnSite Contact VeriSense™
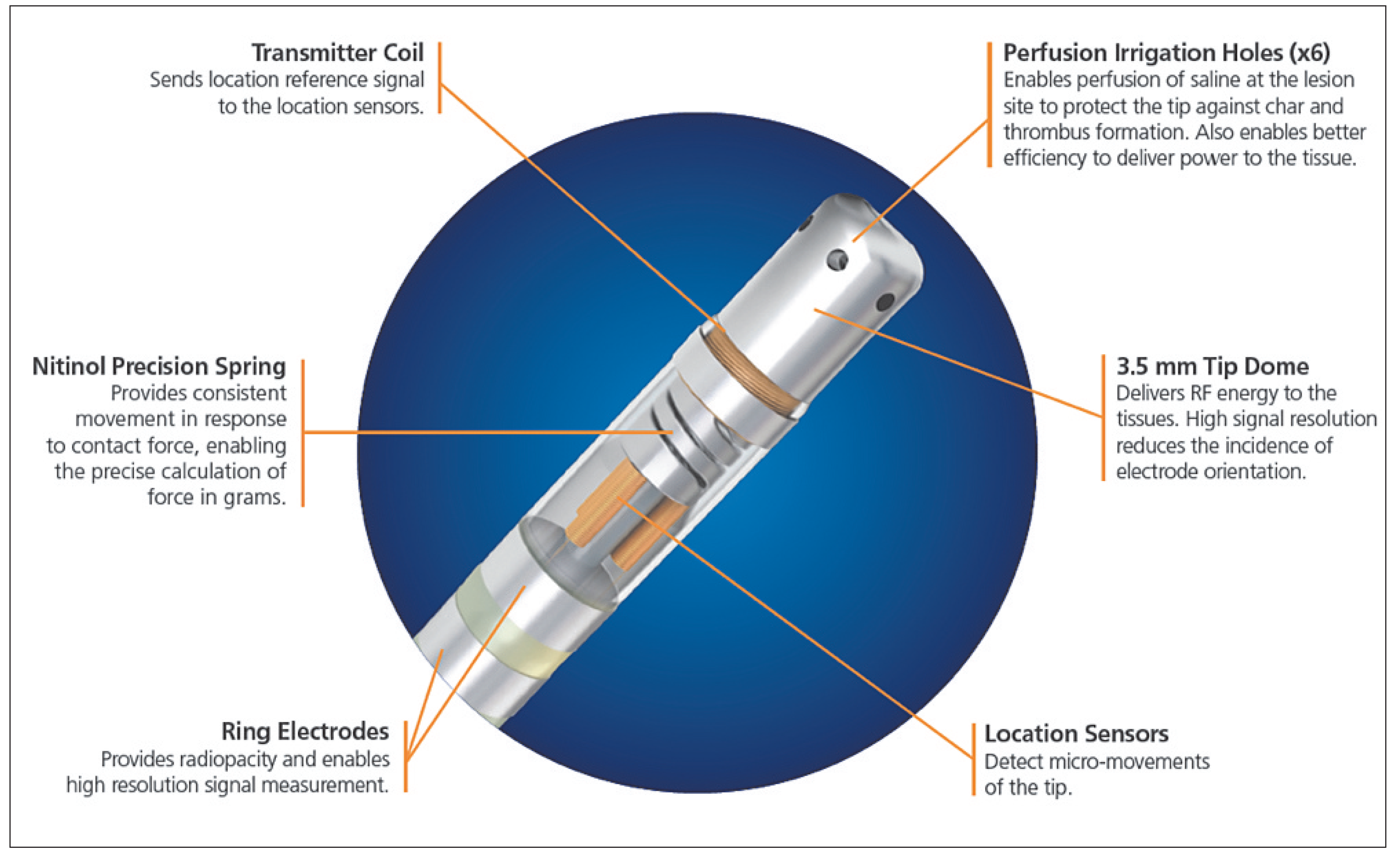
TactiCath® and SmartTouch™
Catheter contact force in mapping and ablation of ventricular tachycardia
Conclusion
Funding / potential competing interests
References
- Huang, S.K.; Graham, A.R.; Lee, M.A.; Ring, M.E.; Gorman, G.D.; Schiffman, R. Comparison of catheter ablation using radiofrequency versus direct current energy: biophysical, electrophysiologic and pathologic observations. J Am Coll Cardiol 1991, 18, 1091–1097. [Google Scholar] [CrossRef]
- Nath, S.; DiMarco, J.P.; Haines, D.E. Basic aspects of radiofrequency catheter ablation. J Cardiovasc Electrophysiol 1994, 5, 863–876. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of skin, muscle and brachial arterial blood temperatures in the resting normal human forearm. Am J Med Sci 1948, 215, 354. [Google Scholar]
- Nath, S.; Haines, D.E. Biophysics and pathology of catheter energy delivery systems. Prog Cardiovasc Dis 1995, 37, 185–204. [Google Scholar] [CrossRef]
- Wittkampf, F.H.; Hauer, R.N.; Robles de Medina, E.O. Control of radiofrequency lesion size by power regulation. Circulation 1989, 80, 962–968. [Google Scholar] [CrossRef]
- Haines, D. Biophysics of ablation: application to technology. J Cardiovasc Electrophysiol 2004, 15, S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.E.; Verow, A.F. Observations on electrode tissue interface temperature and effect on electrical impedance during radio frequency ablation of ventricular myocardium. Circulation 1990, 82, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.E.; Watson, D.D.; Verow, A.F. Electrode radius predicts lesion radius during radiofrequency energy heating. Validation of a proposed thermodynamic model. Circ Res 1990, 67, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.E. The biophysics of radiofrequency catheter ablation in the heart: the importance of temperature monitoring. Pacing Clin Electrophysiol 1993, 16, 586–591. [Google Scholar] [CrossRef]
- Langberg, J.J.; Gallagher, M.; Strickberger, S.A.; Amirana, O. Temperature guided radiofrequency catheter ablation with very large distal electrodes. Circulation 1993, 88, 245–9. [Google Scholar] [CrossRef]
- Panescu, D.; Whayne, J.G.; Fleischman, S.D.; Mirotznik, M.S.; Swanson, D.K.; Webster, J.G. Three dimensional finite element analysis of current density and temperature distributions during radio frequency ablation. IEEE Trans Biomed Eng 1995, 42, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.; Graham, A.R.; Bharati, S.; Lee, M.A.; Gorman, G.; Lev, M. Short and long term effects of transcatheter ablation of the coronary sinus by radiofrequency energy. Circulation 1988, 78, 416–427. [Google Scholar] [CrossRef]
- Haines, D.E. Determinants of Lesion Size During Radiofrequency Catheter Ablation: The Role of Electrode Tissue Contact Pressure and Duration of Energy Delivery. Journal of Cardiovascular Electrophysiology 1991, 2, 509–515. [Google Scholar] [CrossRef]
- Nath, S.; Lynch C3rd Whayne, J.G.; Haines, D.E. Cellular electrophysiological effects of hyperthermia on isolated guinea pig papillary muscle. Implications for catheter ablation. Circulation 1993, 88, 1826–1831. [Google Scholar] [CrossRef]
- Simmers, T.A.; de Bakker, J.M.; Wittkampf, F.H.; Hauer, R.N. Effects of heating with radiofrequency power on myocardial impulse conduction: is radiofrequency ablation exclusively thermally mediated? J Cardiovasc Electrophysiol 1996, 7, 243–247. [Google Scholar] [CrossRef]
- Simmers, T.A.; De Bakker, J.M.; Wittkampf, F.H.; Hauer, R.N. Effects of heating on impulse propagation in superfused canine myocardium. J Am Coll Cardiol 1995, 25, 1457–1464. [Google Scholar] [CrossRef]
- Calkins, H.; Prystowsky, E.; Carlson, M.; Klein, L.S.; Saul, J.P.; Gillette, P. Temperature monitoring during radiofrequency catheter ablation procedures using closed loop control. Atakr Multicenter Investigators Group. Circulation 1994, 90, 1279–1286. [Google Scholar] [CrossRef]
- Eick, O.J.; Gerritse, B.; Schumacher, B. Popping phenomena in temperature controlled radiofrequency ablation: when and why do they occur? Pacing Clin Electrophysiol 2000, 23, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Matsudaira, K.; Nakagawa, H.; Wittkampf, F.H.; et al. High incidence of thrombus formation without impedance rise during radiofrequency ablation using electrode temperature control. Pacing Clin Electrophysiol 2003, 26, 1227–1237. [Google Scholar] [CrossRef]
- Jais, P.; Shah, D.C.; Haissaguerre, M.; et al. Efficacy and safety of septal and left atrial linear ablation for atrial fibrillation. Am J Cardiol 1999, 84, 139R 46R. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.K.; Klein, G.J.; Yee, R.; Zardini, M. Embolic complications after radiofrequency catheter ablation. Am J Cardiol 1994, 74, 278–279. [Google Scholar] [CrossRef]
- Epstein, M.R.; Knapp, L.D.; Martindill, M.; et al. Embolic complications associated with radiofrequency catheter ablation. Atakr Investigator Group. Am J Cardiol 1996, 77, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Keane, D.; Reed, G.; Ruskin, J. Thromboembolic complications of cardiac radiofrequency catheter ablation: a review of the reported incidence, pathogenesis and current research directions. J Cardiovasc Electrophysiol 1999, 10, 611–620. [Google Scholar] [CrossRef]
- Yokoyama, K.; Nakagawa, H.; Wittkampf, F.H.; Pitha, J.V.; Lazzara, R.; Jackman, W.M. Comparison of electrode cooling between internal and open irrigation in radiofrequency ablation lesion depth and incidence of thrombus and steam pop. Circulation 2006, 113, 11–19. [Google Scholar] [CrossRef]
- Otomo, K.; Yamanashi, W.S.; Tondo, C.; et al. Why a large tip electrode makes a deeper radiofrequency lesion: effects of increase in electrode cooling and electrode tissue interface area. J Cardiovasc Electrophysiol 1998, 9, 47–54. [Google Scholar] [CrossRef]
- Nakagawa, H.; Yamanashi, W.S.; Pitha, J.V.; et al. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline irrigated electrode versus temperature control in a canine thigh muscle preparation. Circulation 1995, 91, 2264–2273. [Google Scholar] [CrossRef]
- Nakagawa, H.; Wittkampf, F.H.; Yamanashi, W.S.; et al. Inverse relationship between electrode size and lesion size during radiofrequency ablation with active electrode cooling. Circulation 1998, 98, 458–465. [Google Scholar] [CrossRef]
- Avitall, B.; Mughal, K.; Hare, J.; Helms, R.; Krum, D. The effects of electrode tissue contact on radiofrequency lesion generation. Pacing Clin Electrophysiol 1997, 20, 2899–2910. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, J.D.; Natale, A. New technologies in atrial fibrillation ablation. Circulation 2009, 120, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.C.; Lambert, H.; Nakagawa, H.; Langenkamp, A.; Aeby, N.; Leo, G. Area under the real time contact force curve (force time integral) predicts radiofrequency lesion size in an in vitro contractile model. J Cardiovasc Electrophysiol 2010, 21, 1038–1043. [Google Scholar] [CrossRef]
- Saliba, W.; Cummings, J.E.; Oh, S.; et al. Novel robotic catheter remote control system: feasibility and safety of transseptal puncture and endocardial catheter navigation. J Cardiovasc Electrophysiol 2006, 17, 1102–1105. [Google Scholar] [CrossRef]
- Di Biase, L.; Natale, A.; Barrett, C.; et al. Relationship between catheter forces, lesion characteristics, “popping,” and char formation: experience with robotic navigation system. J Cardiovasc Electrophysiol 2009, 20, 436–440. [Google Scholar] [CrossRef]
- Yokoyama, K.; Nakagawa, H.; Shah, D.C.; et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol 2008, 1, 354–362. [Google Scholar] [CrossRef]
- Neuzil, P.; Reddy, V.Y.; Kautzner, J.; et al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol 2013, 6, 327–33. [Google Scholar] [CrossRef]
- Nakagawa, H.; Kautzner, J.; Natale, A.; et al. Locations of high contact force during left atrial mapping in atrial fibrillation patients: electrogram amplitude and impedance are poor predictors of electrode tissue contact force for ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2013, 6, 746–753. [Google Scholar] [CrossRef]
- Gaspar, T.; Sih, H.; Hindricks, G.; et al. Use of electrical coupling information in AF catheter ablation: a prospective randomized pilot study. Heart Rhythm 2013, 10, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Al Ahmad, A.; Grossman, J.D.; Wang, P.J. Early experience with a computerized robotically controlled catheter system. J Interv Card Electrophysiol 2005, 12, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Kanagaratnam, P.; Koa Wing, M.; Wallace, D.T.; Goldenberg, A.S.; Peters, N.S.; Davies, D.W. Experience of robotic catheter ablation in humans using a novel remotely steerable catheter sheath. J Interv Card Electrophysiol 2008, 21, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Saliba, W.; Reddy, V.Y.; Wazni, O.; et al. Atrial fibrillation ablation using a robotic catheter remote control system: initial human experience and long term follow up results. J Am Coll Cardiol 2008, 51, 2407–2411. [Google Scholar] [CrossRef]
- Okumura, Y.; Johnson, S.B.; Bunch, T.J.; Henz, B.D.; O’Brien, C.J.; Packer, D.L. A systematical analysis of in vivo contact forces on virtual catheter tip/tissue surface contact during cardiac mapping and intervention. J Cardiovasc Electrophysiol 2008, 19, 632–640. [Google Scholar] [CrossRef]
- Piorkowski, C.; Sih, H.; Sommer, P.; et al. First in human validation of impedance based catheter tip to tissue contact assessment in the left atrium. J Cardiovasc Electrophysiol 2009, 20, 1366–1373. [Google Scholar] [CrossRef]
- Perna, F.; Heist, E.K.; Danik, S.B.; Barrett, C.D.; Ruskin, J.N.; Mansour, M. Assessment of catheter tip contact force resulting in cardiac perforation in swine atria using force sensing technology. Circ Arrhythm Electrophysiol 2011, 4, 218–224. [Google Scholar] [CrossRef]
- Thiagalingam, A.; D’Avila, A.; Foley, L.; et al. Importance of catheter contact force during irrigated radiofrequency ablation: evaluation in a porcine ex vivo model using a force sensing catheter. J Cardiovasc Electrophysiol 2010, 21, 806–811. [Google Scholar] [CrossRef]
- Haqqani, H.M.; Marchlinski, F.E. Creating lesions and indexing transmural ablation: pushing harder to find the Holy Grail. J Cardiovasc Electrophysiol 2010, 21, 812–814. [Google Scholar] [CrossRef]
- Reichlin, T.; Knecht, S.; Lane, C.; et al. Initial impedance decrease as an indicator of good catheter contact: insights from radiofrequency ablation with force sensing catheters. Heart Rhythm 2014, 11, 194–201. [Google Scholar] [CrossRef]
- Ikeda, A.; Nakagawa, H.; Lambert, H.; et al. Relationship between catheter contact force and radiofrequency lesion size and incidence of steam pop in the beating canine heart: electrogram amplitude, impedance, and electrode temperature are poor predictors of electrode tissue contact force and lesion size. Circ Arrhythm Electrophysiol 2014, 7, 1174–1180. [Google Scholar]
- Kuck, K.H.; Reddy, V.Y.; Schmidt, B.; et al. A novel radiofrequency ablation catheter using contact force sensing: Toccata study. Heart Rhythm 2012, 9, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Lambert, H.; Langenkamp, A.; et al. Catheter tip force required for mechanical perforation of porcine cardiac chambers. Europace 2011, 13, 277–283. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Shah, D.; Kautzner, J.; et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm 2012, 9, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Morton, J.B.; Lee, J.; et al. Prospective characterization of catheter tissue contact force at different anatomic sites during antral pulmonary vein isolation. Circ Arrhythm Electrophysiol 2012, 5, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Martinek, M.; Lemes, C.; Sigmund, E.; et al. Clinical impact of an open irrigated radiofrequency catheter with direct force measurement on atrial fibrillation ablation. Pacing Clin Electrophysiol 2012, 35, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Jarman, J.W.; Panikker, S.; et al. Contact force sensing technology identifies sites of inadequate contact and reduces acute pulmonary vein reconnection: A prospective case control study. Int J Cardiol 2012. [CrossRef] [PubMed]
- Park, C.I.; Lehrmann, H.; Keyl, C.; et al. Mechanisms of pulmonary vein reconnection after radiofrequency ablation of atrial fibrillation: the deterministic role of contact force and interlesion distance. J Cardiovasc Electrophysiol 2014, 25, 701–708. [Google Scholar] [CrossRef]
- Natale, A. Ablation Of Symptomatic Paroxysmal Atrial Fibrillation Using Novel Contact Force Catheter: SMART AF Trial, SP22, Heart Rhyhtm Society Meeting 2013.
- Piorkowski, C.; Eitel, C.; Rolf, S.; et al. Steerable versus nonsteerable sheath technology in atrial fibrillation ablation: a prospective, randomized study. Circ Arrhythm Electrophysiol 2011, 4, 157–165. [Google Scholar] [CrossRef]
- Arentz, T.; Macle, L.; Kalusche, D.; et al. “Dormant” pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2004, 15, 1041–1047. [Google Scholar] [CrossRef]
- Epstein, L.M.; Mitchell, M.A.; Smith, T.W.; Haines, D.E. Comparative study of fluoroscopy and intracardiac echocardiographic guidance for the creation of linear atrial lesions. Circulation 1998, 98, 1796–1801. [Google Scholar] [CrossRef]
- Mizuno, H.; Vergara, P.; Maccabelli, G.; et al. Contact force monitoring for cardiac mapping in patients with ventricular tachycardia. J Cardiovasc Electrophysiol 2013, 24, 519–524. [Google Scholar] [CrossRef]
- Tilz, R.R.; Makimoto, H.; Lin, T.; et al. In vivo left ventricular contact force analysis: comparison of antegrade transseptal with retrograde transaortic mapping strategies and correlation of impedance and electrical amplitude with contact force. Europace 2014. [CrossRef]
- Sacher, F.; Wright, M.; Derval, N.; et al. Endocardial versus epicardial ventricular radiofrequency ablation: utility of in vivo contact force assessment. Circ Arrhythm Electrophysiol 2013, 6, 144–150. [Google Scholar] [CrossRef]
- Wong, M.C.; Edwards, G.; Spence, S.J.; et al. Characterization of catheter tissue contact force during epicardial radiofrequency ablation in an ovine model. Circ Arrhythm Electrophysiol 2013, 6, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Valk, S.D.; de Groot, N.M.; Jordaens, L. Catheter ablation of right ventricular outflow tract tachycardia using contact force guidance. Neth Heart J 2014. [CrossRef] [PubMed][Green Version]
- Dabiri Abkenari, L.; Akca, F.; Van Mieghem, N.M.; Szili Torok, T. The first human experience of a contact force sensing catheter for epicardial ablation of ventricular tachycardia. Neth Heart J 2014. [CrossRef] [PubMed]
© 2015 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Namdar, M.; Shah, D.C. Real-Time Contact Force Measurement for Catheter Ablation of Cardiac Arrhythmias. Cardiovasc. Med. 2015, 18, 155. https://doi.org/10.4414/cvm.2015.00313
Namdar M, Shah DC. Real-Time Contact Force Measurement for Catheter Ablation of Cardiac Arrhythmias. Cardiovascular Medicine. 2015; 18(5):155. https://doi.org/10.4414/cvm.2015.00313
Chicago/Turabian StyleNamdar, Mehdi, and Dipen C. Shah. 2015. "Real-Time Contact Force Measurement for Catheter Ablation of Cardiac Arrhythmias" Cardiovascular Medicine 18, no. 5: 155. https://doi.org/10.4414/cvm.2015.00313
APA StyleNamdar, M., & Shah, D. C. (2015). Real-Time Contact Force Measurement for Catheter Ablation of Cardiac Arrhythmias. Cardiovascular Medicine, 18(5), 155. https://doi.org/10.4414/cvm.2015.00313



