Assisted Reproduction: A Novel Cardiovascular Risk Factor
Abstract
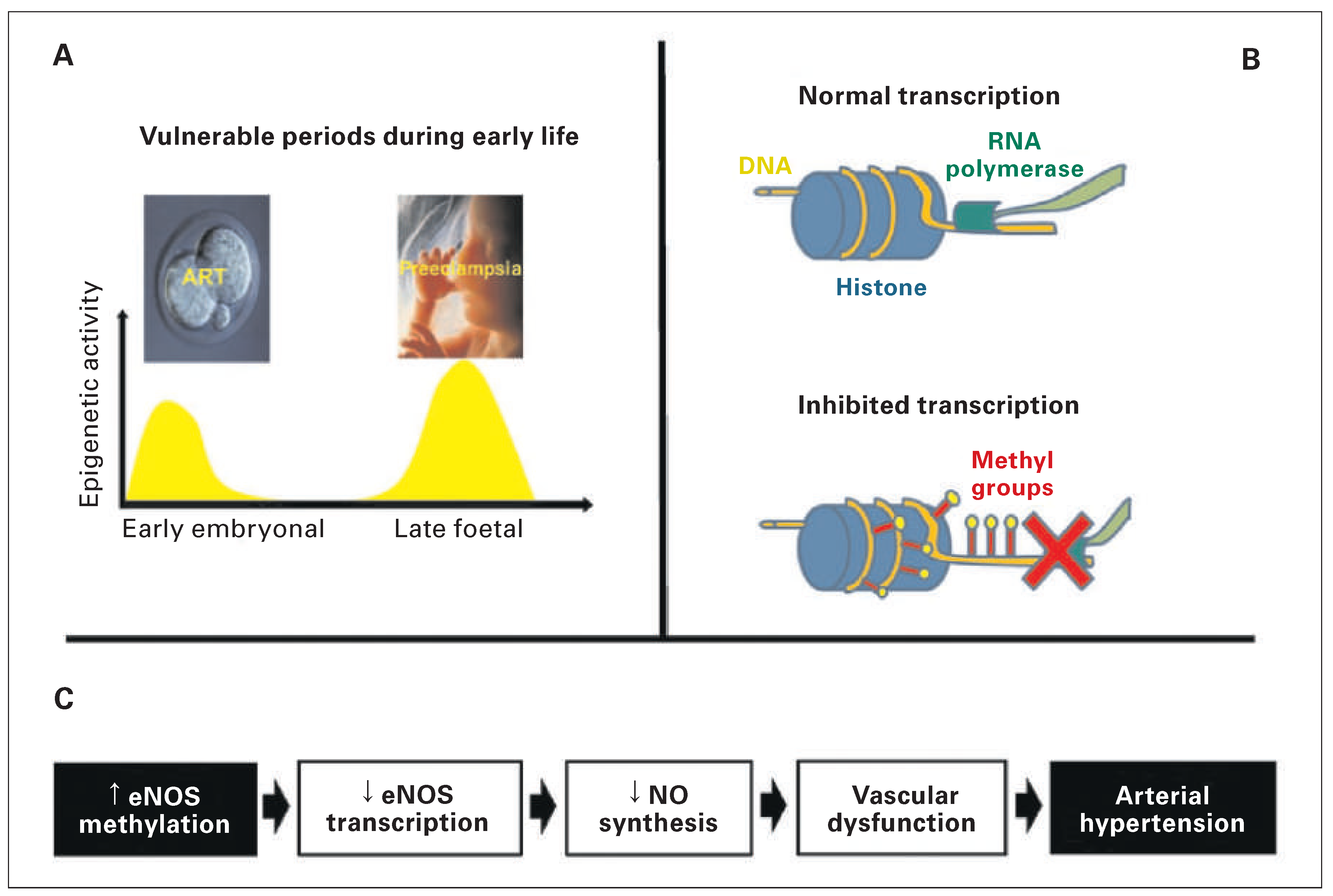
Introduction
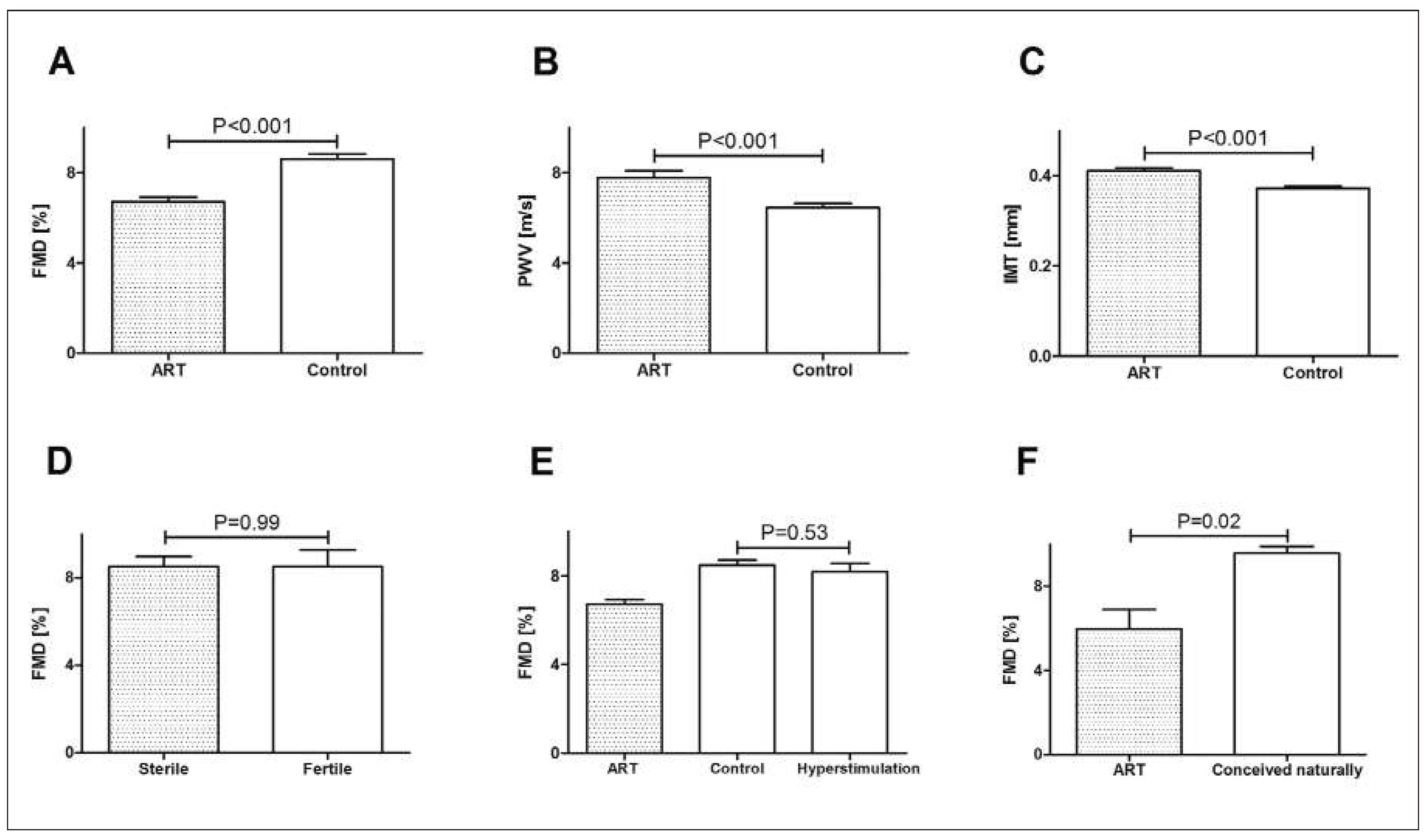
Studies in humans
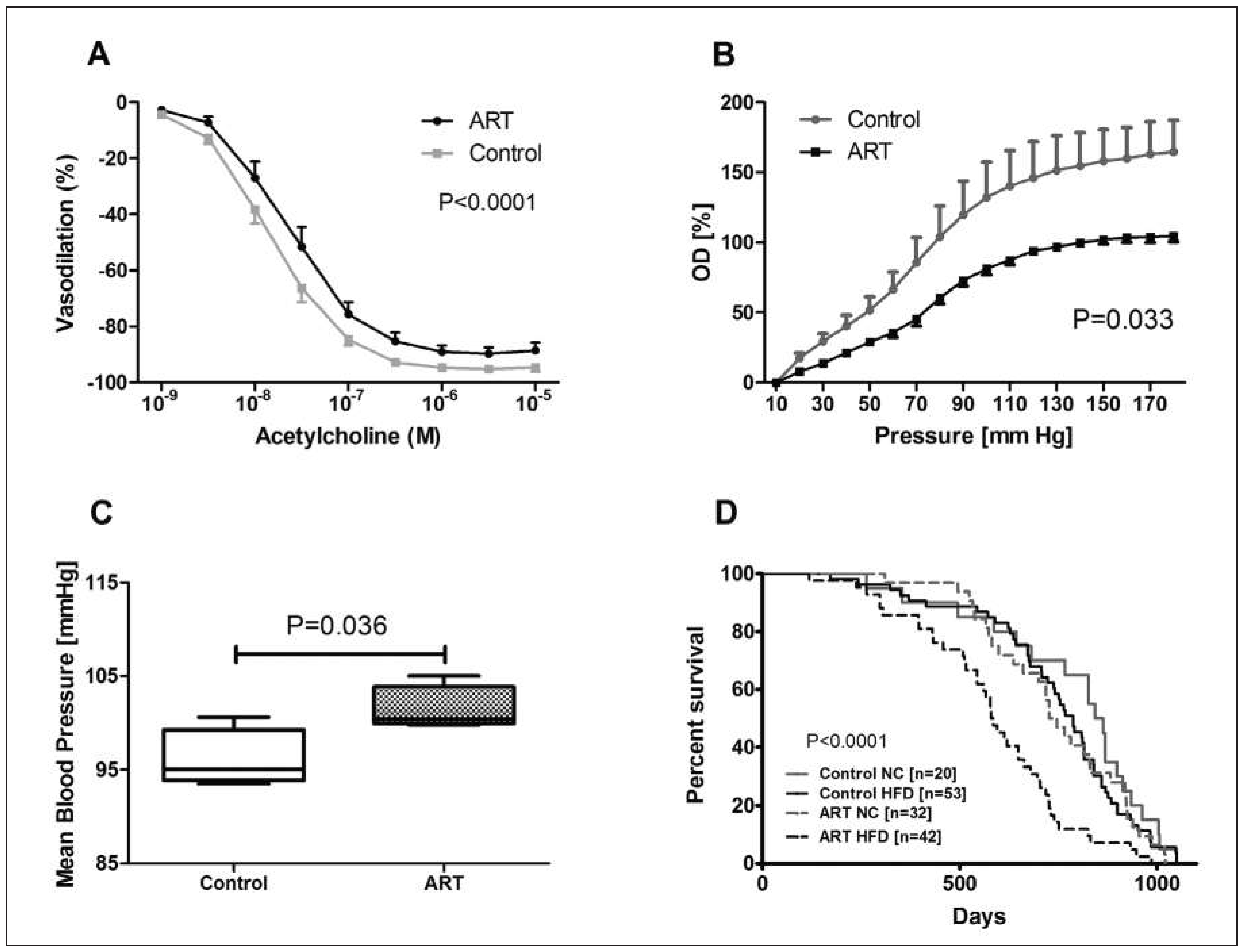
Studies in mice
Conclusion
Funding / potential competing interests:
References
- Barker, D.J. Maternal and fetal origins of coronary heart disease. J R Coll Physicians Lond. 1994, 28, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Sartori, C.; Allemann, Y.; Trueb, L.; Delabays, A.; Nicod, P.; Scherrer, U. Augmented vasoreactivity in adult life associated with perinatal vascular insult. Lancet. 1999, 353, 2205–2207. [Google Scholar] [CrossRef] [PubMed]
- Jayet, P.Y.; Rimoldi, S.F.; Stuber, T.; Salmon, C.S.; Hutter, D.; Rexhaj, E.; et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010, 122, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Rexhaj, E.; Bloch, J.; Jayet, P.Y.; Rimoldi, S.F.; Dessen, P.; Mathieu, C.; et al. Fetal programming of pulmonary vascular dysfunction in mice: role of epigenetic mechanisms. Am J Physiol Heart Circ Physiol. 2011, 301, H247–52. [Google Scholar] [CrossRef] [PubMed]
- Rexhaj, E.; Paoloni-Giacobino, A.; Rimoldi, S.F.; Fuster, D.G.; Anderegg, M.; Somm, E.; et al. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J Clin Invest. 2013, 123, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, S.F.; Sartori, C.; Rexhaj, E.; Cerny, D.; Von Arx, R.; Soria, R.; et al. Vascular dysfunction in children conceived by assisted reproductive technologies: underlying mechanisms and future implications. Swiss Med Wkly. 2014, 144, w13973. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, U.; Rimoldi, S.F.; Rexhaj, E.; Stuber, T.; Duplain, H.; Garcin, S.; et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation. 2012, 125, 1890–1896. [Google Scholar] [PubMed]
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, A.G.; Ludwig, M. Outcome of assisted reproduction. Lancet. 2007, 370, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Engelen, L.; Ferreira, I.; Stehouwer, C.D.; Boutouyrie, P.; Laurent, S. Reference intervals for common carotid intima-media thickness measured with echotracking: relation with risk factors. Eur Heart J. 2013, 34, 2368–2380. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, S.F.; Sartori, C.; Rexhaj, E.; Bailey, D.M.; Marchi, S.F.; McEneny, J.; et al. Antioxidants improve vascular function in children conceived by assisted reproductive technologies: A randomized double-blind placebo-controlled trial. Eur J Prev Cardiol 2014. [CrossRef] [PubMed]
- Hart, R.; Norman, R.J. The longer-term health outcomes for children born as a result of IVF treatment: Part I General health outcomes. Hum Reprod Update. 2013, 19, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Belva, F.; Roelants, M.; De Schepper, J.; Roseboom, T.J.; Bonduelle, M.; Devroey, P.; et al. Blood pressure in ICSI-conceived adolescents. Hum Reprod. 2012, 27, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, M.; van Weissenbruch, M.M.; Vermeiden, J.P.; van Leeuwen, F.E.; Delemarre-van de Waal, H.A. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008, 93, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Alcaraz, B.; Crispi, F.; Bijnens, B.; Cruz-Lemini, M.; Creus, M.; Sitges, M.; et al. Assisted reproductive technologies are associated with cardiovascular remodeling in utero that persists postnatally. Circulation. 2013, 128, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Cowley, A.W.; Jr Nadeau, J.H.; Baccarelli, A.; Berecek, K.; Fornage, M.; Gibbons, G.H.; et al. Report of the National Heart, Lung, and Blood Institute Working Group on epigenetics and hypertension. Hypertension. 2012, 59, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Haaf, T. Aberrant methylation patterns at the two-cell stage as an indicator of early developmental failure. Mol Reprod Dev.. 2002, 63, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Rexhaj, E.R.S.; Giacobino, A.; Sartori, C.; Allemann, Y.; Scherrer, U. Epigenetically-induced systemic vascular dysfunction and hypertension by in vitro fertilization; prevention by addition of melatonin to the culture media. FASEB J. 2012, 26, 874. [Google Scholar]
- Rexhaj, E.R.S.; Brenner, R.; Pireva, A.; Cerny, D.; Von Arx, R.; Sartori, C.; et al. Role of foetal programming and epigenetic mechanisms in the pathogenesis of arterial hypertension. Cardiovascular Medicine. 2014, 42–45. [Google Scholar]

© 2015 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Rexhaj, E.; Rimoldi, S.F.; Bouillet, E.; Cerny, D.; Soria, R.; Von Arx, R.; Allemann, Y.; Sartori, C.; Scherrer, U. Assisted Reproduction: A Novel Cardiovascular Risk Factor. Cardiovasc. Med. 2015, 18, 115. https://doi.org/10.4414/cvm.2015.00311
Rexhaj E, Rimoldi SF, Bouillet E, Cerny D, Soria R, Von Arx R, Allemann Y, Sartori C, Scherrer U. Assisted Reproduction: A Novel Cardiovascular Risk Factor. Cardiovascular Medicine. 2015; 18(4):115. https://doi.org/10.4414/cvm.2015.00311
Chicago/Turabian StyleRexhaj, Emrush, Stefano F. Rimoldi, Elisa Bouillet, David Cerny, Rodrigo Soria, Robert Von Arx, Yves Allemann, Claudio Sartori, and Urs Scherrer. 2015. "Assisted Reproduction: A Novel Cardiovascular Risk Factor" Cardiovascular Medicine 18, no. 4: 115. https://doi.org/10.4414/cvm.2015.00311
APA StyleRexhaj, E., Rimoldi, S. F., Bouillet, E., Cerny, D., Soria, R., Von Arx, R., Allemann, Y., Sartori, C., & Scherrer, U. (2015). Assisted Reproduction: A Novel Cardiovascular Risk Factor. Cardiovascular Medicine, 18(4), 115. https://doi.org/10.4414/cvm.2015.00311



