Abstract
Late valve thrombosis is uncommon after trans catheter aortic valve implantation. Herein, we describe such a complication after transcatheteraortic valve implantation of an Edwards SAPIEN XT aortic bioprosthesis valve.
Case report
A 70-year-old caucasian male was admitted for elective aortic valve replacement (AVR) because of severe, symptomatic aortic stenosis. Preoperative transthoracic echocardiography (TTE) showed a concentric hypertrophic left ventricle with preserved left ventricular ejection fraction (EF 65%) and a severely calcified aortic valve with a maximum pressure gradient (PG) of 82 mm Hg and a mean PG of 52 mm Hg. The preoperative left and right heart catheterisation revealed a mean PG of 54 mm Hg and an aortic valve area (AVA, according to the Gorlin Equation) of 0.74 cm2. Preprocedural coronary angiography revealed only minor coronary artery disease. The EuroSCORE II was 2.15% and the STS score for mortality was 1.11%. Owing to a heavily calcified ascending aorta and peripheral vascular disease, the patient was scheduled for transcatheter aortic valve implantation (TAVI) via a transapical approach.
Transcatheter aortic valve implantation was successfully performed with a 29 mm, Edwards SAPIEN XT bioprosthesis (Edwards Lifesciences Corp., Irvine, CA, USA). After an uneventful postoperative period, the patient was discharged on day 10 on aspirin and clopidogrel and advised to stop the clopidogrel after 3 months. Discharge TTE showed normal function of the aortic valve prosthesis with a mean PG of 9 mm Hg and no paravalvular regurgitation. TTE performed at 3-month follow-up revealed normal function of the bioprosthetic aortic valve with a mean PG of 10 mm Hg. Because of a transient ischaemic attack (TIA) 7 months after TAVI the antiplatelet therapy was changed from aspirin to clopidogrel monotherapy. Thirteen months after TAVI the patient suddenly became symptomatic, with dyspnoea (New York Heart Association [NYHA] functional class III) and angina pectoris (Canadian Cardiovascular Society [CCS] functional class II). TTE revealed thickened cusps of the aortic valve prosthesis, a mean PG of 57 mm Hg, and an AVA (2D planimetry) of 0.7 cm2. These findings were confirmed by a transoesophageal echocardiography (TEE), where a homogeneous thickening of all three cusps of the aortic valve prosthesis with significant reduced mobility was observed (Figure 1 and Figure 2). Obstructive valve thrombosis was suspected and oral anticoagulation was initiated. In accordance with the consensus document of the European Society of Cardiology on the management of valvular heart disease [1] unfractioned intravenous heparin and a vitamin K antagonist (VKA) were started and heparin was stopped after 8 days when the patient’s international normalised ratio (INR) was 2.34. The antiplatelet therapy with clopidogrel was continued. Over the next few days the patient’s symptoms improved rapidly and no more dyspnoea or angina pectoris occurred. The patient was discharged on day 8 on a VKA and clopidogrel.
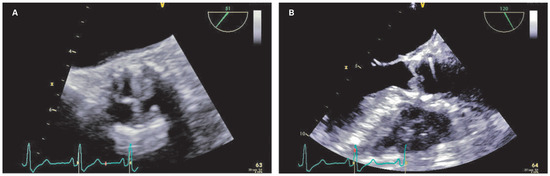
Figure 1.
Transoesophageal echocardiography views of the prosthetic aortic valve before starting anticoagulation (short axis [A] and long axis [B]).
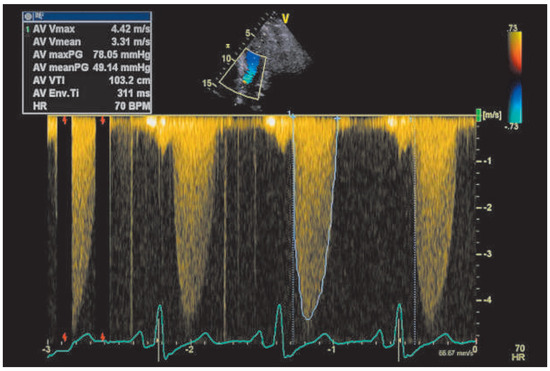
Figure 2.
Continuous-wave Doppler of the prosthetic aortic valve before starting anticoagulation.
At a follow-up visit 9 weeks later the patient remained asymptomatic. TTE showed completely restored function of the aortic valve prosthesis with a significant reduction of the mean PG to 18 mm Hg. In addition, TEE showed only a minimal residual thickening of the cusps and no more restriction of the cusp excursion (Figure 3 and Figure 4). Five months later at a further follow-up visit the mean PG remained at 17 mm Hg.
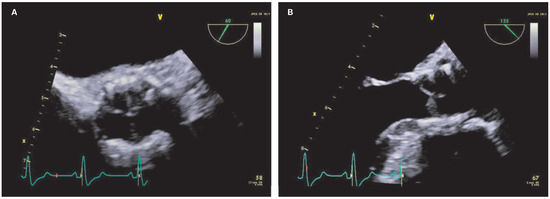
Figure 3.
Transoesophageal echocardiography views of the prosthetic aortic valve after starting anticoagulation (short axis [A] and long axis [B]).
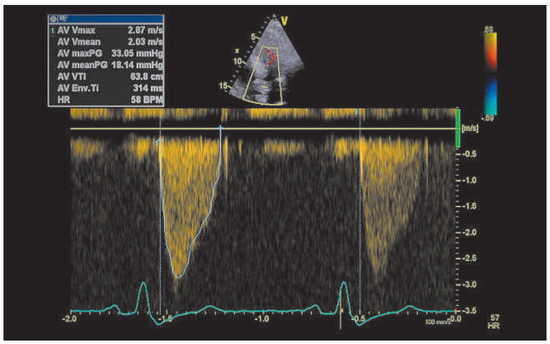
Figure 4.
Continuous-wave Doppler of the prosthetic aortic valve after starting anticoagulation.
Discussion
Thrombotic obstruction of a bioprosthesis aortic valve is a rare complication after transcatheter aortic valve implantation. Edwards SAPIEN transcatheter aortic valve prostheses have been implanted since 2007 [2] and consist of a cobalt chromium frame with bovine pericardial tissue valves [3]. To date, there have been only 12 reports concerning thrombotic obstruction of Edwards SAPIEN transcatheter aortic valve prostheses in 18 patients so far (Table 1) [4,5,6,7,8,9,10,11,12,13,14,15,16]. A recent retrospective multicentre study reported valve thrombosis after transcatheter aortic valve implantation in 26 of 4 266 patients (0.61%). In 20 patients valve thrombosis occurred in the Edwards SAPIEN cohort, in 6 patients valve thrombosis occurred in the Medtronic CoreValve (Medtronic PLC, Dublin, Ireland) cohort [17]. In a single-centre cohort after TAVI (Edwards SAPIEN and Medtronic CoreValve) prosthetic valve thrombosis was found in 2 of 130 patients (2.7%) at 1 year [18].

Table 1.
Cases of Edwards SAPIEN bioprosthesis valve thrombosis reported in the literature.
Although the description of the appearance varies, there seem to be two different morphologies to be distinguished in thrombotic bioprosthesis valve obstruction: a definite thrombus inhibiting normal movement of the leaflets, or thrombotic thickening of the cusps resulting in reduced mobility of the leaflets. Latib et al. [17] found thickened leaflets or thrombotic apposition in leaflets in 20 (77%) patients and a thrombotic mass on the leaflets in 6 (23%) patients. Most of the cases occurred within the first year after implantation, with a median time of 181 days after implantation [17].
There are several pathophysiological mechanisms possibly related to restenosis after transcatheter aortic valve implantation. Leaflet fracture during crimping or implantation, geometric deformation of the annulus of the implanted valve due to oversizing, and valve thrombosis. Nonetheless histological changes with fragmentation and disruption of collagen fibres have been described [19]. Furthermore, valve endocarditis and coagulation disorders should be considered. Inadequate antiplatelet therapy may also have an impact on the occurrence of valve thrombosis. In the present case, no signs of structural damage of the valve were present and no transvalvular or paravalvular leakage was seen. There were no signs of infection. Dual antiplatelet therapy (DAPT) with aspirin (prescribed indefinitely) and clopidogrel (prescribed for 3 months) was given, although after 7 months antiplatelet therapy was switched from aspirin to clopidogrel as a result of a TIA. Resistance to clopidogrel with high platelet reactivity index was not excluded. Switching to clopidogrel monotherapy was possibly an incriminating factor for valve thrombosis either through clopidogrel resistance or by simply not offering as good protection as aspirin monotherapy. Compliance with medical therapy was good. Evidence concerning antiplatelet therapy in transcatheter aortic valve implantation is scarce and is historically based on the management of coronary stenting. In the AHA/ACC Guidelines for Management of Patients with Valvular Heart Disease 2014 [20] DAPT with aspirin (indefinitely continued) and clopidogrel (continued for 6 months) is rated as reasonable (Class IIb, Evidence level C). In the present case, administration of anticoagulation completely restored the function of the bioprosthesis as it did in most of the cases reported in literature. In three cases surgical replacement with a conventional aortic valve bioprosthesis was required [5,8,9]. The optimal duration of anticoagulant therapy in absence of a clear cause for valve thrombosis is unknown. In most cases reported in literature, anticoagulant therapy was continued indefinitely. Anticoagulant therapy resulted in a significant decrease of the aortic pressure gradient within 2 months in 88% of patients with valve thrombosis [17].
Oversizing of the implanted bioprosthesis valve could be a risk factor for valve thrombosis due to injury to the pericardial tissue of the valve. In this patient, mean annulus diameter measured with multislice computed tomography was 24 mm. According to sizing recommendations for the Edwards SAPIEN XT valve, a 26 mm valve is recommended, although 24 mm is the upper limit for this size [21]. After balloon valvuloplasty with a 25 mm Edwards balloon, the decision was made to implant a 29 mm valve. So oversizing could play a role in the present case.
In summary, in the case of restenosis occurring after transcatheter aortic valve implantation, it is necessary to consider the appearance of the valve prosthesis together with the possible pathophysiological mechanisms when deciding the best treatment strategies. Larger studies with longer follow-up are needed to demonstrate the frequency and the best management strategies of patients presenting with obstructive valve thrombosis following transcatheter aortic valve implantation.
Disclosures
No financial support and no other potential conflict of interest relevant to this article was reported.
References
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; BarónEsquivias, G.; Baumgartner, H.; et al. ESC Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012, 33, 2451–2496. [Google Scholar] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010, 363, 1597–1607. [Google Scholar] [PubMed]
- http://www.edwards.com/eu/Products/Transcatheter Valves/Pages/sapienxt.aspx.
- Pache, G.; Blanke, P.; Zeh, W.; Jander, N. Cusp thrombosis after transcatheter aortic valve replacement detected by computed tomography and echocardiography. Eur Heart J. 2013, 34, 3546. [Google Scholar] [CrossRef] [PubMed]
- Trepels, T.; Martens, S.; Doss, M.; Fichtlscherer, S.; Schächinger, V. Images in cardiovascular medicine. Thrombotic restenosis after minimally invasive implantation of aortic valve stent. Circulation. 2009, 120, e23–4. [Google Scholar] [PubMed]
- Cota, L.; Stabile, E.; Agrusta, M.; Sorropago, G.; Pucciarelli, A.; Ambrosini, V.; Mottola, G.; Esposito, G.; Rubino, P. Bioprostheses "thrombosis" after transcatheter aortic valve replacement. J Am Coll Cardiol. 2013, 61, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Kefer, J.; Astarci, P.; Renkin, J.; Glineur, D.; Pierard, S.; Seldrum, S.; Vanoverschelde, J.L. Images and case reports in interventional cardiology. Thrombotic aortic restenosis after transapical Sapien valve implantation. Circ Cardiovasc Interv. 2010, 3, 289–292. [Google Scholar] [PubMed]
- Greason, K.L.; Mathew, V.; Sarano, M.E.; Maleszewski, J.J.; Suri, R.M.; Rihal, C.S. Early transcatheter aortic valve thrombosis. J Card Surg. 2013, 28, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Pingpoh, C.; Pache, G.; Nawras, D.; Guenkel, L.; Sami, K.; Zeh, W.; Zimmer, E.; Jander, N.; Siepe, M.; Beyersdorf, F. Valve thrombosis 7 months after transcatheter aortic valve implantation. Ann Thorac Surg. 2014, 98, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Leetmaa, T.H.; Poulsen, S.H.; Nørgaard, B.L.; Klaaborg, K.E.; Terp, K.; Høyer, S.; Christiansen, E.H.; Andersen, H.R. Two cases of thrombotic stenosis in SAPIEN XT valves after transapical implantation. EuroIntervention. 2014, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Descoux, J.; Gautier-Pignonblanc, P.; Innorta, A.; Durel, N.; Camilleri, L.; Motreff, P.; Lusson, J.R.; Souteyrand, G. Effectiveness of anticoagulant therapy in the treatment of post-TAVI bioprosthetic thrombosis. J Cardiothorac Surg. 2015, 10, 50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orbach, A.; Karkabi, B.; Shiran, A. Reversible restenosis after transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging. 2014, 15, 350. [Google Scholar][Green Version]
- Tay, E.L.; Gurvitch, R.; Wijeysinghe, N.; Nietlispach, F.; Wood, D.; Allard, M.; Munt, B.; Cheung, A.; Webb, J.G. Valve thrombosis after transcatheter heart valve implantation. EuroIntervention. 2011, 7, 170–171. [Google Scholar] [PubMed][Green Version]
- Pergolini, A.; Pino, P.G.; Zampi, G.; Polizzi, V.; Musumeci, F. Thrombotic aortic restenosis after transapical SAPIEN valve implantation. J Card Surg. 2014, 29, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Latib, A.; Messika-Zeitoun, D.; Maisano, F.; Himbert, D.; Agricola, E.; Brochet, E.; Alfieri, O.; Colombo, A.; Vahanian, A. Reversible Edwards Sapien XT dysfunction due to prosthesis thrombosis presenting as early structural deterioration. J Am Coll Cardiol. 2013, 61, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Mylotte, D.; Andalib, A.; Thériault-Lauzier, P.; Dorfmeister, M.; Girgis, M.; Alharbi, W.; Chetrit, M.; Galatas, C.; Mamane, S.; Sebag, I.; et al. Transcatheter heart valve failure: a systematic review. Eur Heart J. 2014. [Google Scholar] [CrossRef]
- Latib, A.; Naganuma, T.; Abdel-Wahab, M.; Danenberg, H.; Cota, L.; Barbanti, M.; Baumgartner, H.; Finkelstein, A.; Legrand, V.; de Lezo, J.S.; et al. Treatment and clinical outcomes of transcatheter heart valve thrombosis. Circ Cardiovasc Interv. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Stähli, B.E.; Bünzli, R.; Grünenfelder, J.; Bühler, I.; Felix, C.; Bettex, D.; Biaggi, P.; Tanner, F.C.; Nguyen-Kim, D.L.; Plass, A.; et al. Transcatheter aortic valve implantation (TAVI) outcome according to standardized endpoint definitions by the Valve Academic Research Consortium (VARC). J Invasive Cardiol. 2011, 23, 307–312. [Google Scholar] [PubMed]
- Zegdi, R.; Bruneval, P.; Blanchard, D.; Fabiani, J.N. Evidence of leaflet injury during percutaneous aortic valve deployment. Eur J Cardiothorac Surg. 2011, 40, 257–259. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin JP3rd Guyton, R.A.; O'Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; Sundt, T.M.; et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014, 129, e521–643. [Google Scholar] [CrossRef]
- Kasel, A.M.; Cassese, S.; Bleiziffer, S.; Amaki, M.; Hahn, R.T.; Kastrati, A.; Sengupta, P.P. Standardized imaging for aortic annular sizing: implications for transcatheter valve selection. JACC Cardiovasc Imaging. 2013, 6, 249–262. [Google Scholar]
© 2015 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.