Pregnancy-Associated Cardiomyopathies
Summary
Introduction
Normal physiological changes during pregnancy
Hypertensive complications in pregnancy and impact on the cardiovascular system
Familial predisposition and genetic forms of pregnancy-associated cardiomyopathies
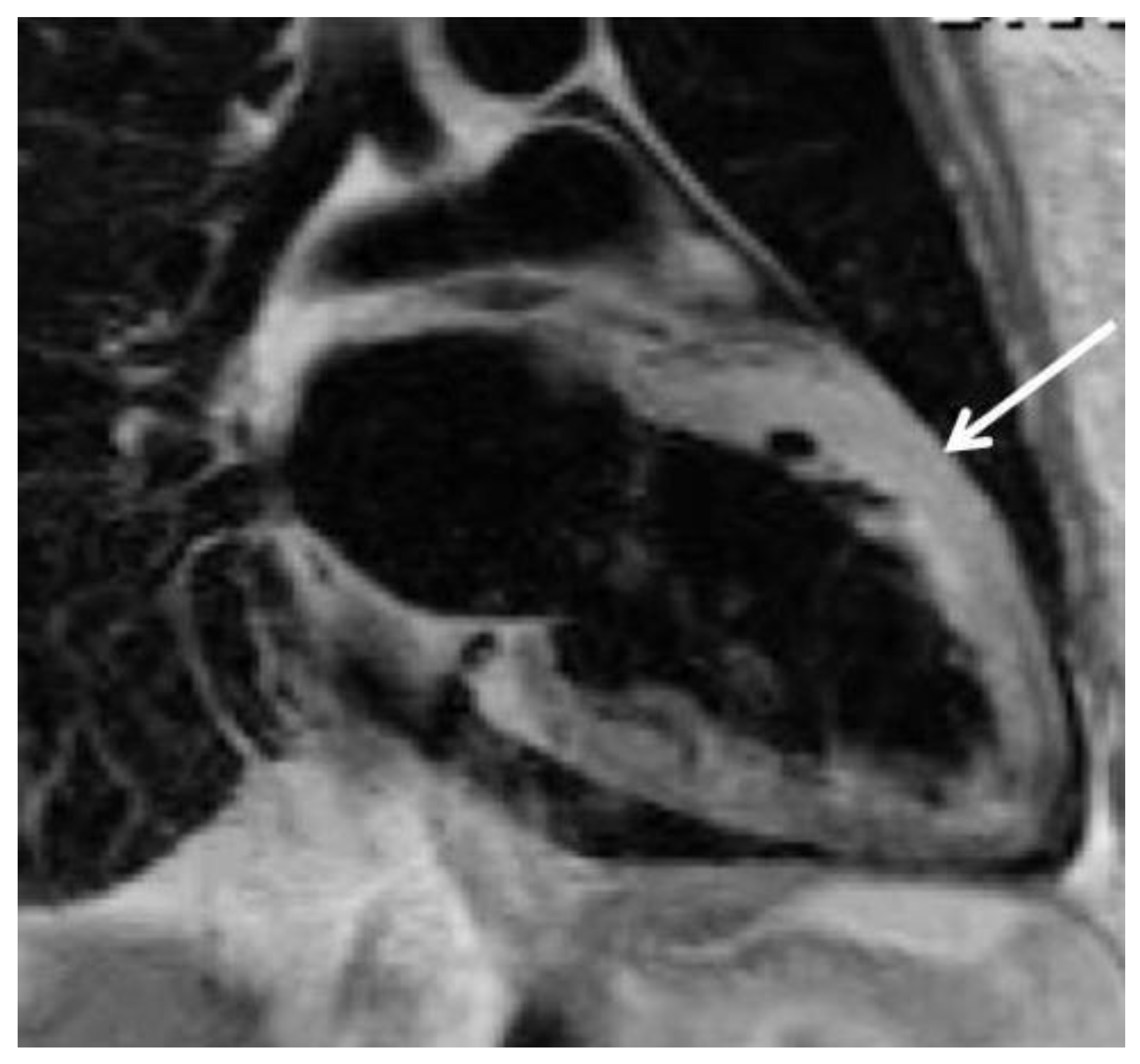
Peripartum cardiomyopathy (PPCM)
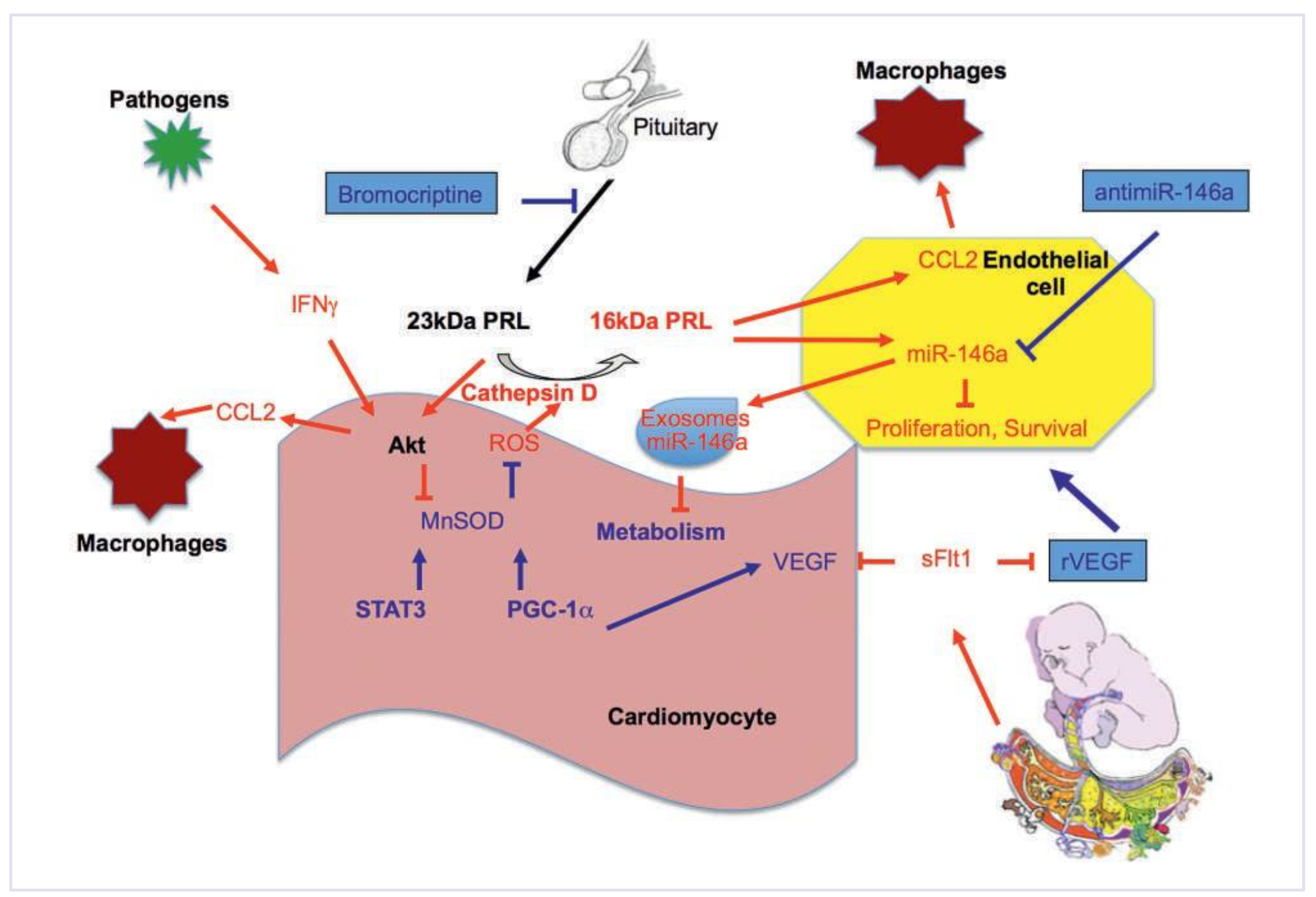
Pathomechanisms of PPCM
Biomarkers
Therapeutic concepts and management for PPCM
Conclusion
Funding
Potential Competing Interests
References
- Regitz-Zagrosek, V.; Blomstrom Lundqvist, C.; Borghi, C.; Cifkova, R.; Ferreira, R.; Foidart, J.M.; et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011, 32, 3147–3197. [Google Scholar] [PubMed][Green Version]
- Ruys, T.P.; Roos-Hesselink, J.W.; Hall, R.; Subirana-Domenech, M.T.; GrandoTing, J.; Estensen, M.; et al. Heart failure in pregnant women with cardiac disease: data from the ROPAC. Heart 2014, 100, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Leinwand, L.A. Pregnancy as a cardiac stress model. Cardiovasc Res. 2014, 101, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.P.; Davison, J.M. The renal circulation in 1 normal pregnancy and preeclampsia. Is there a place for relaxin? Am. J. Physiol. Ren. Physiol. 2014, 306, F1121–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.X.; Arany, Z. Maternal cardiac metabolism in pregnancy. Cardiovasc Res. 2014, 101, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Bello, N.; Rendon, I.S.; Arany, Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Coll Cardiol. 2013, 62, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Powe, C.E.; Salahuddin, S.; Verlohren, S.; Perschel, F.H.; Levine, R.J.; et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012, 125, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Dunford, J.; Mehran, R.; Robson, S.; Kunadian, V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014, 63, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Mannisto, T.; Mendola, P.; Vaarasmaki, M.; Jarvelin, M.R.; Hartikainen, A.L.; Pouta, A.; et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation 2013, 127, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Vikse, B.E. Pre-eclampsia and the risk of kidney disease. Lancet 2013, 382, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. Long-term complications of preeclampsia. Semin Nephrol. 2011, 31, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Podewski, E.; Libhaber, E.; Labidi, S.; Fischer, D.; Roentgen, P.; et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013, 108, 366. [Google Scholar] [CrossRef] [PubMed]
- Patten, I.S.; Rana, S.; Shahul, S.; Rowe, G.C.; Jang, C.; Liu, L.; et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012, 485, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker-Kleiner, D.; Sliwa, K. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol. 2014, 11, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Painter, T.; Li, R.; Siegfried, J.D.; Li, D.; Norton, N.; et al. Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation 2010, 121, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- van Spaendonck-Zwarts, K.Y.; van Tintelen, J.P.; van Veldhuisen, D.J.; van der Werf, R.; Jongbloed, J.D.; Paulus, W.J.; et al. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation 2010, 121, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Swamy, A.; Sharma, Y.; Kumar, N. Isolated noncompaction of left ventricle presenting as peripartum cardiomyopathy. Int J Cardiol. 2006, 109, 422–423. [Google Scholar] [CrossRef] [PubMed]
- Lea, B.; Bailey, A.L.; Wiisanen, M.E.; Attili, A.; Rajagopalan, N. Left ventricular noncompaction presenting as peripartum cardiomyopathy. Int J Cardiol. 2012, 154, e65–6. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker-Kleiner, D.; Struman, I.; Hoch, M.; Podewski, E.; Sliwa, K. 16-kDa prolactin and bromocriptine in postpartum cardiomyopathy. Curr. Heart Fail. Rep. 2012, 9, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Fett, J.; Elkayam, U. Peripartum cardiomyopathy. Lancet 2006, 368, 687–693. [Google Scholar] [CrossRef]
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.C.; Mebazaa, A.; Pieske, B.; Buchmann, E.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010, 12, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.; Mebazaa, A.; Pieske, B.; Buchmann, E.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on Peripartum Cardiomyopathy. Eur Heart J. 2010, 12, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.D.; Veille, J.C.; Rahimtoola, S.; Hsia, J.; Oakley, C.M.; Hosenpud, J.D.; et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA 2000, 283, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Mielniczuk, L.M.; Williams, K.; Davis, D.R.; Tang, A.S.; Lemery, R.; Green, M.S.; et al. Frequency of peripartum cardiomyopathy. Am J Cardiol. 2006, 97, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Halkein, J.; Tabruyn, S.P.; Ricke-Hoch, M.; Haghikia, A.; Nguyen, N.Q.; Scherr, M.; et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013, 123, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker-Kleiner, D.; Kaminski, K.; Podewski, E.; Bonda, T.; Schaefer, A.; Sliwa, K.; et al. A Cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007, 128, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Hilfiker-Kleiner, D.; Mebazaa, A.; Petrie, M.C.; Maggioni, A.P.; Regitz-Zagrosek, V.; et al. EURObservational Research Programme: a worldwide registry on peripartum cardiomyopathy (PPCM) in conjunction with the Heart Failure Association of the European Society of Cardiology Working Group on PPCM. Eur J Heart Fail. 2014, 16, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011, 58, 659–670. [Google Scholar] [PubMed]
- Safirstein, J.G.; Ro, A.S.; Grandhi, S.; Wang, L.; Fett, J.D.; Staniloae, C. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int J Cardiol. 2012, 154, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Selle, T.; Renger, I.; Labidi, S.; Bultmann, I.; Hilfiker-Kleiner, D. Reviewing peripartum cardiomyopathy: current state of knowledge. Future Cardiol. 2009, 5, 175–189. [Google Scholar] [PubMed]
- Sliwa, K.; Forster, O.; Libhaber, E.; Fett, J.D.; Sundstrom, J.B.; Hilfiker-Kleiner, D.; et al. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J. 2006, 27, 441–446. [Google Scholar]
- Toescu, V.; Nuttall, S.L.; Martin, U.; Kendall, M.J.; Dunne, F. Oxidative stress and normal pregnancy. Clin Endocrinol 2002, 57, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Levine, R.J.; Karumanchi, S.A. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef] [PubMed]
- Forster, O.; Hilfiker-Kleiner, D.; Ansari, A.A.; Sundstrom, J.B.; Libhaber, E.; Tshani, W.; et al. Reversal of IFN-gamma, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur J Heart Fail. 2008, 10, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Walenta, K.; Schwarz, V.; Schirmer, S.H.; Kindermann, I.; Friedrich, E.B.; Solomayer, E.F.; et al. Circulating microparticles as indicators of peripartum cardiomyopathy. Eur Heart J. 2012, 33, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Habedank, D.; Kuhnle, Y.; Elgeti, T.; Dudenhausen, J.W.; Haverkamp, W.; Dietz, R. Recovery from peripartum cardiomyopathy after treatment with bromocriptine. Eur J Heart Fail. 2008, 10, 1149–1151. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.P.; Labidi, S.; Podewski, E.; Sliwa, K.; Drexler, H.; Hilfiker-Kleiner, D. Bromocriptine treatment associated with recovery from peripartum cardiomyopathy in siblings: two case reports. J Med Case Reports. 2010, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Forster, O.; Tibazarwa, K.; Libhaber, E.; Becker, A.; Yip, A.; et al. Long-term outcome of peripartum cardiomyopathy in a population with high seropositivity for human immunodeficiency virus. Int J Cardiol. 2011, 147, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, U.; Tummala, P.P.; Rao, K.; Akhter, M.W.; Karaalp, I.S.; Wani, O.R.; et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001, 344, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Hilfiker-Kleiner, D.; Mebaaza, A.; Petrie, M.C.; Maggioni, A.; RegitzZagrosek, V.; et al. EURObservational Research Programme: a worldwide registry on peripartum cardiomyopathy (PPCM) in conjunction with the Heart Failure Association of the European Society of Cardiology Working Group on PPCM. Eur J Heart Fail. 2014, 16, 583–591. [Google Scholar] [CrossRef]
© 2014 by the author. Attribution-Non-Commercial-NoDerivatives 4.0.
Share and Cite
Hilfiker-Kleiner, D.; Bauersachs, J. Pregnancy-Associated Cardiomyopathies. Cardiovasc. Med. 2014, 17, 245. https://doi.org/10.4414/cvm.2014.00262
Hilfiker-Kleiner D, Bauersachs J. Pregnancy-Associated Cardiomyopathies. Cardiovascular Medicine. 2014; 17(9):245. https://doi.org/10.4414/cvm.2014.00262
Chicago/Turabian StyleHilfiker-Kleiner, Denise, and Johann Bauersachs. 2014. "Pregnancy-Associated Cardiomyopathies" Cardiovascular Medicine 17, no. 9: 245. https://doi.org/10.4414/cvm.2014.00262
APA StyleHilfiker-Kleiner, D., & Bauersachs, J. (2014). Pregnancy-Associated Cardiomyopathies. Cardiovascular Medicine, 17(9), 245. https://doi.org/10.4414/cvm.2014.00262



