Mechanical Thrombectomy After Embolic Internal Carotid Artery Occlusion in Acute Stroke
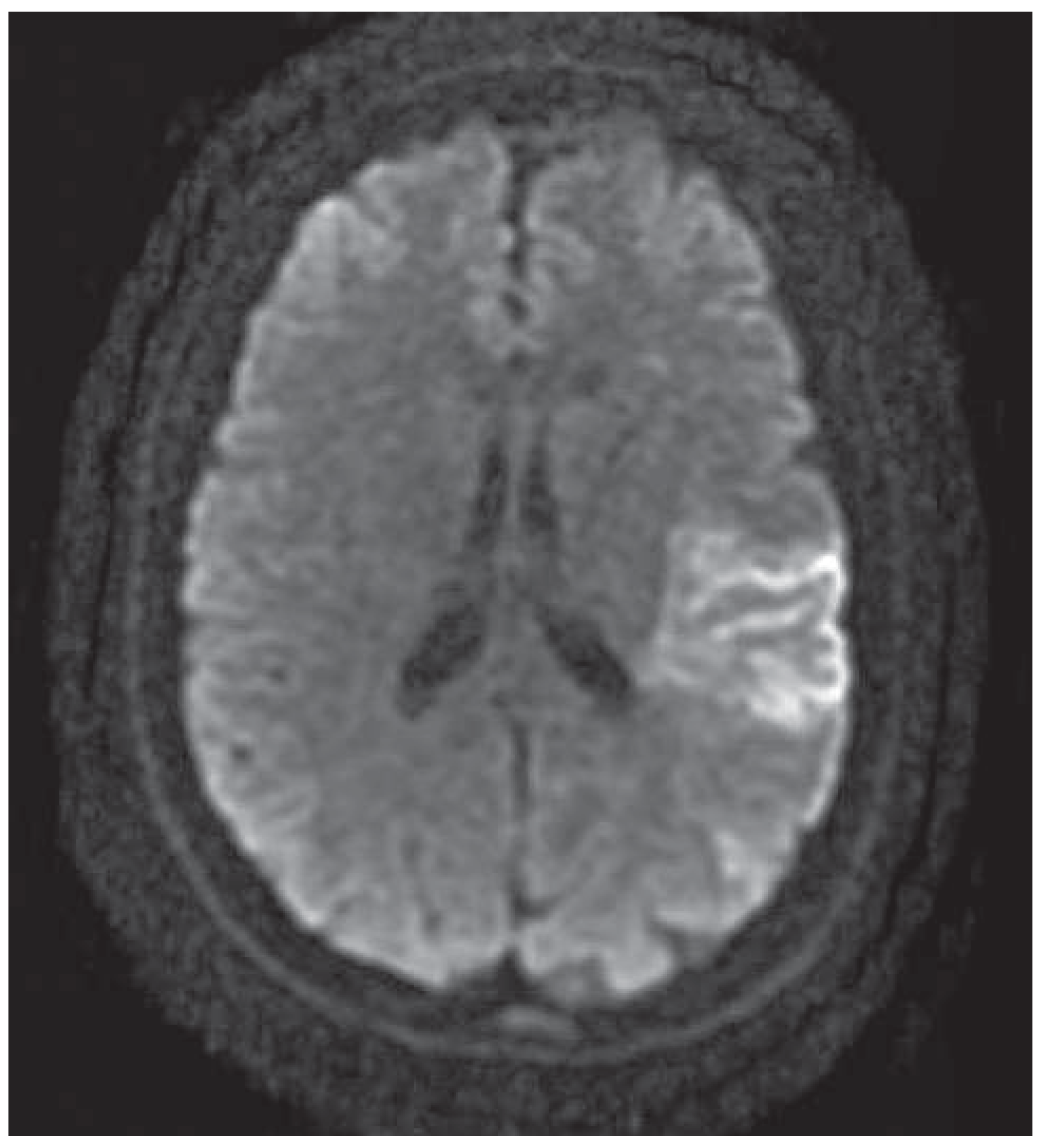
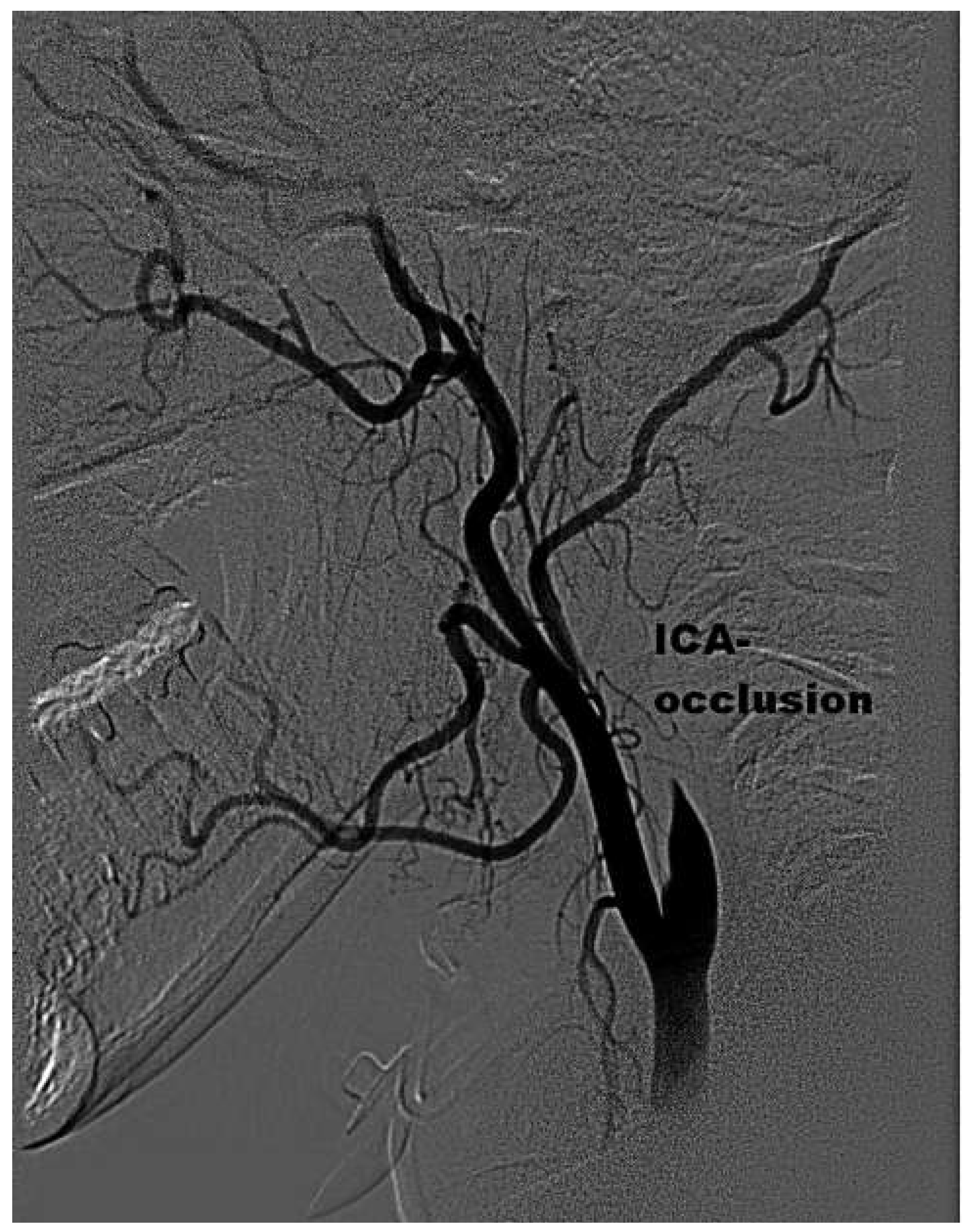
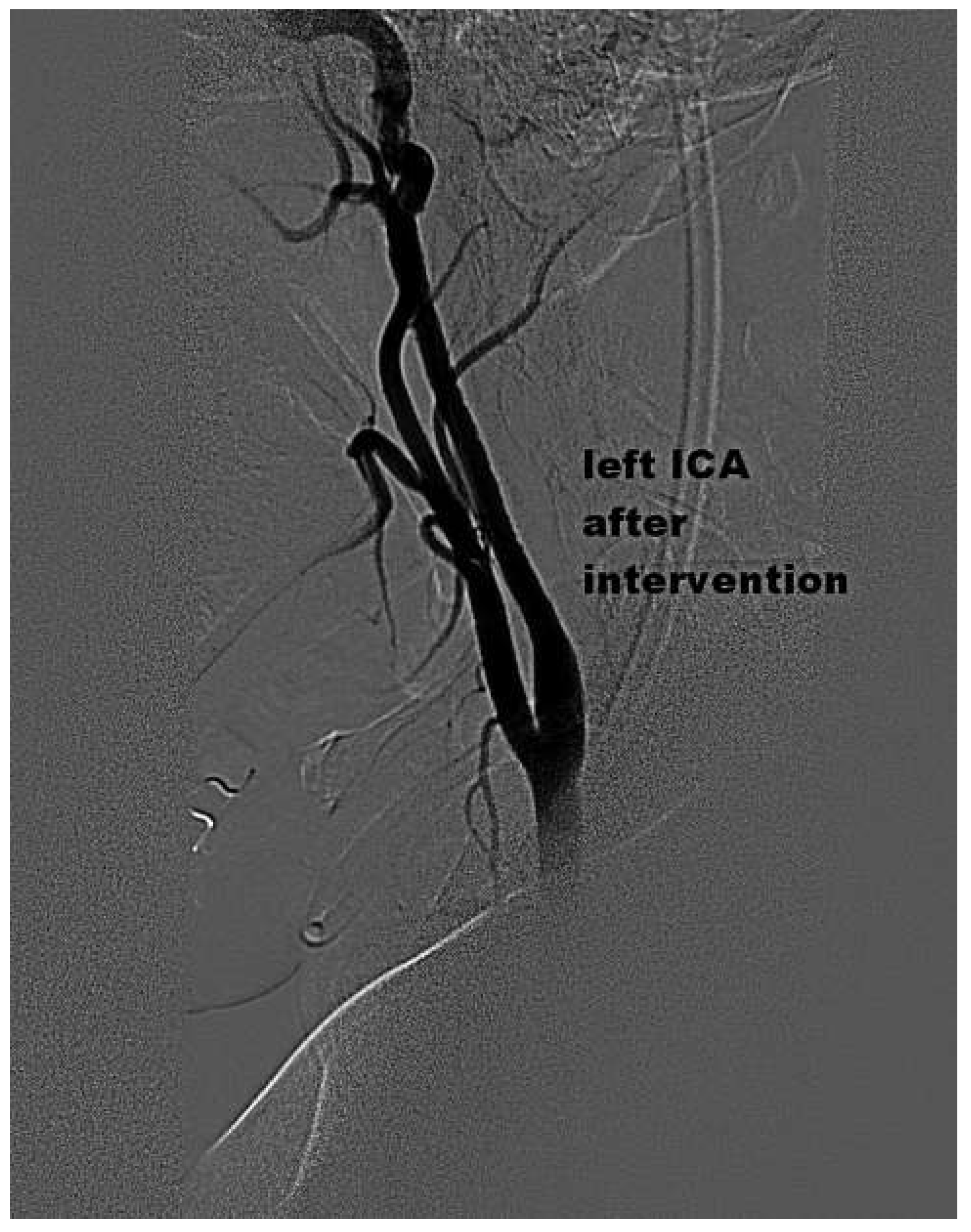
Case presentation
Discussion
Funding / potential competing interests
References
- Meyer, F.B.; Sundt, T.M., Jr.; Piepgras, D.G.; Sandok, B.A.; Forbes, G. Emergency carotid endarterectomy for patients with acute carotid occlusion and profound neurological deficits. Ann Surg. 1986, 203, 82–89. [Google Scholar] [CrossRef]
- Endo, S.; Kuwayama, N.; Hirashima, Y.; Akai, T.; Nishijima, M.; Takaku, A. Results of urgent thrombolysis in patients with major stroke and atherothrombotic occlusion of the cervical internal carotid artery. AJNR Am J Neuroradiol. 1998, 19, 1169–1175. [Google Scholar]
- Rebecca, M. Sugg, Marc D. Malkoff, Elizabeth A. Noser, Hashem M. Shaltoni, Raymond Weir et al. Endovascular Recanalization of Internal Carotid Artery Occlusion in Acute Ischemic Stroke. AJNR Am J Neuroradiol. 2005, 26, 2591–2594. [Google Scholar]
- Imai, K.; Mori, T.; Izumoto, H.; Takabatake, N.; Kunieda, T.; et al. Clot removal therapy by aspiration and extraction for acute embolic carotid occlusion. AJNR Am J Neuroradiol. 2006, 27, 1521–1527. [Google Scholar] [PubMed]
- Bellon, R.J.; Putman, C.M.; Budzik, R.F.; Pergolizzi, R.S.; Reinking, G.F.; Norbash, A.M. Rheolytic thrombectomy of the occluded internal carotid artery in the setting of acute ischemic stroke. AJNR Am J Neuroradiol. 2001, 22, 526–530. [Google Scholar] [PubMed]
- Wade S. Smith, Gene Sung, Jeffrey Saver, Ronald Budzik, Gary Duckwiler, David S. Liebeskind, et al. Mechanical Thrombectomy for Acute Ischemic Stroke. Final Results of the Multi MERCI Trial. Stroke. 2008, 39, 1205–1212.
- Natarajan, S.K.; Snyder, K.V.; Siddiqui, A.H.; Ionita, C.C.; Hopkins, L.N.; Levy, E.I. Safety and effectiveness of endovascular therapy after 8 hours of acute ischemic stroke onset and wake-up strokes. Stroke. 2009, 40, 3269–3274. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, A.; Norris, G.M.; Xavier, A.R. Acute endovascular recanalization therapy in wake-up stroke. J Neurol Sci. 2011, 300, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, A.; Valvassori, L.; Nichelatti, M.; Sgoifo, A.; Ponzio, M.; et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013, 368, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Draga Jichici. Alison Elizabeth Baird, Anterior Circulation Stroke; Medscape 21.6.2013.
- Furlan, A.J.; Reisman, M.; Massaro, J.; Mauri, L.; Adams, H.; Albers GWet, a.l. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012, 366, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Saver, J.L.; Thaler, D.E.; Smalling, R.W.; Berry, S.; MacDonald, L.A.; et al. Closure of Patent Foramen Ovale versus Medical Therapy after Cryptogenic Stroke. N Engl J Med. 2013, 368, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.; Kalesan, B.; Mattle, H.P.; Khattab, A.A.; Hildick-Smith, D.; et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013, 368, 1083–1091. [Google Scholar] [CrossRef] [PubMed]


© 2014 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Wyss, C.A.; Dastoor, A.; Corti, R. Mechanical Thrombectomy After Embolic Internal Carotid Artery Occlusion in Acute Stroke. Cardiovasc. Med. 2014, 17, 51. https://doi.org/10.4414/cvm.2014.00204
Wyss CA, Dastoor A, Corti R. Mechanical Thrombectomy After Embolic Internal Carotid Artery Occlusion in Acute Stroke. Cardiovascular Medicine. 2014; 17(2):51. https://doi.org/10.4414/cvm.2014.00204
Chicago/Turabian StyleWyss, Christophe A., Anahita Dastoor, and Roberto Corti. 2014. "Mechanical Thrombectomy After Embolic Internal Carotid Artery Occlusion in Acute Stroke" Cardiovascular Medicine 17, no. 2: 51. https://doi.org/10.4414/cvm.2014.00204
APA StyleWyss, C. A., Dastoor, A., & Corti, R. (2014). Mechanical Thrombectomy After Embolic Internal Carotid Artery Occlusion in Acute Stroke. Cardiovascular Medicine, 17(2), 51. https://doi.org/10.4414/cvm.2014.00204



