Quantification of Differential ECMO Return Flow Through an Axillary Artery Anastomosis Graft with Spectral Doppler Echocardiographyphy
Summary
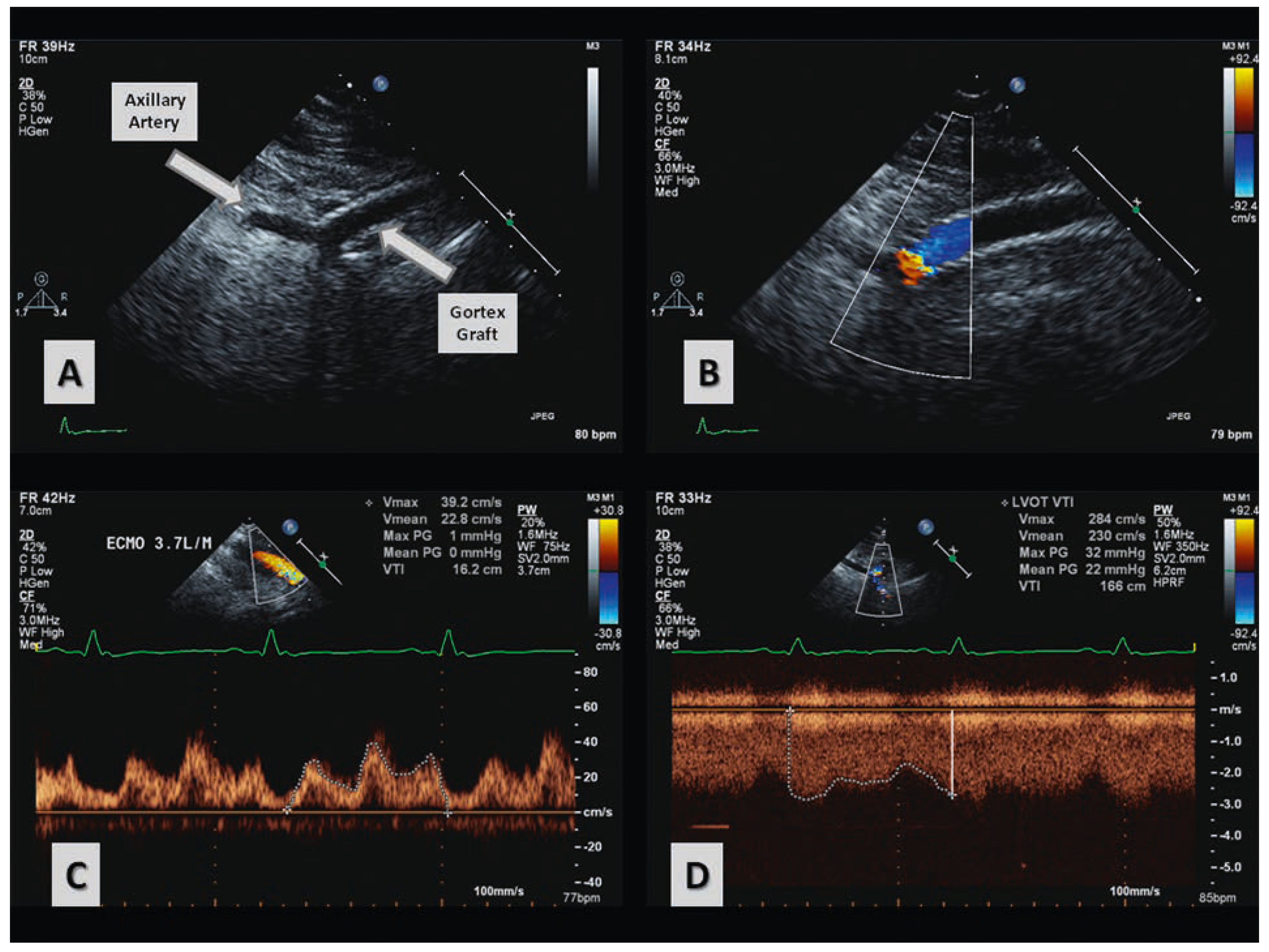
Case report
Discussion
Funding / potential competing interests
References
- Anderson, B. Echocardiography: The Normal Examination and Echocardiographic Measurements, 1st ed.; Wiley-Blackwell; 2002.
- Navia, J.L.; Atik, F.A.; Beyer, E.A.; Ruda Vega, P. Extracorporeal membrane oxygenation with right axillary artery perfusion. Ann Thorac Surg. 2005, 79, 2163–2165. [Google Scholar] [CrossRef] [PubMed]
- Moazami, N.; Moon, M.R.; Lawton, J.S.; Bailey, M.; Damiano, R., Jr. Axillary artery cannulation for extracorporeal membrane oxygenator support in adults: an approach to minimize complications. J Thorac Cardiovasc Surg. 2003, 126, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Chamogeorgakis, T.; Lima, B.; Shafii, A.E.; Nagpal, D.; Pokersnik, J.A.; Navia, J.L.; et al. Outcomes of axillary artery side graft cannulation for extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2013, 145, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Platts, D.G.; Sedgwick, J.F.; Burstow, D.J.; Mullany, D.V.; Fraser, J.F. The role of echocardiography in the management of patients supported by extracorporeal membrane oxygenation. J Am Soc Echocardiogr. 2012, 25, 131–141. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Platts, D.G.; Shekar, K.; Thomson, B.; Fraser, J.F. Quantification of Differential ECMO Return Flow Through an Axillary Artery Anastomosis Graft with Spectral Doppler Echocardiographyphy. Cardiovasc. Med. 2014, 17, 48. https://doi.org/10.4414/cvm.2014.00217
Platts DG, Shekar K, Thomson B, Fraser JF. Quantification of Differential ECMO Return Flow Through an Axillary Artery Anastomosis Graft with Spectral Doppler Echocardiographyphy. Cardiovascular Medicine. 2014; 17(2):48. https://doi.org/10.4414/cvm.2014.00217
Chicago/Turabian StylePlatts, David G., Kiran Shekar, Bruce Thomson, and John F. Fraser. 2014. "Quantification of Differential ECMO Return Flow Through an Axillary Artery Anastomosis Graft with Spectral Doppler Echocardiographyphy" Cardiovascular Medicine 17, no. 2: 48. https://doi.org/10.4414/cvm.2014.00217
APA StylePlatts, D. G., Shekar, K., Thomson, B., & Fraser, J. F. (2014). Quantification of Differential ECMO Return Flow Through an Axillary Artery Anastomosis Graft with Spectral Doppler Echocardiographyphy. Cardiovascular Medicine, 17(2), 48. https://doi.org/10.4414/cvm.2014.00217



