Introduction
“Ablate and pace” (A and P) is an established procedure for selected patients with drug-refractory atrial fibrillation (AF) and rapid ventricular response who are not candidates for pulmonary vein isolation [
1,
2,
3]. It involves catheter ablation of the atrioventricular node and implantation of a DDDR (for paroxysmal AF) or VVIR pacemaker (for permanent AF). In current guidelines, this procedure receives a Class IIa level B indication [
4]. A high level of right-ventricular pacing has been shown to exhibit deleterious effects on left ventricular ejection fraction (LVEF) in some patients [
5]. Therefore, cardiac resynchronisation therapy (CRT) has been studied as an alternative to conventional right-ventricular pacing [
6]. In current guidelines, CRT is indicated as class IIa level B if LVEF is <35% and New York Heart Association (NYHA) class III/IV, but only class IIB level C if NYHA class is I/II [
4].
The aim of this study was to evaluate the long-term follow-up of LVEF of patients with A and P and conventional pacing, and to determine the frequency of patients with a decline of LVEF into a region where pacemaker upgrade to CRT might be necessary or considered.
Methods
All 55 patients in whom “A and P” was performed at our hospital between January 2001 and May 2009 were identified. We had to exclude 15 patients with insufficient echo data and 4 with a follow-up of less than 12 months. Nine patients in whom a CRT pacemaker was implanted were also excluded. The LVEF was <40% in all nine and <35% in seven of the nine patients.
The pacemakers were implanted with a standard procedure and leads were placed in the right ventricular apex and the right atrial appendage. A dual chamber device was used in patients with paroxysmal AF, a single chamber device in patients with persistent/permanent AF. The programming mode was usually DDD with activated mode-switch to DDIR, otherwise it was VVIR. Atrioventricular node radiofrequency catheter ablation was performed in a fast pathway position with the aim of maintaining a junctional escape rhythm. The powers aimed at were 50 Watts with a nonirrigated and 30 Watts with an irrigated catheter. Directly after ablation, the minimum pacing rate was set to 85/min for a period of 4 weeks and then reduced again to 60/min. In a subanalysis, patients were stratified as to whether the ablation procedure was performed at the time of pacemaker implantation or not more than 30 days afterwards (“early group”), or later during follow-up (“late group”). The LVEF was measured, using Simpson’s method, by a fellow in echocardiography and supervised by a senior. LVEF taken as the baseline value was the one determined before atrioventricular nodal ablation and not the one determined before pacemaker implantation. With this approach, the direct effect of ablation-induced permanent pacemaker stimulation could be studied.
Echocardiographic follow-up was performed in all patients. We considered all available measurements of LVEF. In cases with multiple LVEF measurements, the last measurement was used for the purpose of this study. The date of last follow-up was December 2011. Follow-up duration was calculated as the time between the echo performed closest to atrioventricular node ablation and the last available echocardiogram. In April 2013 all 18 patients alive at this time were contacted by a study nurse, and NYHA class and hospitalisations for heart failure were evaluated. Data were available for 17 of them.
For a univariate analysis to determine predictors for a decrease in LVEF by ≥10%, the following parameters were used. NYHA class >II, diabetes, hypertension, coronary artery disease, treatment with beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, amiodarone and diuretics.
Continuous variables are reported as mean ± standard deviation (SD) and were compared with an unpaired t-test; p-values <0.05 were considered statistically significant. Calculations were done using GraphPad Software.
Results
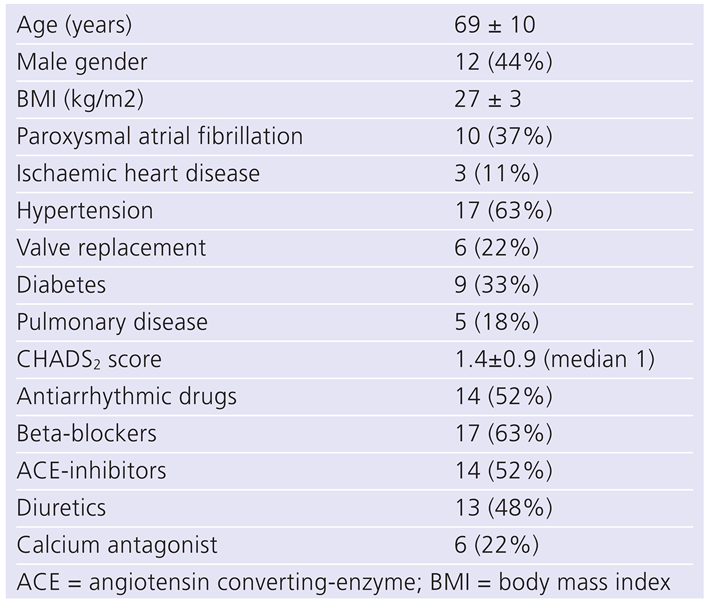
The study population consisted of 27 patients, 13 in the “early” and 14 in the “late” group (median delay 247 days). Baseline characteristics are shown in
Table 1. AF type was paroxysmal in 10 patients and persistent/permanent in 17. Atrioventricular nodal ablation was performed in ten patients with a previously implanted pacemaker for a brady-tachy-syndrome, whereas in 17 patients pacemaker implantation was performed with the intention of subsequent ablation. Mean follow-up was 65 ± 32 months. No patient showed recovery of atrioventricular nodal conduction.
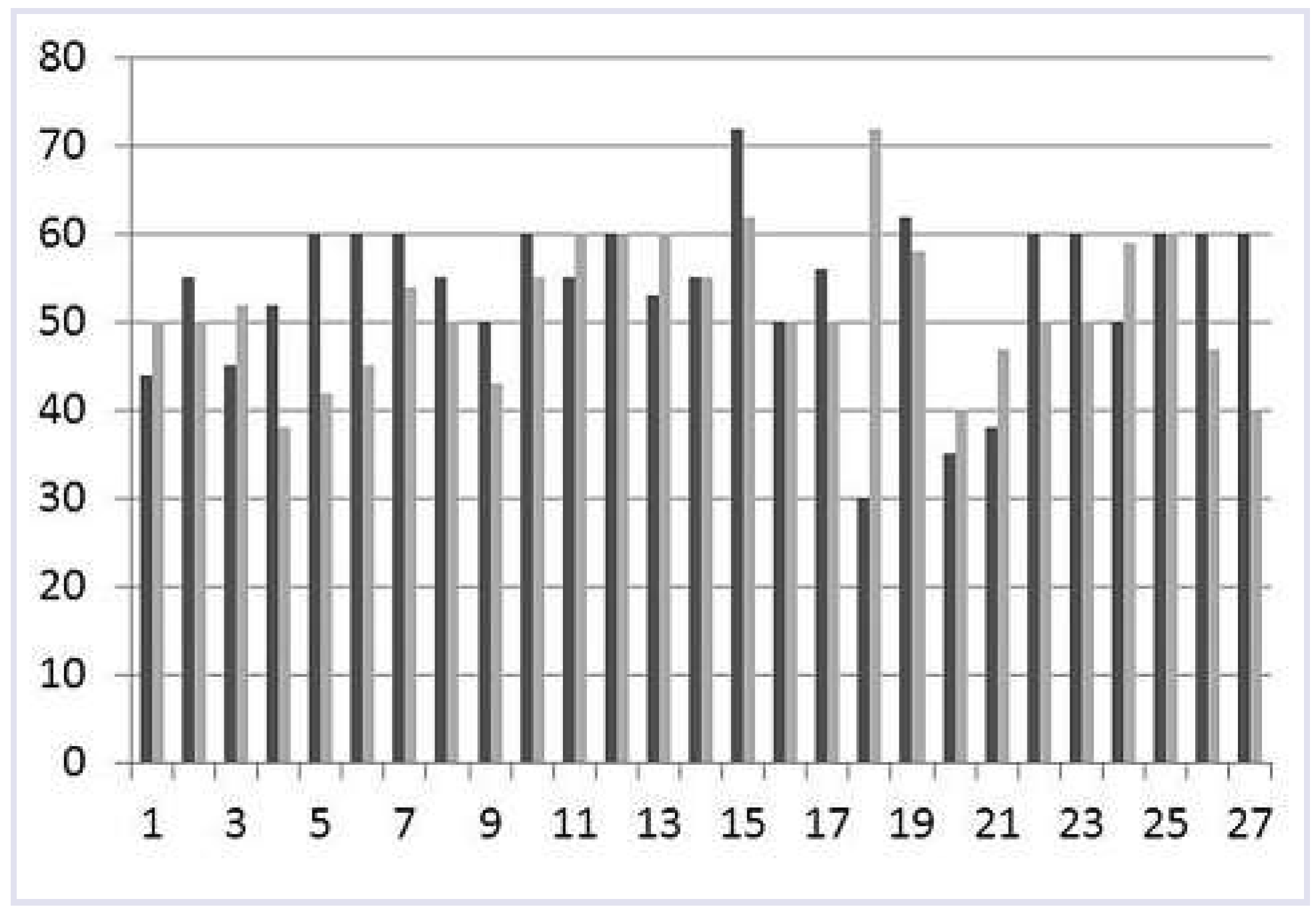
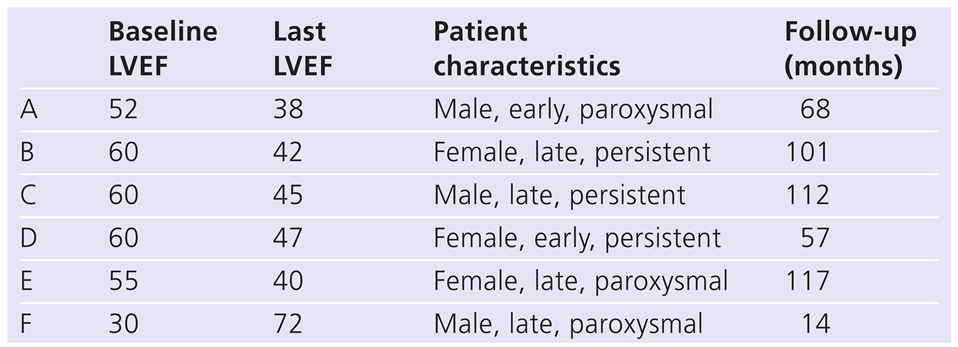
Mean LVEF at implant was 53%±9%, without a difference between the early and the late groups (51% ± 10% and 56% ± 8%, p-value 0.17). A LVEF of ≤40% (30%, 35% and 38%) was seen in three patients. At last echocardiographic follow-up, after 61 ± 30 months, mean LVEF was 52% ± 8%. The three patients with a LVEF initially ≤40% remained stable or LVEF increased (30% to 72%; 35% to 40%; 38% to 40%).
Table 2 gives details of those patients with a change in either direction of >10%.
Figure 1 shows the individual changes in all 27 patients.
Comparisons according to several baseline factors are shown in
Table 3.
The only predictors for a decrease in LVEF by ≥10% were lack of betablocker or ACE-inhibitor therapy at implant (p values 0.02 and 0.003). Due to the small number of events, we did not perform a multivariate analysis.
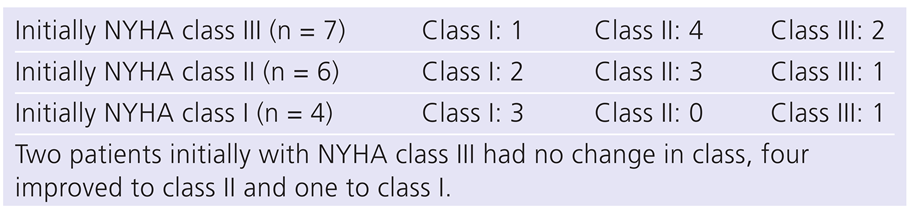
During follow-up nine patients died, four due to advanced age >85 years, two for noncardiac medical reasons, two for unknown reasons and one for cardiogenic shock after a prolonged period of pacing at upper tracking limit owing to a malfunctioning mode switch. In one patient, a dual chamber implantable cardioverter difibrillator (ICD) was implanted for a primary ventricular fibrillation and preserved LVEF. Of the 17 patients who could be contacted (1 had returned to his native country), one patient had been admitted several times for heart failure. He had bypass surgery and was also upgraded to a CRT-D due to an increase of his NYHA class from I to III and a borderline LVEF of 38%. Changes of individual NYHA class are shown in
Table 4.
Discussion
The main findings of this study are: (1.) with a desired high level of right ventricular pacing after atrioventricular nodal ablation, mean LVEF remained stable during long-term follow-up with a mean duration of five years; (2.) a decrease by ≥10% was seen in five patients (18%); (3.) in no patient was a decrease of LVEF to <35% observed and thus no patient required an upgrade to a CRT device in accordance with current guidelines
Why is it important to focus on LVEF at baseline and during follow-up? A LVEF <35% at the time of A and P indicates CRT, even though in mildly symptomatic patients the evidence for CRT is weak. On the other hand, cardiologists might argue that, due to the fact that continuous right ventricular pacing impairs left ventricular function, up-front CRT is indicated in every patient. Even though we present only a small number of patients, none of them had a LVEF <35% after a mean observation period of more than five years. Mean LVEF was not lower than at baseline, but a minority (18% of patients) experienced a decrease in LVEF by ≥10%.
Similar results with regard to LVEF were seen in the APT trial in 82 patients [
1]. Overall, LVEF remained stable (50%), but improved in those patients with a baseline LVEF <45% from 30% to 41%. Results are weakened by a short follow-up of only 12 months.
AIRCRAFT [
7] included 23 patients with A and P, but echocardiographic data after five years were available for only 12 of them. Again, LVEF remained stable (54% vs 51%), but with a wide individual spread (changes from +30% to –31%). In only one patient did LVEF decrease to <35%. PAVE [
8] randomised 184 patients to right ventricular or biventricular pacing. The primary endpoint (distance walked in 6 minutes) was improved in patients in NYHA class II or III or with LVEF <45%. Mean LVEF was better in the biventricular group (46% vs 41%, p-value 0.03), but follow-up was short (six months) and no individual data are given. Two other smaller studies looked for predictors of LVEF decrease during long-term pacing. Vernooy et al. [
9] reported that patients with a preserved left ventricular diameter (i.e. left ventricular end-diastolic dimension <50mm) were prone to LVEF decrease (from 64% to 55%), whereas no changes were seen in the others, even though their baseline LVEF showed a mild reduction to 50%. Left ventricular dyssynchrony, determined with echocardiography (septal-to posterior wall motion delay) after a mean of four years of continuous right ventricular pacing predicted LVEF decrease. However, the decrease was only small (48% vs 43%) and no individual data is given [
10]. The largest series was published by Chen et al. [
2]. They followed up 286 patients for a mean of 20 months and had data on LVEF at least one year after the procedure for 152 of them. Mean LVEF remained stable with 48% both at baseline and at last follow-up. In 15% a decrease by >10% was observed, except for one case, only in patients with a baseline LVEF of >40%. Unfortunately, again no data are available as to how many patients decreased to < 35% and the study provides no long-term follow-up.
The strength of our study is the long-term follow-up and the fact that we do not only report the mean, but also individual, LVEF. This is important, as the detrimental effect of right ventricular pacing in some patients might be counterbalanced by the positive effect of rate control in others, leading to unchanged mean LVEF. Tachymyopathy may be one of the reasons of depressed left ventricular function and may mimic idiopathic dilated cardiomyopathy, but is often difficult to diagnose at first patient encounter [
11].
Brignole et al. [
6] randomised 97 patients to CRT and 87 to conventional apical pacing before atrioventricular nodal ablation. Mean LVEF was 38% and thus lower compared with all other studies. They showed a reduction of the combined endpoint of heart failure death, hospitalisation for or progression of heart failure after a median follow-up of 20 months. However, differences were small (3% vs 1%), and even smaller in the subgroup of patients with baseline LVEF of >35% (1% vs 2.2%). LVEF improved in both groups, 4.7% in the right-ventricular and 6.6% in the CRT group. This suggests that two-thirds of the improvement is due to rate control and one-third to CRT alone. Interestingly, improvement in those patients with a baseline of LVEF <35% was larger in the right-ventricular paced group (6.9% vs. 5.7%). Unfortunately no data are given with regard to individual patients, for example, how many patients decreased to <35%.
The recently published BLOCK-HF trial [
12] showed a 23% reduction of the combined secondary endpoint of death or hospitalisation for heart failure over about three years. However, the setting was not A and P, but patients were in 55% in sinus rhythm and had acquired atrioventricular block. Included were patients with a LVEF of <50%. In A and P, the possible negative effect of right ventricular pacing might be counterbalanced by a normofrequent heart rate.
From a safety point of view, the additional lead in a CRT system prevents syncope in the case of lead dislodgement of the right ventricular lead in patients without escape rhythm. However, this rarely occurs, implies higher costs, more complications at implant and earlier battery depletion [
13].
The only predictors identified for a decrease of LVEF by ≥10% were lack of beta-blocker and ACE-inhibitor therapy at implant. Owing to the small number of patients, this analysis must be interpreted with care, but can give a direction for future research in a larger population.
A surprising finding was that patients with non-paroxysmal AF experienced a significant decline in LVEF (–5%) compared with those with paroxysmal AF (+3%). One might speculate that atrial myopathy and loss of atrial kick are also part of the global process and not only permanent right ventricular pacing.
This paper has some limitations that need to be addressed. The number of patients included is rather small and the design is retrospective. LVEF was measured using a single measurement and not verified in a core laboratory. In those patients in whom both echocardiograms were not performed in sinus rhythm or AF, some differences in LVEF might be explained by this phenomenon [
14]. Mean heart rate just before ablation is unknown. Patients with a rate >100–120/min might show a more pronounced recovery of tachymyopathy. Data interpretation might be impaired by the fact that 9 patients were directly implanted with CRT. However, all patients had a LVEF <40%.