Balloon Angioplasty Using the “GRIP™” Scoring Balloon for Treatment of Coronary In-Stent Restenosis—Immediate and 12-Month Clinical Outcomes
Abstract
Introduction
Materials and methods
Quantitative angiography
Follow-up
Definitions
Statistical analysis
Results
Baseline data
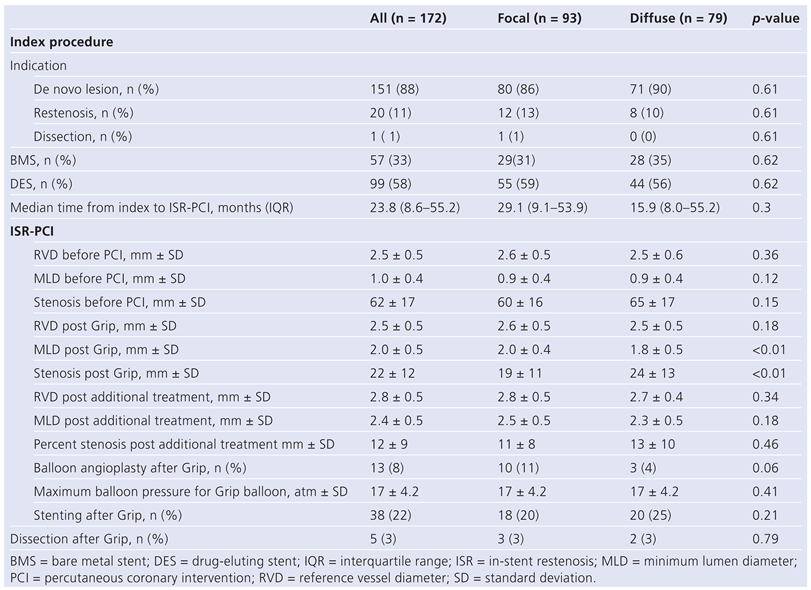
Index procedure
ISR procedure
Safety outcome
Clinical follow up
ISR prediction
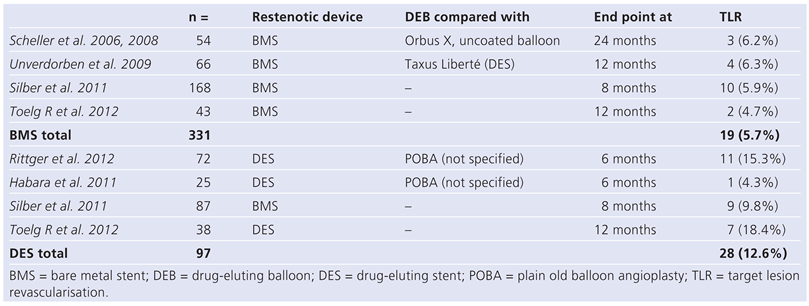
Discussion
Limitations
Conclusions
Funding / potential competing interests
Abbreviations
| BMS | Bare metal stent |
| DEB | Drug-eluting balloon |
| DES | Drug-eluting stent |
| ISR | In-stent restenosis |
| MACE | Major adverse cardiac events |
| MLD | Minimum lumen diameter |
| PCI | Percutaneous coronary intervention |
| RVD | Reference vessel diameter |
| ST | Stent thrombosis |
| TLR | Target lesion revascularisation |
References
- Komatsu, R.; Ueda, M.; Naruko, T.; Kojima, A.; Becker, A.E. Neointimal tissue response at sites of coronary stenting in humans: Macroscopic, histological, and immunohistochemical analyses. Circulation. 1998, 98, 224–233. [Google Scholar] [CrossRef]
- Lee, M.S.; Pessegueiro, A.; Zimmer, R.; Jurewitz, D.; Tobis, J. Clinical presentation of patients with in-stent restenosis in the drug-eluting stent era. J Invasive Cardiol. 2008, 20, 401–403. [Google Scholar] [PubMed]
- Mehilli, J.; Byrne, R.A.; Tiroch, K.; Pinieck, S.; Schulz, S.; Kufner, S.; et al. Randomized trial of paclitaxelversus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents: The isar-desire 2 (intracoronary stenting and angiographic results: Drug eluting stents for in-stent restenosis 2) study. J Am Coll Cardiol. 2010, 55, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, A.; Mehilli, J.; von Beckerath, N.; Dibra, A.; Hausleiter, J.; Pache, J.; et al. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary instent restenosis: A randomized controlled trial. JAMA. 2005, 293, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zahn, R.; Hamm, C.W.; Schneider, S.; Zeymer, U.; Nienaber, C.A.; Richardt, G.; et al. Incidence and predictors of target vessel revascularization and clinical event rates of the sirolimus-eluting coronary stent (results from the prospective multicenter german cypher stent registry). Am J Cardiol. 2005, 95, 1302–1308. [Google Scholar] [CrossRef]
- Zahn, R.; Hamm, C.W.; Schneider, S.; Richardt, G.; Kelm, M.; Levenson, B.; et al. Coronary stenting with the sirolimus-eluting stent in clinical practice: Final results from the prospective multicenter german cypher stent registry. J Interv Cardiol. 2010, 23, 18–25. [Google Scholar] [CrossRef]
- Kastrati, A.; Dibra, A.; Mehilli, J.; Mayer, S.; Pinieck, S.; Pache, J.; et al. Predictive factors of restenosis after coronary implantation of sirolimusor paclitaxel-eluting stents. Circulation. 2006, 113, 2293–2300. [Google Scholar] [CrossRef]
- Bossi, I.; Klersy, C.; Black, A.J.; Cortina, R.; Choussat, R.; Cassagneau, B.; et al. In-stent restenosis: Long-term outcome and predictors of subsequent target lesion revascularization after repeat balloon angioplasty. J Am Coll Cardiol. 2000, 35, 1569–1576. [Google Scholar] [CrossRef]
- Chen, M.S.; John, J.M.; Chew, D.P.; Lee, D.S.; Ellis, S.G.; Bhatt, D.L. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006, 151, 1260–1264. [Google Scholar] [CrossRef]
- Rathore, S.; Kinoshita, Y.; Terashima, M.; Katoh, O.; Matsuo, H.; Tanaka, N.; et al. A comparison of clinical presentations, angiographic patterns and outcomes of in-stent restenosis between bare metal stents and drug eluting stents. EuroIntervention. 2010, 5, 841–846. [Google Scholar] [CrossRef]
- Assali, A.R.; Moustapha, A.; Sdringola, S.; Denktas, A.E.; Willerson, J.T.; Holmes, D.R., Jr.; Smalling, R.W. Acute coronary syndrome may occur with in-stent restenosis and is associated with adverse outcomes (the presto trial). Am J Cardiol. 2006, 98, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.L.; Harding, S.A.; Walsh, C.R.; Wong, P.; Pomerantsev, E.; Jang, I.K. Acute coronary syndrome is a common clinical presentation of in-stent restenosis. Am J Cardiol. 2002, 89, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Kawamura, A.; Nesto, R.W.; Davis, G.; Jarbeau, J.; Pyne, C.T.; et al. Myocardial infarction as a presentation of clinical in-stent restenosis. Circ J. 2006, 70, 1026–1029. [Google Scholar] [CrossRef]
- Adamian, M.; Colombo, A.; Briguori, C.; Nishida, T.; Marsico, F.; Di Mario, C.; et al. Cutting balloon angioplasty for the treatment of in-stent restenosis: A matched comparison with rotational atherectomy, additional stent implantation and balloon angioplasty. J Am Coll Cardiol. 2001, 38, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B.; Hehrlein, C.; Bocksch, W.; Rutsch, W.; Haghi, D.; Dietz, U.; et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006, 355, 2113–2124. [Google Scholar] [CrossRef]
- Guidon, A.; Cook, S.; Berger, A.; Goy, J.J. Long-term clinical outcome after sirolimus-stent implantation for in sirolimuseluting stent restenosis. Clinical Medicine: Cardiology. 2008, 2, 161–163. [Google Scholar]
- Waksman, R.; White, R.L.; Chan, R.C.; Bass, B.G.; Geirlach, L.; Mintz, G.S.; et al. Intracoronary gamma-radiation therapy after angioplasty inhibits recurrence in patients with in-stent restenosis. Circulation. 2000, 101, 2165–2171. [Google Scholar] [CrossRef]
- Dangas, G.D.; Claessen, B.E.; Caixeta, A.; Sanidas, E.A.; Mintz, G.S.; Mehran, R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010, 56, 1897–1907. [Google Scholar] [CrossRef]
- Scheller, B.; Hehrlein, C.; Bocksch, W.; Rutsch, W.; Haghi, D.; Dietz, U.; et al. Two year follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2008, 97, 773–781. [Google Scholar] [CrossRef]
- Wijns, W.; Kolh, P.; Danchin, N.; Di Mario, C.; Falk, V.; Folliguet, T.; et al. Guidelines on myocardial revascularization. Eur Heart J. 2010, 31, 2501–2555. [Google Scholar] [CrossRef]
- Roques, F.; Michel, P.; GoldstonNashef, S.A. The logistic euroscore. Eur Heart J. 2003, 24, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Dangas, G.; Abizaid, A.S.; Mintz, G.S.; Lansky, A.J.; Satler, L.F.; et al. Angiographic patterns of in-stent restenosis: Classification and implications for long-term outcome. Circulation. 1999, 100, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.H.; Lasala, J.M. Coronary artery dissection and perforation complicating percutaneous coronary intervention. J Invasive Cardiol. 2004, 16, 493–499. [Google Scholar] [PubMed]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation. 2007, 115, 2344–2351. [Google Scholar] [CrossRef]
- Bauters, C.; Banos, J.L.; Van Belle, E.; Mc Fadden, E.P.; Lablanche, J.M.; Bertrand, M.E. Six-month angiographic outcome after successful repeat percutaneous intervention for in-stent restenosis. Circulation. 1998, 97, 318–321. [Google Scholar] [CrossRef][Green Version]
- Eltchaninoff, H.; Koning, R.; Tron, C.; Gupta, V.; Cribier, A. Balloon angioplasty for the treatment of coronary in-stent restenosis: Immediate results and 6-month angiographic recurrent restenosis rate. J Am Coll Cardiol. 1998, 32, 980–984. [Google Scholar] [CrossRef]
- Sanchez-Recalde, A.; Galeote, G.; Martin-Reyes, R.; Moreno, R. Angiosculpt ptca balloon entrapment during dilatation of a heavily calcified lesion. Rev Esp Cardiol. 2008, 61, 1361–1363. [Google Scholar] [CrossRef]
- Reimers, B.; Moussa, I.; Akiyama, T.; Tucci, G.; Ferraro, M.; Martini, G.; et al. Long-term clinical follow-up after successful repeat percutaneous intervention for stent restenosis. J Am Coll Cardiol. 1997, 30, 186–192. [Google Scholar] [CrossRef][Green Version]
- Scheller, B.; Clever, Y.P.; Kelsch, B.; Hehrlein, C.; Bocksch, W.; Rutsch, W.; et al. Long-term follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. JACC Cardiovasc Interv. 2012, 5, 323–330. [Google Scholar] [CrossRef]
- Unverdorben, M. Trend towards lower tlr and mace rates in patients treated with deb compared to des. EuroPCR 2011.
- Habara, S.; Mitsudo, K.; Kadota, K.; Goto, T.; Fujii, S.; Yamamoto, H.; et al. Effectiveness of paclitaxel-eluting balloon catheter in patients with sirolimus-eluting stent restenosis. JACC Cardiovasc Interv. 2011, 4, 149–154. [Google Scholar] [CrossRef]
- Rittger, H.; Brachmann, J.; Sinha, A.M.; Waliszewski, M.; Ohlow, M.; Brugger, A.; et al. A randomized, multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with plain balloon angioplasty in drug-eluting stent restenosis: The pepcad-des study. J Am Coll Cardiol. 2012, 59, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Unverdorben, M.; Vallbracht, C.; Cremers, B.; Heuer, H.; Hengstenberg, C.; Maikowski, C.; et al. Paclitaxel-coated balloon catheter versus paclitaxelcoated stent for the treatment of coronary in-stent restenosis. Circulation. 2009, 119, 2986–2994. [Google Scholar] [CrossRef] [PubMed]
- Toelg, R. Paclitaxel releasing balloon with an inert bthc excipient: Sixmonth results on 1’064 patients of the international delux registry; EuroPCR: Paris, France, 2012. [Google Scholar]
- Appleby, C.E.; Khattar, R.S.; Morgan, K.; Clarke, B.; Curzen, N.; Neyses, L.; et al. Drug eluting stents for the treatment of bare metal in-stent restenosis: Long-term outcomes in real world practice. EuroIntervention. 2011, 6, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Beijk, M.A.; Claessen, B.E.; Koch, K.T.; Henriques, J.P.; Baan, J.; Vis, M.M.; et al. One-year clinical outcome after treatment of bare-metal stent in-stent restenosis with the paclitaxel-eluting stent in an unselected cohort. Int J Cardiol. 2010, 145, 608–609. [Google Scholar] [CrossRef]
- Steinberg, D.H.; Gaglia, M.A., Jr.; Pinto Slottow, T.L.; Roy, P.; Bonello, L.; De Labriolle, A.; et al. Outcome differences with the use of drug-eluting stents for the treatment of in-stent restenosis of bare-metal stents versus drug-eluting stents. Am J Cardiol. 2009, 103, 491–495. [Google Scholar] [CrossRef]
- Pohl, T.; Kupatt, C.; Steinbeck, G.; Boekstegers, P. Angiographic and clinical outcome for the treatment of in-stent restenosis with sirolimus-eluting stent compared to vascular brachytherapy. Z Kardiol. 2005, 94, 405–410. [Google Scholar] [CrossRef]
- Tagliareni, F.; La Manna, A.; Saia, F.; Marzocchi, A.; Tamburino, C. Longterm clinical follow-up of drug-eluting stent restenosis treatment: Retrospective analysis from two high volume catheterisation laboratories. EuroIntervention. 2010, 5, 703–708. [Google Scholar] [CrossRef]
- Rathore, S.; Terashima, M.; Katoh, O.; Matsuo, H.; Tanaka, N.; Kinoshita, Y.; et al. Predictors of angiographic restenosis after drug eluting stents in the coronary arteries: Contemporary practice in real world patients. EuroIntervention. 2009, 5, 349–354. [Google Scholar] [CrossRef]
- Cosgrave, J.; Melzi, G.; Biondi-Zoccai, G.G.; Airoldi, F.; Chieffo, A.; Sangiorgi, G.M.; et al. Drug-eluting stent restenosis the pattern predicts the outcome. J Am Coll Cardiol. 2006, 47, 2399–2404. [Google Scholar] [CrossRef][Green Version]
- Mishkel, G.J.; Moore, A.L.; Markwell, S.; Shelton, M.C.; Shelton, M.E. Longterm outcomes after management of restenosis or thrombosis of drugeluting stents. J Am Coll Cardiol. 2007, 49, 181–184. [Google Scholar] [CrossRef]


 |
 |
 |
 |
© 2012 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Puricel, S.; Schoepke, L.; Togni, M.; Oberhänsli, M.; Moschovitis, A.; Meier, B.; Windecker, S.; Cook, S. Balloon Angioplasty Using the “GRIP™” Scoring Balloon for Treatment of Coronary In-Stent Restenosis—Immediate and 12-Month Clinical Outcomes. Cardiovasc. Med. 2012, 15, 354. https://doi.org/10.4414/cvm.2012.00128
Puricel S, Schoepke L, Togni M, Oberhänsli M, Moschovitis A, Meier B, Windecker S, Cook S. Balloon Angioplasty Using the “GRIP™” Scoring Balloon for Treatment of Coronary In-Stent Restenosis—Immediate and 12-Month Clinical Outcomes. Cardiovascular Medicine. 2012; 15(12):354. https://doi.org/10.4414/cvm.2012.00128
Chicago/Turabian StylePuricel, Serban, Linus Schoepke, Mario Togni, Markus Oberhänsli, Aris Moschovitis, Bernhard Meier, Stephan Windecker, and Stéphane Cook. 2012. "Balloon Angioplasty Using the “GRIP™” Scoring Balloon for Treatment of Coronary In-Stent Restenosis—Immediate and 12-Month Clinical Outcomes" Cardiovascular Medicine 15, no. 12: 354. https://doi.org/10.4414/cvm.2012.00128
APA StylePuricel, S., Schoepke, L., Togni, M., Oberhänsli, M., Moschovitis, A., Meier, B., Windecker, S., & Cook, S. (2012). Balloon Angioplasty Using the “GRIP™” Scoring Balloon for Treatment of Coronary In-Stent Restenosis—Immediate and 12-Month Clinical Outcomes. Cardiovascular Medicine, 15(12), 354. https://doi.org/10.4414/cvm.2012.00128




