Abstract
We report the case of a Bolivian woman with a complete atrioventricular block due to Chagas cardiomyopathy. Diagnosis was established by echocardiography and highly positive serological results for Trypanosoma cruzi americanum. Treatment included initiation of an ACE inhibitor and implantation of a dual-chamber pacemaker, as well as an anti-parasitic medication with benznidazole. A follow-up after one year exhibited improved left ventricular function and intermittent sinus rhythm.
Case report
A 49-year-old Bolivian woman presented with progressive fatigue, dyspnoea NYHA II, and mild leg oedema. Her cardiovascular medical history was unremarkable. Originating from La Paz in Bolivia, she had been living in Switzerland for the past 14 years and had never returned to her home country. Apart from permanent bradycardia of 30 bpm and mild leg oedema, the clinical examination was normal. We diagnosed alternating second degree (type Mobitz 2) and complete atrioventricular block (AVB) with a narrow QRS complex without bundle branch block (Figure 1). Echocardiography in a bradycardial escape rhythm (32 bpm) revealed a slightly dilated left ventricle (end diastolic volume index [EDVI] 80 ml/m2) with a preserved systolic function (left ventricular ejection fraction [LVEF] 57%) and an apical ventricular aneurysm (Figure 2). The troponin level was normal and other signs of coronary heart disease were absent. For both rheumatic syndromes and Lyme disease, negative serologic test results were obtained. A chest x-ray and clinical examination did not show any signs of sarcoidosis.
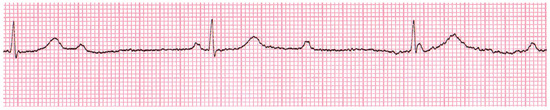
Figure 1.
ECG at initial presentation: Complete atrioventricular block, no bundle branch block.

Figure 2.
Echocardiography 4-chamber apical view after pacemaker implantation. (A) Dilated cardiomyopathy (enddiastolic freeze-frame). (B) Ventricular aneurysm (arrows) in the apex of the left ventricle (endsystolic freeze-frame). LV = left ventricle; LA = left atrium; LV apex = apex of left ventricle.
Considering the patient’s South American background, serological analysis for Trypanosoma cruzi americanum was initiated and showed highly positive results (ELISA and IFAT titre). Thus, diagnosis of chronic Chagas disease with consequent cardiomyopathy and complete AVB was confirmed.
A dual-chamber pacemaker (Medtronic®, AAIRDDDR) was implanted. In addition, we initiated heart failure treatment with an ACE inhibitor and referred the patient to the Swiss Tropical and Public Health Institute, Basel, where she received an ambulant antiparasitic medication with benznidazole (300 mg daily for 30 days).
At the one-year follow-up pacemaker interrogation revealed intermittent sinus rhythm and pacemaker dependency of 55%. The left ventricular volume was unchanged (EDVI 81 ml/m2) and its function had increased (LVEF 67%) in sinus rhythm (55 bum). The apical aneurysm was unchanged. The patient did not show heart failure symptoms anymore. Serological titres were unchanged compared to the initial blood sample.
Discussion
Chagas disease is mainly endemic in Central and South America where about 8 million infected patients are suspected. The estimated prevalence of Trypanosoma cruzi in Latin American immigrants is 8–50 per 1000 in the USA, 9 per 1000 in Canada, 25 per 1000 in Spain, and 16 per 1000 in Australia [1]. Data for Switzerland is not available.
Chagas disease is a parasitosis, commonly transmitted to humans by a blood sucking assassin bug (Triatominae) serving as an insect vector. The bug’s faeces – contaminated with Trypanosoma cruzi – come in contact with human circulation by being rubbed into the puncture from the bug’s bite or onto mucus membranes.
Since symptoms of the acute infection are unspecific, the initial phase often remains unrecognised. If in this phase no specific treatment is applied, the disease evolves into a chronic (usually lifelong) stage. Chronic infection can be present without the development of related symptoms or findings, which is then referred to as an indeterminate stage. However, about 30% of chronic infections lead to damage of the heart and/or the gastrointestinal tract [2]. Even though many pathophysiological concepts are currently discussed, parasite persistence is likely to be the main mechanism causing myocardial injury in patients with chronic chagasic cardiomyopathy [3].
Heart related clinical findings after years or even decades of chronic infection often include dilated cardiomyopathy and arrhythmia. About 17–28% of all infected patients develop heart failure. Dilatation of the left ventricle and apical aneurysms are echocardiographic key features for confirmation of the diagnosis [4]. In end stage disease patients present with fullblown dilated cardiomyopathy and corresponding heart failure.
Defects of the conduction system are usually present many years before clinically manifested heart failure. While conduction disturbances like right bundle branch block (RBBB, 50%) and left anterior hemi-block (LAHB, 26%) are common, higher grade conduction abnormalities like in the presented case are rare [5]. Complex premature ventricular contractions, ventricular tachycardia, sinus node dysfunction and higher degree AVB normally develop in late stage disease but may be present even in clinically non-overt patients. Together with ventricular dysfunction and syncope they are major predictors of sudden death in chronic chagasic heart disease [6].
Since only few controlled studies have been performed, optimal treatment of Chagas cardiomyopathy remains controversial. Whether recovery of an altered conduction system can be expected from anti-parasitic medication remains unclear. Thus, in symptomatic bradycardia derived from third-degree and advanced second-degree AVB at any anatomic level, implantation of a permanent cardiac pacemaker is the treatment of choice [7,8]. As appropriate, ACE inhibitors or amiodarone should be prescribed and heart transplantation may be considered. In addition to these approaches, encouraging data suggests that benznidazole, an anti-parasitic drug, decelerates progression of the disease [1]. Negative seroconversion can be expected after this treatment but may take years to decades. After anti-parasitic treatment our patient showed a partial recovery of the conduction disturbance. This is remarkable since chronic Chagas cardiomyopathy seemed to be in an advanced stage and there was no change of the serological titres compared to the initial blood sample. A multicentre study (BENEFIT trial) currently being conducted will presumably provide more data about efficacy and safety of benznidazole [9].
This report underlines that in unclear cases of heart failure and conduction abnormalities Chagas disease should be taken into consideration. In view of migration from and tourism to endemic regions, Chagas disease may be seen more often in European countries in the future.
Conclusions
This case underlines the necessity to take Chagas disease into diagnostic consideration in patients with severe conduction abnormalities in the absence of a known underlying cardiac disease – especially in patients with Latin American background or respective travel history.
Funding / potential competing interests
We report no financial support and no other potential conflict of interest relevant to this article.
Acknowledgments
The authors thank J. Blum (Swiss Tropical and Public Health Institute, Basel, Switzerland) for his clinical help and advice.
References
- Blum, J.A.; Zellweger, M.J.; Burri, C.; Hatz, C. Cardiac involvement in African and American trypanosomiasis. Lancet Infect Dis. 2008, 8, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Montgomery, S.P.; Herwaldt, B.L.; Rassi, A., Jr.; Marin-Neto, J.A.; Dantas, R.O.; et al. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA. 2007, 298, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Marin-Neto, J.A.; Cunha-Neto, E.; Maciel, B.C.; Simões, M.V. Pathogenesis of Chronic Chagas Heart Disease. Circulation. 2007, 115, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Acquatella, H. Echocardiography in Chagas heart disease. Circulation. 2007, 115, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.H.; Hoff, R.; Sherlock, I.; Guimarães, A.C.; Sleigh, A.C.; Ramos, N.B.; et al. Cardiac morbidity and mortality due to Chagas’ disease: prospective electrocardiographic study of a Brazilian community. Circulation. 1987, 75, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A.J.; Rassi, S.G.; Rassi, A. Sudden death in Chagas’ disease. Arq Bras Cardiol. 2001, 76, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Gascón, J.; Albajar, P.; Cañas, E.; Flores, M.; Gómez i Prat, J.; Herrera, R.N.; et al. Diagnosis, management and treatment of chronic Chagas’ heart disease in areas where Trypanosoma cruzi infection is not endemic. Enfermedades Infecc. Y Microbiol. Clínica 2008, 26, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.E.; Dimarco, J.P.; Ellenbogen, K.A.; Mark Estes, N.A.; Freedman, R.A.; Gettes, L.S.; et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008, 51, e1–e62. [Google Scholar] [PubMed]
- Marin-Neto, J.A.; Rassi, A., Jr.; Morillo, C.A.; Avezum, A.; Connolly, S.J.; SosaEstani, S.; et al. BENEFIT Investigators. Rationale and design of a randomized placebo-controlled trial assessing the effects of etiologic treatment in Chagas’ cardiomyopathy: the BENznidazole Evaluation For Interrupting Trypanosomiasis (BENEFIT). Am Heart J. 2008, 156, 37–43. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.