Abstract
The aim of the present review article is to provide an overview of the evaluation of the right ventricle and its function using conventional measurements and newer echocardiographic modalities. Because of the complex geometry of the right ventricle calculation of volumes and ejection fraction using 2D measurements is not useful in clinical practice. Echocardiographic assessment of systolic right ventricular function using 2D guided M-mode measurements of systolic long axis motion of the tricuspid annulus (tricuspid annular plane systolic motion, TAPSE) is a simple and established tool also providing prognostic information in a number of settings. The main limitation of this method is that it only represents the inflow free wall segments but not outflow tract and the septum which also contribute to the overall function of the right ventricle. Additional measurements of the right ventricular outflow tract fractional shortening and fractional area change add great value in the assessment of the right ventricle function. Doppler tissue imaging is a relatively new echocardiographic tool for the assessment of right ventricular function, however, similarly to TAPSE its main caveat is its angle dependency. The most recent imaging technique is based on detecting speckles from the myocardium with 2D echocardiography analysing motion in different directions (longitudinal, radial and circumferential). One of the greatest advantages of speckle tracking compared to Doppler tissue imaging is the lack of angle dependency. Finally, 3D imaging for functional assessment and volume calculations overcomes the limitations of 2D echocardiography and has a similar accuracy as magnetic resonance imaging for the assessment of right ventricular function.
Anatomy and physiology
The normal right ventricle (RV) is a complex crescentshaped structure wrapped around the left ventricle (LV) [1] with separate inflow and outflow portions [2]. The inflow portion contains the tricuspid valve, and from the outflow portion originates the pulmonary trunk [3]. The demarcation between these two parts is formed by a number of prominent muscular bands: the parietal band, the crista supraventricularis (the word, crista, denotes a crest, and this characteristic is only seen if a sagittal section passes through this junction point [4]), the septal band, and the moderator band [2,3]. The moderator band runs from the septum to the anterior RV wall [5]. Together, these muscle bands form an almost circular orifice which, in the normal heart, is wide and forms no impediment to flow [3]. The wall of the inflow portion is heavily trabeculated, particularly in its most apical portion [3]. The outflow portion of the right ventricle, often called the infundibulum, contains only a few trabeculae [3]. The subpulmonic area is smooth-walled [3]. The muscular architecture of the outflow tract is described as a bulbar musculature, which is anatomically and functionally different from the rest of the RV [6]. The right ventricle has superficial circumferential muscle fibres responsible for its inward bellows movement, as well as inner longitudinal fibres which mediate the base-toapex contraction [7]. Compared with the left ventricle, the base-to-apex shortening contributes more to RV emptying [8].
The right and left ventricles are closely related because they share the inter-ventricular septum which predominantly functions as part of the left ventricle in normal hearts [5]. With right-sided volume or pressure overload, the interventricular septal motion is reversed, and the septum functions as a part of the right ventricle [5]. After open heart surgery and a subsequent fall in RV free wall function, septal movement reverses and thus the septum contributes to right ventricular function [5]. Ischaemic or mechanical heart damage and/or an attempt of the heart to maintain RV stroke volume were proposed as mechanisms underlying this phenomenon [5,9]. The term ventricular interdependence has been used to describe dysfunction of one ventricle due to an effect on ventricular septal motion secondary to structural and/or functional abnormalities of the opposite ventricle [2,10]. The right and left ventricles are also linked due to the fact that they are both enclosed within the pericardium. In patients with pericardial constriction, the end-diastolic pressure equalises between the two ventricles. A similar physiology can be seen in patients with large pleural effusion and with end-stage pulmonary disease [11].
There are several clinical conditions where evaluation of right-sided heart morphology and function is of great clinical importance.
The thin wall of the RV (3–4 mm) makes it sensitive to alterations in the pulmonary artery pressure and with a chronic increase in afterload (pulmonary diseases, mitral valve diseases etc.) the RV dilates and develops muscular hypertrophy [12,13]. However, once irreversible pulmonary vascular disease develops, pulmonary artery pressures may remain elevated even after a successful valve or myocardial surgery [14,15]. With a rapid increase of pulmonary artery pressure e.g., in acute pulmonary embolism, the RV acutely dilates resulting in dilatation of the tricuspid annulus and hence tricuspid regurgitation and clinical signs of right ventricular failure [11,16].
Right ventricular dysfunction is present in at least one third of patients with inferior myocardial infarction and is associated with a significant increase in mortality [17]. Reduced RV function is a common finding after open heart surgery [15]. Pericardectomy with the loss of lubricating surface at the anterior surface of the heart, “stunned myocardium”, ischaemic damage due to non-optimal RV myocardial preservation during cardiac surgery, and right atrial damage due to placement of bypass cannulae have all been proposed as possible explanations [15,18]. In the early postoperative period (first days after operation) this might be secondary to the presence of chest tubes, oedema, and bleeding (transient changes combined with mechanical impediment) and in the later postoperative period (six months) it could be due to adhesions between heart and surrounding tissues (mainly mechanical impediment) [15]. This latter hypothesis is supported by the lack of complete recovery of the tricuspid annulus motion after cardiac surgery [15].
The evaluation of right ventricular function in congenital heart disease is of prognostic importance as well. Patients with a systemic right ventricle and bad RV function carry a worse prognosis than the patients with a clearly preserved RV function [19]. In cases of pulmonary valve stenosis, pulmonary valve replacement should be performed before severe RV dysfunction occurs, otherwise RV function never recovers [19]. In patients with repaired Tetralogy of Fallot, a decreasing RV function in the setting of severe pulmonary regurgitation drives the indication for pulmonary valve replacement, before the irreversible RV dysfunction occurs [19]. An isolated large atrial septal defect results in left-to-right shunting and volume overload of the RV [20]. Although the RV generally tolerates chronic volume overload well, long-standing volume overload in the setting of an atrial septal defect is associated with increased mortality and morbidity [21]. Older age at repair or closure (>40 years of age) also is associated with incomplete RV and right atrial remodelling [21].
Conventional two-dimensional assessment of the right heart structure
RV hypertrophy is usually the result of the RV systolic pressure overload [22]. Usually, flattening of the interventricular septum (or even D shaping of the RV) in these cases can be seen (Figure 1) [23]. In the absence of pulmonary hypertension, increased RV thickness can be due to infiltrative or hypertrophic cardiomyopathies [22]. RV free wall thickness, normally less than 0.5 cm, is measured using either M-mode or 2-D imaging [23]. Although RV free wall thickness can be assessed from the apical and parasternal long axis views, the measurement at the level of the tricuspid valve chordae tendinae in the subcostal view is associated with less variability and closely correlates with RV peak systolic pressure [20]. Every effort must be made to avoid overestimation of RV thickness due to the presence of epicardial fat deposition or coarse trabeculations within the right ventricle [8,23]. In cases of significant thickening of the visceral pericardium, measurement of RV wall thickness may be very challenging [8].
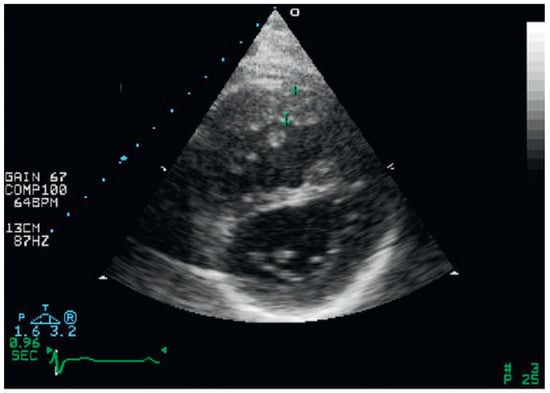
Figure 1.
Right ventricular hypertrophy and flattening of the interventricular septum from parasternal short-axis view.
The RV dilates in response to chronic volume and/ or pressure overload [7]. RV dilatation can also be seen in presence of RV myocardial infarction [24]. In a normal heart, RV area or mid cavity diameter measured in the apical four chamber view is smaller than that of the left ventricle [23]. In cases of RV enlargement, the RV cavity area is similar to that of the left ventricle or exceeds it, and the RV will be “apex forming” [23]. An end-diastolic diameter >42 mm at the RV base and >35 mm at the mid level indicates RV dilatation, and a maximal longitudinal dimension >86 mm indicates RV enlargement as well [8]. However, normal values for RV size are under debate, and in some recommendations [23], a longitudinal RV dimension ≥80 mm is already regarded as abnormal. Care should be taken to obtain a non-foreshortened and properly rotated apical four-chamber view, oriented to obtain the maximum RV dimension, before making the measurements [23].
The apical 4-chamber view allows the estimation of the dimensions of the right atrium (RA): RA area >18 cm2, RA length >53 mm, and RA diameter (minor dimension) >44 mm indicate RA enlargement [8].
The RV outflow tract is best imaged from the parasternal long-axis view angled superiorly and the parasternal short-axis at the base of the heart [23]. It can also be imaged from the subcostal long and transverse windows as well as the apical window [23]. An RV outflow tract diameter >29 mm at the level of the aortic valve, >23 mm just below the pulmonic valve at end-diastole (parasternal short-axis view) indicates RV outflow tract dilatation [25]. Reference values for the size of pulmonary artery diameter are 15–21 mm [25]. Data on normal values of the diameter of the right and left pulmonary artery are sparse. According to Weyman [27], the diameter of the right pulmonary artery at the end diastole in normal adults is 1.2 ± 0.2 cm (range 0.9–1.3 cm) and that of the left pulmonary artery is 1.1 ± 0.4 (range 0.8–1.6 cm).
Assessment of the right ventricular function in M-mode (tricuspid annular excursion, RV outflow fractional shortening)
Due to the complex RV shape assessment, the right ventricular function remains challenging [26,28]. Echocardiographic assessment of systolic RV function using 2D guided M-mode measurements of systolic motion of the tricuspid annulus (tricuspid annular plane systolic excursion or tricuspid annular motion [TAPSE, TAM]) is an attractive tool and a simple measurement [29]. Because the main contraction of the RV occurs in a longitudinal fashion [7,8], assessment of longitudinal function as a substitute of overall RV function is justified in clinical practice. TAPSE is the maximal longitudinal excursion of the tricuspid annulus at peak systole measured by M-mode [8] (Figure 2). In a study of 750 patients with a variety of cardiac conditions and 150 age-matched normal controls, a TAPSE cut-off <17 mm yielded high specificity, though low sensitivity to identify abnormal RV function [30]. According to current guidelines [8], TAPSE <16 mm indicates RV systolic dysfunction (Table 1). The main limitation of this method is that it only represents the function of the inflow free wall segments but not that of the outflow tract and the septum [5]. RV outflow tract fractional shortening obtained in the parasternal short axis view, can be used as a simple additional measure of RV function (Figure 3). Linquist et al. [31] found, that right ventricular outflow tract fractional shortening was reduced in patients with pulmonary hypertension (37% ± 18%), compared to the subjects without (61% ± 13%). There is a good correlation between right ventricular outflow tract fractional shortening and TAPSE, pulmonary artery acceleration time, and estimated systolic pulmonary artery pressure gradient [31].
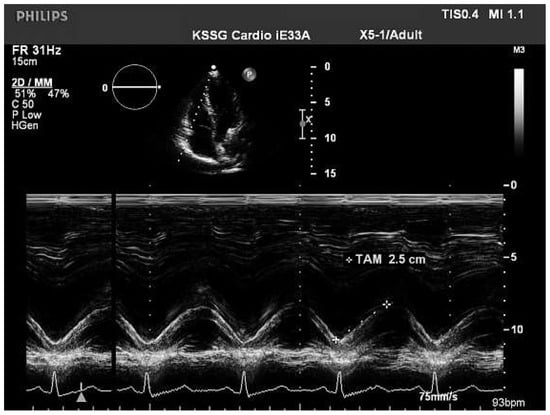
Figure 2.
Tricuspid annulus excursion (TAPSE, TAM) from 4-chamber apical view.

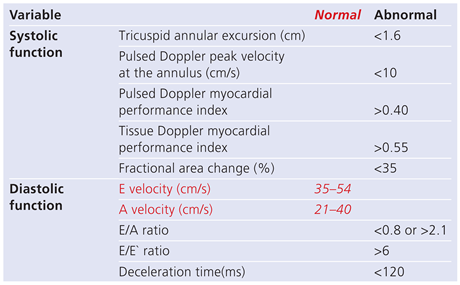
Table 1.
Reference limits for recommended measures of right heart function according to the guidelines for the echocardiographic assessment of the right heart in adults [8].
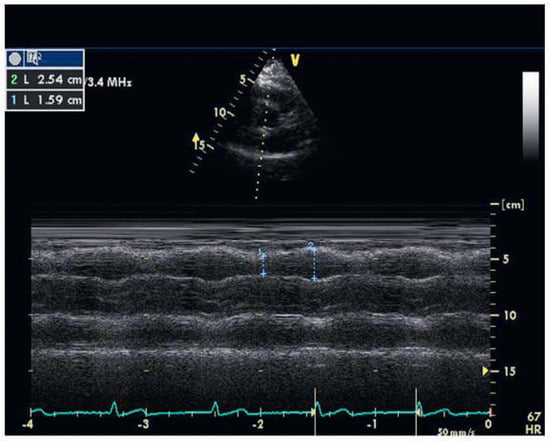
Figure 3.
Right ventricular outflow tract fractional shortening (63%) from parasternal short axis view (right ventricular outflow tract systolic diameter / right ventricular outflow tract diastolic diameter × 100%).
Assessment of the right ventricular function in two-dimensional echocardiography (fractional area change)
The percentage RV fractional area change (FAC), defined as end-diastolic area – end-systolic area/end-diastolic area × 100, is a monoplane measure of RV systolic function that has been shown to correlate with RV ejection fraction as assessed by magnetic resonance imaging [32]. FAC is obtained by tracing the RV endocardium both in systole and diastole [8]. Care must be taken to trace the free wall beneath the trabeculations [8]. Two-dimensional FAC is one of the recommended methods to assess RV function. An RV FAC of less than 35% is considered abnormal [8]. In contrast, further volumetric estimation of RV ejection fraction based on 2D measurement is not recommended, because of the complex form of the RV and the numerous geometric assumptions [8].
Conventional Doppler echocardiography and vena cava inferior measurement
The broadest application of Doppler echocardiography in the assessment of the right heart is that of the estimation of pulmonary artery systolic pressure by measuring the peak velocity of a tricuspid regurgitant jet, applying the modified Bernoulli equation, and combining this value with an estimate of the right atrial pressure. A peak velocity >2.8–2.9 m/s corresponds to systolic pulmonary artery pressure of approximately 36 mm Hg, assuming a right atrial pressure of 3 to 5 mmHg, which may indicate elevated RV systolic and pulmonary artery pressure [8,33]. It is recommended to estimate right atrial pressure based on size and respiratory variability of the inferior vena cava rather than arbitrarily assigning a fixed value [8]. According to Lang et al [23] and Otto [34] the diameter of the inferior vena cava should be measured just below the right atrium (normally 1.0–2.0 cm from the junction with the right atrium) in the subcostal view. If M-mode is used, care must be taken that the measurement is perpendicular to the course of the vessel. To accurately assess inferior vena cava collapsibility, the change in diameter of the vein with a sniff and also with quiet respiration should be measured, thereby making sure that the change in diameter does not reflect a transition of the inferior vena cava into another plane [8,34]. There are several conditions to be considered in evaluating the inferior vena cava [25]. Athletes, especially highly trained swimmers, have been shown to have dilated inferior vena cava (2.31 ± 0.46 cm, compared to 1.14 ± 0.13 cm in age-matched normals) with normal collapsibility index [35,36]. A dilated inferior vena cava in mechanically ventilated patients does not always indicate a high right atrial pressure. However, a small inferior vena cava (<1.2 cm) has a 100% specificity for a right atrial pressure of less than 10 mm Hg [37]. A better correlation between right atrial pressure and size and respiratory variability of the inferior vena cava was found when inferior vena cava diameter was measured at end-expiration and end-diastole in this setting [38]. Therefore this could be advised for the measurement, though guidelines [8,25] do not give any directions of the measurement concerning the heart cycle. According to the results of Mookadam et al. [39], there were no significant changes in the calibre of inferior vena cava with regard to the cardiac cycle phase, as well.
In the absence of a gradient across the pulmonicvalve or right ventricular outflow tract, systolic pulmonary artery pressure is equal to right ventricular systolic pressure. In cases of elevated right ventricular systolic pressure, obstruction at the level of the right ventricular outflow tract or pulmonic valve should be excluded especially in patients with congenital heart disease or post pulmonic valve surgery [8].
If the Doppler signal is not satisfactory, it may be enhanced with agitated saline contrast, but it is important to avoid overestimation of the spectral envelope by ensuring that only the well-defined, dense spectral profile is measured [8].
Pulmonary artery diastolic pressure can be estimated from velocity of the end-diastolic pulmonary regurgitant jet (adding the right atrium pressure), and mean pulmonary artery pressure (normally <25 mm Hg at rest [40]) from the early pulmonary regurgitation velocity or pulmonary artery flow acceleration time (mean pulmonary artery pressure = 90 – (0.45 × pulmonary artery flow acceleration time), when the acceleration time <120 ms and heart rate is in the normal range of 60 to <100 beats/min. [41]. Pulmonary artery flow acceleration time may not be fully reliable for accurate pulmonary artery pressure predictions, but it has been found to be of prognostic value in patients with chronic lung disease [42], and its shortening could indicate an enhanced stiffness of pulmonary vessels [23]. In our experience, pulmonary artery flow acceleration time may differ considerably when measured in the parasternal or subcostal view. A possible explanation for this may be different investigation angles to the flow.
The Tei or myocardial performance index (MPI) [43] calculated as the ratio between total RV isovolumic time (contraction and relaxation) divided by pulmonary ejection time is a combined measure of systolic and diastolic function RV function. The intervals are measured from the tricuspid inflow and pulmonary ejection profiles. The RV MPI has been found to correlate with pulmonary pressures (patients with increased right ventricular afterload) and can be used to identify early global RV dysfunction in congenital heart disease [44], pulmonary arterial hypertension [45], chronic respiratory disease [46], and connective tissue diseases [23,47]. Severely reduced RV ejection fraction (≤30%, as obtained by magnetic resonance imaging) was found to be best detected by MPI at a value of >0.50 [48]. The main limitation of the MPI is the fact that with increased right atrial pressure MPI falls due to shortened isovolumic relaxation time [49].
Doppler tissue imaging and regional strain and strain rate
Doppler tissue imaging (TDI) is a relatively new echocardiographic tool in the assessment of RV function. The following components can be measured: isovolumic contraction peak positive velocity (IVCPPV), isovolumic contraction time (IVCT), (time period between the end of atrial peak velocity [A’] and the onset of systolic ejection peak velocity [S’]); isovolumic relaxation time (IVRT), defined as time difference between the end of ejection and the onset of early diastolic peak velocity (E’) (Figure 4). IVRT (TDI measurements performed in the apical four chamber view at the lateral aspect of the tricuspid annulus) appears to be helpful in evaluating pulmonary hypertension. A normal IVRT (<40 ms) can exclude pulmonary hypertension with high negative predictive value. A prolonged IVRT is suggestive of pulmonary hypertension [50]. Longitudinal RV myocardial velocities are usually obtained from the apical four-chamber view and tracings are best recorded from the annulus (A), basal (B) and mid (M) segmental levels of the RV free wall [51]. Any evidence of asynchrony appears in the form of wrongly timed segmental movements [5].
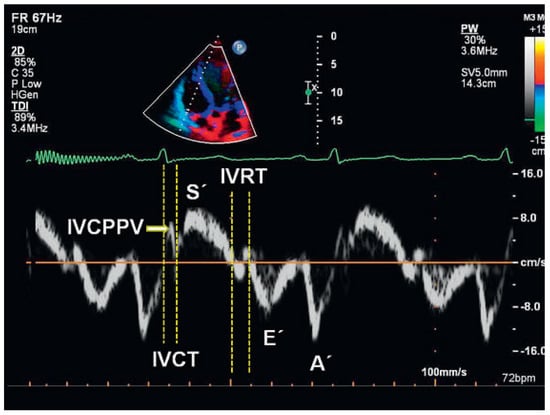
Figure 4.
Tissue Doppler measurements of right ventricular lateral tricuspid annulus. IVCPPV = isovolumic contraction peak positive velocity; IVCT = isovolumic contraction time (time period between the end of atrial peak velocity [A’] and the onset of systolic ejection peak velocity [S’]); IVRT = isovolumic relaxation time, defined as time difference between the end of ejection and the onset of early diastolic peak velocity (E’).
Longitudinal velocity of excursion of the basal free wall segment of the RV has been termed the RV S’ (or Sm, where m - myocardial) or systolic excursion velocity and is a simple and reproducible measure to evaluate basal RV free wall function and RV function as well [8]. The velocity S’ is read as the highest systolic velocity without overgaining the Doppler envelope [8]. S’ <10 cm/s should raise the suspicion of abnormal RV function, particularly in a younger adult patient, but there are insufficient data in the elderly [8]. According to the study of Pavlicek et al. [48], the velocity S’ of <11 cm/s at the lateral tricuspid annulus most accurately detects impaired RV ejection fraction as obtained by magnetic resonance image – currently assumed to be the most reliable and reproducible method of assessing RV function.
Another parameter, myocardial acceleration time during isovolumic contraction is defined as the peak isovolumic myocardial velocity divided by time to peak velocity and is measured at the lateral tricuspid annulus [8]. For the calculation of isovolumic acceleration, the onset of myocardial acceleration is at the zero crossing point of myocardial velocity during isovolumic contraction [8]. Isovolumic acceleration appears to be less load dependent than ejection period indices [52,53]. It appeared to be the most consistent TDI variable for the evaluation of RV function measured by either transthoracic echocardiography (lateral wall) or transoesophageal echocardiography (inferior wall) [54] and correlated well with the severity of illness in situations affecting right heart function, including obstructive sleep apnoea [55], mitral stenosis [56], repaired tetralogy of Fallot with pulmonary regurgitation [57] and transposition of the great arteries following an atrial switch procedure [58]. Isovolumic acceleration time is age dependent and varies with heart rate. Therefore, indexing of this parameter to heart rate may be appropriate in some clinical situations [8,53]. Because of the broad confidence interval around its lower reference limit, the guidelines do not recommend any reference value at present [8].
The major disadvantages of pulsed TDI are poor spatial resolution due to movement of the heart, while the sample volume is fixed and apical velocities from the apical long axis projection are difficult to measure [59]. Colour TDI is an alternative approach for the measurement of myocardial motion and can be used off-line [5]. The limitation of colour TDI is the lower temporal resolution; however, a frame rate above 100 frames per second is considered acceptable [60].
Unfortunately, TDI is dependent on the angle at which the region of interest is imaged [5]. Overall heart motion, cardiac rotation and wall motion from tethering segments limit the use of TDI [5]. One-dimensional strain echocardiography is a dimensionless measurement which represents the fractional or percentage change in myocardial fibre shortening. As this myocardial deformation or strain is caused by fibre shortening, it can be used as a measure of „true“ segmental systolic performance [61]. Strain measurements of the RV are best performed from the apical four-chamber view, assessing the RV free wall from the base to the apical level [5]. Dambrauskaite et al. [62] found, that in patients with pulmonary hypertension, deformation parameters were significantly lower compared with that of control subjects: basal strain rate –2.28 ± 0.9 vs –2.94 ± 0.9 s (–1); strain –28 ± 13% vs –42 ± 11%; apical strain rate –1.05 ± 1.38 vs –2.60 ± 0.9 s (–1); strain –13 ± 16% vs –41 ± 11%, respectively. The deformation parameters in the apical segment were reduced more than in the basal segment (the segment-wise comparison with p <0.002 for strain rate and p <0.0001 for strain) in the patient group [62]. Strain and strain rate have shown significant RV abnormalities in patients with amyloidosis, pulmonary stenosis, atrial septum defects and arrhythmogenic RV dysplasia as well [63,64]. In conclusion, strain echocardiography might have a potential impact for quantifying regional RV function in terms of regional myocardial shortening and lengthening [5]. However, how these advantages overcome the disadvantages of signal to noise ratio and angle dependence are still not thoroughly evaluated, thus limiting its clinical application [5].
RV diastolic function
There are many conditions, associated with RV diastolic dysfunction, including both pressure and volume overload situations, ischaemic heart disease (especially inferior wall myocardial infarction with RV involvement), cardiomyopathies, congenital heart diseases, arterial hypertension, systemic and endocrine diseases, primary lung diseases, physiologic aging process, left ventricular dysfunction (via ventricular interdependence), heart transplantation etc. [8,65,66,67,68]. In investigations of RV diastolic function by means of TDI at the tricuspid annulus in ischemic heart disease, the utility of E’, A’, and the E’/A’ ratio is usually evaluated [67,68,69]. Patients with RV diastolic dysfunction usually have reduced E’ velocity and a reduced E’/A’ ratio [69]. A’ velocity may be increased in the early course of diastolic dysfunction, whereas with increased RV late diastolic pressures, it may decrease [69]. Care must be taken to measure at held end-expiration and/or take the average of ≥5 consecutive beats [70]. According to the guidelines [8], grading of RV diastolic dysfunction is recommended as follows: tricuspid E/A ratio <0.8 suggests impaired relaxation, a tricuspid E/A ratio of 0.8 to 2.1 with an E/E’ ratio >6 or diastolic flow predominance in the hepatic veins suggests pseudonormal filling, and a tricuspid E/A ratio >2.1 with a deceleration time <120 ms suggests restrictive filling. Further studies would be useful to validate the sensitivity and specificity and the prognostic implications of this classification [8].
Speckle tracking and three-dimensional echocardiography
The most recent image acquisition techniques are based on detecting speckles from the myocardium with 2D echocardiography (Figure 5) analysing motion in different directions, longitudinal, radial and circumferential [71]. Three dimensional (3D)-wall motion tracking provides a more complete and probably accurate evaluation of global and segmental ventricular function, assessing real 3D strain, rotation and twist movement [72]. One of the greatest advantages of speckle tracking compared to TDI is the lack of angle dependency – this allows the separation of myocardial motion patterns into the basic components (longitudinal-, radial-, circumferential motion) [73]. Similar to TDI technology, postprocessing variables include velocity, displacement, strain rate and strain analysis [73]. Whereas the results of TDI are best with apical views and longitudinal motion patterns, speckle tracking offers the possibility to use almost all standard views and seems to have a higher degree of reproducibility [73]. Assessment of right ventricular free wall longitudinal myocardial deformation using speckle tracking imaging in normal subjects showed, that older subjects had lower early diastolic strain rate (SRe) and higher late diastolic strain rate (SRa) than the younger subjects [74]. Normal values [75] of speckle tracking parameters are presented in Table 2. Regional RV free wall longitudinal myocardial strain calculated by speckle tracking can be used to assess RV function in pulmonary hypertension: RV free wall longitudinal myocardial strain was significantly associated with WHO functional group (–25.4 ± 4.9%, –21.2 ± 7.9%, –17.4 ± 6.3%, –13.4 ± 4.9% for groups I–IV respectively) [76].
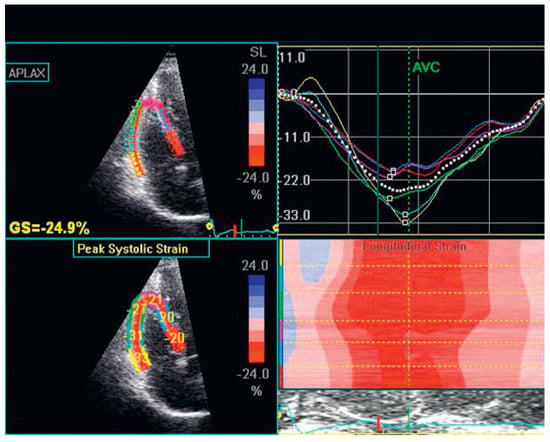
Figure 5.
Speckle tracking imaging of the right ventricle.

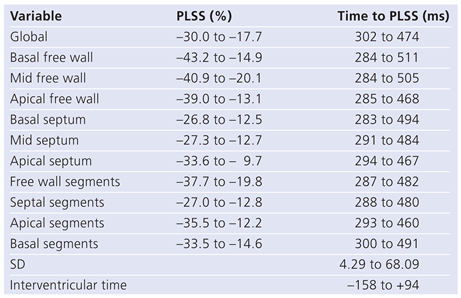
Table 2.
Ranges of normality of right ventricle for global and regional peak longitudinal systolic stress (PLSS) and time to PLSS in normal subjects according to Meris et al. [75].
3D-echocardiography makes it possible to visualise the entire RV and provide its volumetric assessment [77]. Niemann et al. [78] report on the use of semiautomated border detection software (TomTec), which provides accurate RV volumes and RV ejection fraction measurements. But all techniques (including computer tomography or magnetic resonance imaging as well) struggle with the heavy trabecularisation of the RV and with the demarcation of the valvular structures [79]. Further studies are needed to assess the technical aspects of acquiring data sets and to determine the range of RV volumes and RV ejection fraction in health and pathology [80].
In conclusion, 3D-echocardiography and speckle tracking are new methods and could provide simultaneous quantification of global and regional RV-function that is not angle dependent and can be applied retrospectively to digitally stored images [81]. Possibly, these methods could mark the beginning of a new era in RV investigations. For the everyday practice at present, the guidelines [8] recommend several simple and reproducible methods of assessing right ventricular systolic function (FAC, TAPSE, pulsed tissue Doppler S`, and MPI).
Funding / potential competing interests
No financial support and no other potential conflict of interest relevant to this article were reported.
References
- Haddad, F.; Doyle, R.; Murphy, D.J.; Hunt, S.A. Right ventricle function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricle failure. Circulation. 2008, 117, 1717–1731. [Google Scholar] [CrossRef]
- Burgess, M.I.; Bright-Thomas, R.J.; Ray, S.G. Echocardiographic evaluation of right ventricular function. Eur J Echocardiography. 2002, 3, 252–262. [Google Scholar] [CrossRef][Green Version]
- Netter, F.H. Atria and ventricles In: Netter, F.H.; ed. Yonkman, F.F.; The Netter Collection of Medical illustrations Vol.5 Heart. Novartis publication, Ninth Printing by Union Graphics, Inc., Linden, NJ 1999:8–10.[Green Version]
- McAlpine, W.A. The right ventricle. In: McAlpine, W.A.; Heart and coronary arteries. An Anatomical Atlas for Clinical Diagnosis, Radiological Investigation, and Surgical Treatment. Springer-Verlag Berlin Heidelberg New York 1975: 65–86.[Green Version]
- Lindqvist, P.; Calcutteea, A.; Henein, M. Echocardiography in assessment of right heart function. Eur J Echocardiogr. 2008, 9, 225–234. [Google Scholar] [CrossRef]
- Goldstein, J.A. Right heart ischemia: pathophysiology, natural history and clinical management. Prog Cardiovasc Dis. 1998, 40, 325–341. [Google Scholar] [CrossRef]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging and functional assessment of the right ventricle. Circulation. 2008, 117, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from American Society of Echocardiography Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Wranne, B.; Pinto, F.J.; Hammarstrom, E.; St. Goar, F.G.; Puryear, J.; Popp, R.L. Abnormal right heart filling after cardiac surgery: time course and mechanisms. Br Heart J. 1991, 66, 435–442. [Google Scholar] [CrossRef]
- Yu, C.M.; Sanderson, J.E.; Chan, S.; Yeung, L.; Hung, Y.T.; Woo, K.S. Right ventricular diastolic dysfunction in heart failure. Circulation. 1996; 93, 1509–1514, Clyne, C.A.; Alpert, J.S.; Benotti, J.R. Interdependence of the left and right ventricles in health and disease. Am Heart J. 1989, 117, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.; Sheppard, M.; Pepper, J.; Rigby, M. Clinical echocardiography; Springer: Berlin, Germany, 2004; p. 240. [Google Scholar]
- Dell’’Italia, L.J. The right ventricle: anatomy, physiology, and clinical importance. Curr Probl Cardiol. 1991, 16, 653–720. [Google Scholar] [PubMed]
- Armour, J.A.; Randall, W.C. Structural basis for cardiac function. Am J Physiol. 1970, 218, 1517–1523. [Google Scholar] [CrossRef]
- Nagel, E.; Stuber, M.; Hess, O.M. Importance of the right ventricle in valvular heart disease. Eur Heart J. 1996, 17, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Fuster, V. What we do gain from the analysis of right ventricular function? J Am Coll Cardiol. 1984, 3, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Casazza, F.; Bongarzoni, A.; Capozi, A.; Agostoni, O. Regional right ventricular dysfunction in acute pulmonary embolism and right ventricular infarction. Eur J Echocardiogr. 2005, 6, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Zehender, M.; Kasper, W.; Kauder, E.; Schönthaler, M.; Geibel, A.; Olschewski, M.; Just, H. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med. 1993, 328, 981–988. [Google Scholar] [CrossRef]
- Carr-White, G.S.; Kon, M.; Koh, T.W.; Glennan, S.; Ferdinand, F.D.; De Souza, A.C.; et al. Right ventricular function after pulmonary outograft replacement of the aortic valve. Circulation. 1999, 100 (Suppl. 19), 1136–1141. [Google Scholar] [CrossRef]
- Warnes, C.A. Adult congenital heart disease, importance of right ventricle. J Am Coll Cardiol. 2009, 54, 1903–1910. [Google Scholar] [CrossRef]
- Haddad, F.; Doyle, R.; Murphy, D.J.; Hunt, S.A. Right ventricular function in cardiovascular disease, part II. Pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008, 117, 1717–1731. [Google Scholar] [CrossRef]
- Davlouros, P.A.; Niwa, K.; Webb, G.; Gatzoulis, M.A. The right ventricle in congenital heart disease. Heart. 2006, 92, i27–i38. [Google Scholar] [CrossRef]
- Matsukubo, H.; Matsuura, T.; Endo, N.; Asayama, J.; Watanabe, T. Echocardiographic measurement of right ventricular wall thickness. A new application of subxiphoid echocardiography. Circulation. 1977, 56, 278–284. [Google Scholar] [CrossRef]
- Zeydabadinejad, M. Echokardiographie des rechten Herzens. Georg Thieme Verlag KG Stuttgart; 2006: 75.
- Gottdiener, J.S.; Gay, J.A.; Maron, B.J.; Fletcher, R.D. Increased right ventricular wall thickness in left ventricular pressure overload: echocardiographic determination of hypertrophic response of the “nonstressed” ventricle. J Am Coll Cardiol. 1985, 6, 550–555. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; et al. Recommendations for chamber quantification. Eur J Echocardiography. 2006, 7, 79–108. [Google Scholar] [CrossRef]
- Brookes, C.; Ravn, H.; White, P.; Moeldrup, U.; Oldershaw, P.; Redington, A. Acute right ventricular dilatation in response to ischemia significantly impairs left ventricular systolic performance. Circulation. 1999, 100, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Weyman, A.E. Principles and practice of echocardiography. Lea and Febiger, Philadeplphia. A Waverly Company. 1994, 1289–1298.
- Kovalova, S.; Necas, J.; Cerbak, M.P.; Vespalec, J. Echocardiographic volumetry of the right ventricle. Eur J Echocardiography. 2005, 6, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Lindmarker, P.; Johnsson, H.; Juhlin-Dannfelt, A.; Jorfeldt, L. Pulmonary embolism: a follow up study of the relation between the degree of right ventricle overload and the extent of perfusion defects. J Intern Med. 1999, 245, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, G.; Pepi, M.; Galli, C.A.; Maltagliati, A.; Celeste, F.; Muratori, M.; et al. Feasibility and accuracy of a routine echocardiographic assessment of right ventricular function. Int J Cardiol. 2007, 115, 86–89. [Google Scholar] [CrossRef]
- Lindqvist, P.; Henein, M.; Kazzam, E. Right ventricular outflow-tract fractional shortening: an applicable measure of right ventricular systolic function. Eur J Echocardiography. 2003, 4, 29–35. [Google Scholar] [CrossRef]
- Lai, W.W.; Gauvreau, K.; Rivera, E.S.; Saleeb, S.; Powell, A.J.; Geva, T. Accuracy of guideline recommendations for two-dimensional quantification of the right ventricle by echocardiography. Int J Cardiovasc Imaging. 2008, 24, 691–698. [Google Scholar] [CrossRef]
- Yock, P.G.; Popp, R.L. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984, 70, 657–662. [Google Scholar] [CrossRef]
- Wong, S.P.; Otto, C.M. Echocardiographic findings in acute and chronic pulmonary disease. In The practice of clinical echocardiography, 2nd ed.; Otto, C.M., Ed.; W.B. Saunders Company: Philadelphia, 2002; pp. 739–760. [Google Scholar]
- Kircher, B.J.; Himelman, R.B.; Schiller, N.B. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990, 66, 493–496. [Google Scholar] [CrossRef]
- Goldhammer, E.; Mesnick, N.; Abinader, E.G.; Sagiv, M. Dilated inferior vena cava: a common echocardiographic finding in highly trained elite athletes. J Am Soc Echocardiogr. 1999, 12, 988–993. [Google Scholar] [CrossRef]
- Jue, J.; Chung, W.; Schiller, N.B. Does inferior vena cava size predict right atrial pressures in patients receiving mechanical ventilation? J Am Soc Echocardiogr. 1992, 5, 613–619. [Google Scholar] [CrossRef]
- Bendjelid, K.; Romand, J.A.; Walder, B.; Suter, P.M.; Fournier, G. Correlation between measured inferior vena cava diameter and right atrial pressure depends on the echocardiographic method used in patients who are mechanicaly ventilated. J Am Soc Echocardiogr. 2002, 15, 944–949. [Google Scholar] [CrossRef]
- Mookadam, F.; Warsame, T.A.; Yang, H.S.; Emani, U.R.; Appleton, C.P.; Raslan, S.F. Effect of positional changes on inferior vena cava size. Eur J Echocardiogr. 2011, 12, 322–325. [Google Scholar] [CrossRef] [PubMed]
- McLauglin, V.V.; Archer, S.L.; Badesch, D.B.; Barst, R.J.; Farber, H.W.; Lindner, J.R.; Mathier, M.A.; McGoon, M.D.; Park, M.H.; Rosenson, R.S.; et al. ACCF/AHA 2009 Expert consensus document on pulmonary hypertension. A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association Developed in Collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009, 53, 1573–1619. [Google Scholar]
- Dabestani, A.; Mahan, G.; Gardin, J.M.; Takenaka, K.; Burn, C.; Allfie, A.; Henry, W.L. Evaluation of pulmonary artery pressure and resistance by pulsed Doppler echocardiography. Am J Cardiol. 1987, 59, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Torbicki, A.; Pruszczyk, P.; Sliwinski, P.; Kuch-Wochial, A. Pulsed wave Doppler and survival in patients with chronic cor pulmonale. Eur Respir J. 1993, 6, 188. [Google Scholar]
- Tei, C. New non-invasive index for combined systolic and diastolic ventricular function. J Cardiol. 1995, 26, 135–136. [Google Scholar]
- Eidem, B.W.; O’Leary, P.W.; Tei, C.; Seward, J.B. Usefulness of the myocardial performance index for assessing right ventricular function in congenital heart disease. Am J Cardiol. 2000, 86, 654–658. [Google Scholar] [CrossRef]
- Yeo, T.C.; Dujardin, K.S.; Tei, C.; Mahoney, D.W.; McGoon, M.D.; Seward, J.B. Value of a Doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. Am J Cardiol. 1998, 81, 1157–1161. [Google Scholar] [CrossRef]
- Burgess, M.; Mogulkoc, N.; Bright-Thomas, R.; Bishop, P.; Egan, J.; Ray, S. Comparison of echocardiographic markers of right ventricular function in determining prognosis in chronic pulmonary disease. J Am Soc Echocardiogr. 2002, 15, 633–639. [Google Scholar] [CrossRef]
- Vonk, M.C.; Sander, M.H.; van den Hoogen, F.H.; van Riel, P.L.; Verheut, F.W.; van Dijk, A.P. Right ventricle Tei-index: A tool to increase the accuracy of non invasive detection of pulmonary arterial hypertension in connective tissue diseases. Eur J Ecocardiogr. 2006, 8, 317–321. [Google Scholar] [CrossRef]
- Pavlicek, M.; Wahl, A.; Rutz, T.; de Marchi, S.F.; Hille, R.; Wustmann, K.; Steck, H.; Eigenmann, C.; Schwerzmann, M.; et al. Right ventricular systolic function assessment: rank of echocardiographic methods vs. cardiac magnetic resonance imaging. Eur J Echocardiogr. 2011, 12, 871–880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshifuku, S.; Otsuji, Y.; Takasaki, K.; Yuge, K.; Kisanuki, A.; Toyonaga, K.; Lee, S.; Murayama, T.; Nakashima, H.; Kumanohoso, T.; et al. Pseudonormalized Doppler total ejection isovolume (Tei) index in patients with right ventricular acute myocardial infarction. Am J Cardiol. 2003, 91, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Brechot, N.; Gambotti, L.; Lafitte, S.; Roudaut, R. Usefulness of right ventricular isovolumic relaxation time in predicting systolic pulmonary artery pressure. Eur J Echocardiogr. 2008, 9, 547–554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kowalski, M.; Kukulski, T.; Jamal, F.; D’Hooge, J.; Weidemann, F.; Rademakers, F.; et al. Can natural strain and strain rate quantify regional myocardial deformation? A study in healthy subjects. Ultrasound Med Biol. 2001, 27, 1087–1097. [Google Scholar] [CrossRef]
- Kjaergaard, J.; Snyder, E.M.; Hassager, C.; Oh, J.K.; Johnson, B.D. Impact of preload and afterload on global and regional right ventricular function and pressure: a quantitative echocardiography study. J Am Soc Echocardiogr. 2006, 19, 515–521. [Google Scholar] [CrossRef]
- Vogel, M.; Schmidt, M.R.; Kristiansen, S.B.; Cheung, M.; White, P.A.; Sorensen, K.; Redington, A.N. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: comparison with ventricular pressure-volume relations in an animal model. Circulation. 2002, 105, 1693–1699. [Google Scholar] [CrossRef]
- Tousignant, C.P.; Bowry, R.; Levesque, S.; Denault, A.Y. Regional differences in color tissue Doppler-derived measures of longitudinal right ventricular function using transesophageal and transthoracic echocardiography. J Cardiothorac Vasc Anesth. 2008, 22, 400–405. [Google Scholar] [CrossRef]
- Tugcu, A.; Guzel, D.; Yildirimturk, O.; Aytekin, S. Evaluation of right ventricular systolic and diastolic function in patients with newly diagnosed obstructive sleep apnea syndrome without hypertension. Cardiology. 2009, 113, 184–192. [Google Scholar] [CrossRef]
- Sade, L.E.; Ozin, B.; Ulus, T.; Acikel, S.; Pirat, B.; Bilqi, M.; Ulucam, M.; Müderrisoglu, H. Right ventricular contractile reserve in mitral stenosis: implications of heamodynamic burden and clinical outcome. Int J Cardiol. 2009, 135, 193–201. [Google Scholar] [CrossRef]
- Toyono, M.; Harada, K.; Tamura, M.; Yamamoto, F.; Takada, G. Myocardial acceleration during isovolumic contraction as a new index of right ventricular contractile function and its relation to pulmonary regurgitation in patients after repair of tetralogy of Fallot. J Am Soc Echocardiogr. 2004, 17, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.; Derrick, G.; White, P.A.; Cullen, S.; Aichner, H.; Deanfield, J.; Redington, A.N. Systemic ventricular function in patients with transposition of the great arteries after atrial repair: a tissue Doppler and conductance catheter study. J Am Coll Cardiol. 2004, 43, 100–106. [Google Scholar] [PubMed]
- Pellerin, D.; Sharma, R.; Elliott, P.; Veyrat, C. Tissue Doppler, strain, and strain rate echocardiography for the assessment of left and right systolic ventricular function. Heart. 2003, 89 (Suppl. 3), iii9–iii17. [Google Scholar] [PubMed][Green Version]
- Lind, B.; Nowak, J.; Dorph, J.; van der Linden, J.; Brodin, L.A. Analysis of temporal requirements for myocardial tissue velocity imaging. Eur J Echocardiogr. 2002, 3, 214–219. [Google Scholar][Green Version]
- Sutherland, G.R.; Di Salvo, G.; Claus, P.; D’Hooge, J.; Bijnens, B. Strain and strain rate imaging: a new clinical approach to quantifying regional myocardial function. J Am Soc Echocardiogr. 2004, 17, 788–802. [Google Scholar][Green Version]
- Dambrauskaite, V.; Delcroix, M.; Claus, P.; Herbots, L.; D’Hooge, J.; Bijnens, B.; Rademakers, F.; Sutherland, G.R. Regional right ventricular dysfunction in chronic pulmonary hypertension. J Am Soc Echocardiogr. 2007, 20, 1172–1180. [Google Scholar] [CrossRef]
- Lindqvist, P.; Olofsson, B.O.; Backman, C.; Suhr, O.; Waldenstrom, A. Pulsed tissue Doppler and strain imaging discloses early signs of infiltrative cardiac disease: a study on patients with familial amyloidotic polyneuropathy. Eur J Echocardiogr. 2006, 7, 22–30. [Google Scholar]
- Sutherland, G.R.; Di Salvo, G.; Claus, P.; D’Hooge, J.; Bijnens, B. Strain and strain rate imaging: a new clinical approach to quantifying regional myocardial function. J Am Soc Echocardiogr. 2004, 17, 788–802. [Google Scholar] [CrossRef]
- Cicala, S.; Galderisi, M.; Caso, P.; Petrocelli, A.; D’Errico, A.; de Divitiis, O.; et al. Right ventricular diastolic dysfunction in arterial systemic hypertension: analysis by pulsed tissue Doppler. Eur J Echocardiography. 2002, 3, 135–142. [Google Scholar]
- Tektonidou, M.; Ioannidis, J.; Moyssakis, I.; Boki, K.; Vassilliou, V.; Vlachoyiannopoulo, P.; et al. Right ventricular diastolic dysfunction in patients with anticardiolipin antibodies and antiphospholipid syndrome. Ann Rheum Dis. 2001, 60, 43–48. [Google Scholar]
- Hedman, A.M.; Nordlander, R.; Samad, B. Right ventricular function before and after an uncomplicated coronary bypass graft as assessed by pulsed wave Doppler tissue imaging of the trucuspid anulus. Am Heart J. 2003, 146, 520–526. [Google Scholar]
- Dokainish, H.; Abbey, H.; Gin, K.; Ramanathan, K.; Lee, P.K.; Jue, J. Usefulness of tissue Doppler imaging in the diagnosis and prognosis of acute right ventricular infarction with inferior wall acute left ventricular infarction. Am J Cardiol. 2005, 95, 1039–1042. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Zoghbi, W.A. Evaluation of right ventricular diastolic function. In Klein, A.L.; Garcia, M.J. Diastology. Clinical approach to diastolic heart failure, Ed.; Saunders Elsevier: Philadelphia, 2008; pp. 171–180. [Google Scholar]
- Klein, A.L.; Leung, D.Y.; Murray, R.D.; Urban, L.H.; Bailey, K.R.; Tajik, A.J. Effects of age and physiologic variables on right ventricular filling dynamics in normal subjects. Am J Cardiol. 1999, 84, 440–448. [Google Scholar] [CrossRef]
- Helle-Valle, T.; Crosby, J.; Edvardsen, T.; Lyseggen, E.; Amundsen, B.H.; Smith, H.J.; Rosen, B.D.; Lima, J.A.C.; Torp, H.; Ihlen, H.; Smiseth, O.A. New non invasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation. 2005, 112, 3149–3156. [Google Scholar] [CrossRef] [PubMed]
- Perez de Isla, L.; Vivas, D.; Zamorano, J. Three-dimensional wall motion tracking. E-Journal of the ESC Council for Cardiology Practice, 2008, 7, 22, Available from: http://www.escardio.org/communities/councils/ccp/e-journal/volume7/Pages/vol7n7.aspx. [Google Scholar]
- Winter, S.; Nesser, H.-J. Echocardiography for cardiac resynchronization – the next step. Medtronic Oesterreich GmbH, Wien, 2007:18–20.
- Tong, C.; Li, C.; Song, J.; Liu, H.; Deng, Y. Assessment of right ventricular free wall longitudinal myocardial deformation using specle tracking imaging in normal subjects. J Huazhong Univ Sci Technol. 2008, 28, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Meris, A.; Faletra, F.; Conca, C.; Klersy, C.; Regoli, F.; Klimusina, J.; et al. Timing and magnitude of regional right ventricular function: a speckle tracking-derived strain study of normal subjects and patients with right ventricular dysfunction. J Am Soc Echocardiogr. 2010, 23, 823–831. [Google Scholar] [CrossRef]
- Reali, M.; Rajagopalan, N.; Lopez-Candales, A.; Cordero, K.E.; Suffoletto, M.; Shroff, S.G.; et al. Regional right ventricular myocardial strain by echocardiographic speckle tracking distinguishes clinical and hemodynamic RV dysfunction in pulmonary hypertension. J Cardiac Failure. 2008, 14, 17. [Google Scholar] [CrossRef]
- Papavassiliou, D.P.; Parks, W.J.; Hopkins, K.L.; Fyfe, D.A. Three-dimensional echocardiographic measurement of right ventricular volume in children with congenital heart disease validated by magnetic resonance imaging. J Am Soc Echocardiogr. 1998, 11, 770–777. [Google Scholar] [CrossRef]
- Niemann, P.S.; Pinho, L.; Balbach, T.; Galuschky, C.; Blankenhagen, M.; Silberbach, M.; et al. Anatomically oriented right ventricular volume measurements with dynamic three-dimensional echocardiography validated by 3-Tesla magnetic resonance imaging. JACC. 2007, 50, 1668–1676. [Google Scholar] [CrossRef]
- Rademakers, F. Echocardiographic volumetry of the right ventricle. Eur J Echocardiogr. 2005, 6, 4–6. [Google Scholar] [CrossRef][Green Version]
- Jurcut, R.; Giusca, S.; La Gerche, A.; Vasile, S.; Ginghina, C.; Voigt, J.U. The echocardiographic assessment of the right ventricle: what to do in 2010? Eur J Echocardiogr. 2010, 11, 81–96. [Google Scholar] [CrossRef]
- Pirat, B.; Mcculloch, M.L.; Zoghbli, W.A. Evaluation of global and regional right ventricular systolic function in patients with pulmonary hypertension using a novel speckle tracking method. Am J Cardiol. 2006, 98, 699–704. [Google Scholar] [CrossRef]
© 2012 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.