Introduction
Arterial hypertension, defined as a clinic blood pressure ≥140/90 mm Hg and/or a day time ambulatory blood pressure ≥135/85 mm Hg, is quantitatively the most important risk factor for cardiovascular disease and a leading cause of mortality worldwide [
1]. Although major improvements in therapeutic management have been achieved over the last decades, arterial hypertension remains a major public health challenge. Hypertension is only controlled in 38% of adults in practice [
2] and similar figures are available from the NHANES [
3]. Resistant hypertension is defined as inadequate blood pressure control (office BP ≥140/90 mm Hg) on treatment with three antihypertensive classes, including a well titrated diuretic [
3]. However, renal denervation is limited to patients with an uncontrolled hypertension (office BP ≥160/100 mm Hg and/or ambulatory BP ≥135/85 mm Hg treated with four treatments including spironolactone 25 mg. While there is a lack of data on the precise prevalence of resistant hypertension, some sources estimate the frequency close to 10% of all hypertensive individuals [
4], other estimates are lower [
5]. Interestingly, the majority of the resistant hypertensive patients exhibit an increased sympathetic drive and there are arguments for a neurogenic component of the blood pressure increase. Failure to reach the target blood pressure puts patients at high risk for cardiovascular complications and is therefore a major concern [
6].
Destruction of sympathetic nerves as a treatment for hypertension was first described several decades ago. In 1931, some case studies demonstrated the successful treatment of severely elevated blood pressure by surgical removal of parts of the sympathetic nervous system [
7]. However, side effects such as impotence and severe orthostatic hypotension occurred. In addition there were many other risks related to the procedure, therefore the approach was abandoned.
The concept to act selectively on the renal efferent and afferent nerves to treat high blood pressure has recently emerged and is attracting considerable attention. Interestingly, the sympathetic nervous system is not only activated in hypertension, but also in glucose metabolism disturbances, metabolic syndrome, and heart failure. As a consequence, targeting the renal sympathetic nervous system may also be a therapeutic option for these and other conditions linked to sympathetic over-activation. The first clinical study on renal denervation for resistant hypertension was published in 2009 in the Lancet [
8], the first of a series of two reports to date, the “Simplicity Trials”. The subject has drawn broad interest with more than 2,000 publications including the keyword “renal denervation” since 2009.
Pathophysiology
The term of “neurogenic hypertension” has been coined for hypertension triggered by activation of sympathetic nerves [
9]. Measuring sympathetic activity non-invasively in a clinical setting is difficult. There is a direct proportional relationship between sympathetic nerve activity and the venous concentration of noradrenaline of a particular organ. However, static measurements of noradrenaline concentration in plasma or urine do not precisely reflect the activity at neural synapses and are therefore limited [
10]. One limitation includes the inability to detect regional differences in sympathetic responses. To overcome these limitations at the level of the kidneys, a regional noradrenaline spillover measurement is performed, the method of choice to measure renal sympathetic activity. After administration of radiolabeled noradrenaline, blood is sampled from the renal veins and sympathetic nerve activity can be estimated from regional isotope dilution [
11].
In the Symplicity HTN-1 study [
8], ten patients underwent measurements of the release of noradrenaline from the renal sympathetic nerves bilaterally with the isotope-dilution renal noradrenaline spillover method, before and 15–30 days after radiofrequency ablation of renal nerves, as a test for the effectiveness of the radiofrequency ablation of renal sympathetic denervation.
The best known method for quantifying superficial sympathetic nerve activity is microneurography. In humans, muscle nerve activity has been shown to be increased when measured by microneurography in hypertensive compared with normotensive patients and after renal denervation [
8].
Both afferent and efferent renal nerves play a role in hypertension. Afferent sympathetic fibers modulate central sympathetic outflow and generate directly neurogenic hypertension. Efferent renal nerves have a complex implication in hypertension triggering stimulation of the renin-angiotensin system via the adreno- receptors α1 and β1 along the renal proximal tubule [
10]. Efferent nerves modulate sodium homoeostasis as well as the tone of the afferent and efferent renal arteries. They also reduce the renal blood flow. Altogether, these mechanisms are of major importance in the regulation of the arterial pressure.
Renal denervation: technical aspects
Medtronic proposes a system based on radiofrequency ablation for renal denervation, and the catheters used are very similar to the catheters used in the ablation of cardiac conduction pathways used to treat arrhythmias. The technique was initially developed by the Californian start-up Ardian which was bought by Medtronic in 2010. The renal sympathetic nervous fibers embedded in the adventitia of the vessel wall of the main renal arteries can be destroyed by applying radiofrequency of sufficient power directly to the endoluminal part of the vessel wall.
Interventional technique
Before starting the renal denervation procedure, the patient will receive an adequate pre-medication in terms of analgesics (e.g., morphine 5–10 mg), as well as sedation (e.g., midazolam 1–3 mg). The denervation is performed by percutaneously inserting a catheter, usually through a standard femoral access (6 French introducer). A short guiding catheter (e.g., 55 cm) is best suited for the engagement of the renal arteries, but other types of guiding catheters or sheaths may also be used. Once the first renal artery is selectively engaged, a selective angiography is performed to permit detailed analysis of the vascular anatomy. Particular attention is given to the presence of a polar artery, tortuosity of the vessel, and the presence of an ostial atherosclerotic narrowing. Nitroglycerine is used to prevent vasospasms.
If no anatomical contraindication is present (two functional kidneys, absence of a past angioplasty, absence of a significant stenosis), the radiofrequency catheter is advanced through the main renal artery. Once in place at the distal portion of the artery, the catheter tip is deflected in order to obtain good wall contact, documented by a continuous elevation of impedance measured through the catheter tip. An adequate impedance level is >250 Ohms. The device is then activated for 120 s. The energy level delivered to the vessel wall is constantly and automatically adjusted based on the impedance values and temperature measurements at the catheter’s tip. Application of radiofrequency to the vessel wall leads to nonselective destruction of both afferent and efferent renal nerves. After the application of radiofrequency, the catheter is retracted by 5 mm and rotated by ~30 degrees, and the procedure is repeated. At least 4–6 energy applications are performed along the intraluminal side of the vessel by progressive retraction of the catheter after rotating the catheter tip so that a large part of the circumference of the renal artery is treated. The superior part of the renal arterial ostium is close to a zone that is particularly rich in nervous fibres. Consequently this specific region should always be carefully treated by application of radiofrequency energy (Dr. Zeller, unpublished).
The procedure is then repeated on the contralateral kidney. The procedure should not be performed on renal arteries measuring less than 4 mm in diameter. Consequently the technique cannot be used in polar arteries or in branches far distal to the main renal artery bifurcation. During the procedure pain control has to be adapted individually by adding further analgesic or sedation according to patient pain.
Progress in technology
Whereas the catheter initially developed by Ardian is a catheter that requires multiple single applications of radiofrequency current as described above, new developments have produced catheters that permit a “single shot” treatment, with multiple radiofrequency applications applied to the circumference of the vessel wall. It is estimated that close to 50 companies are developing renal denervation systems currently.
Table 1 shows the currently available catheters and their key features.
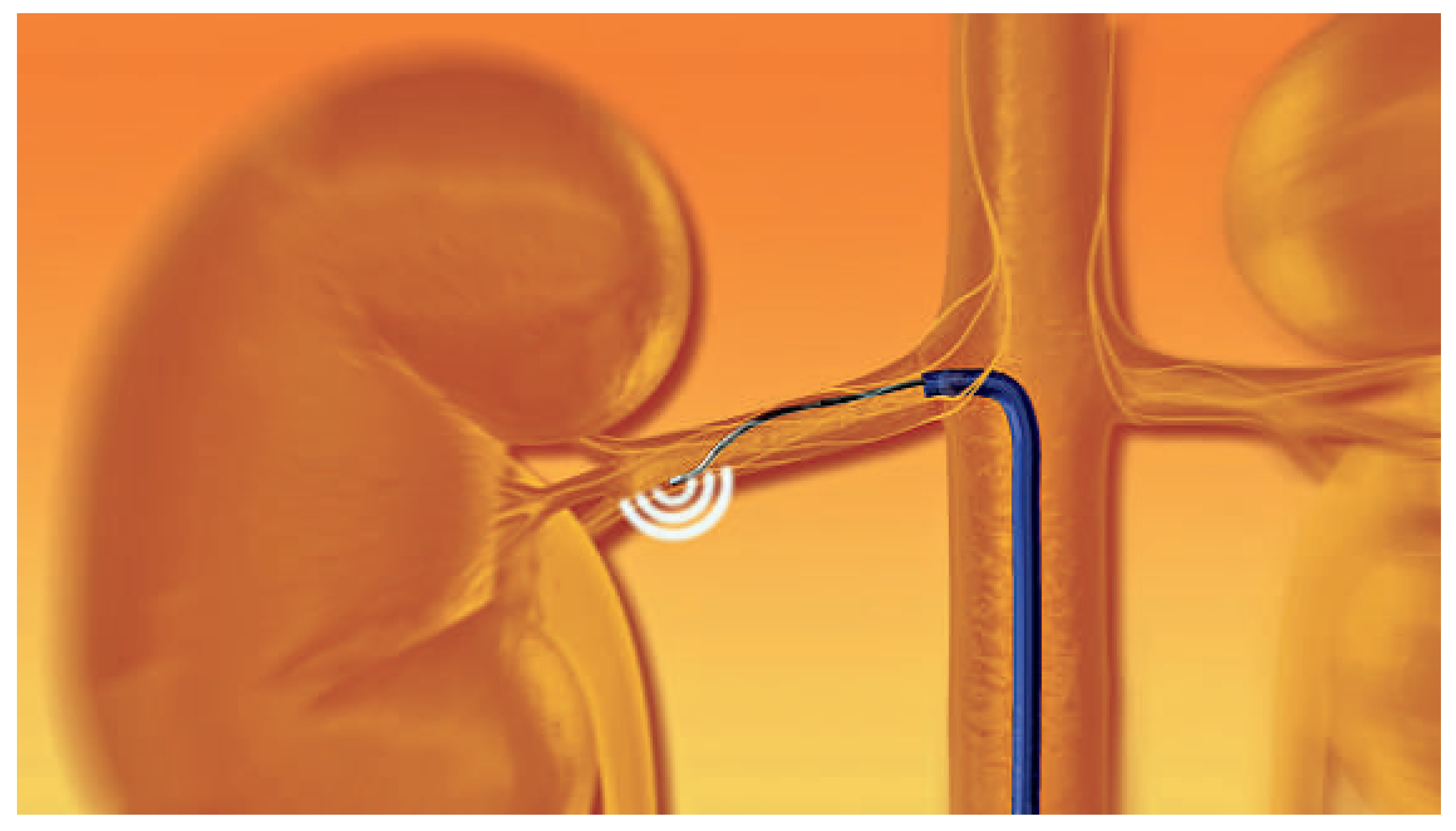
Figure 1.
Schematic locations of radiofrequency application along the renal artery. For illustration purposes only a subset of afferent and efferent nerves are depicted the schematic figure. (© 2012, Ardian/Medtronic. Reprinted with permission.).
Figure 1.
Schematic locations of radiofrequency application along the renal artery. For illustration purposes only a subset of afferent and efferent nerves are depicted the schematic figure. (© 2012, Ardian/Medtronic. Reprinted with permission.).
Clinical studies in the human
The first study, a proof of concept trial, was the Symplicity HTN-1 study designed to assess the safety and efficacy on blood pressure lowering in patients with resistant hypertension. Fifty patients underwent catheter-based percutaneous radiofrequency treatment, mainly in Australia. Successful efferent renal sympathetic denervation was documented by measurements of bilateral renal noradrenaline spillover in a subgroup of patients before and after the procedure. Denervation effectively reduces renal noradrenaline spillover from elevated pre-treatment levels to normal values [
8].
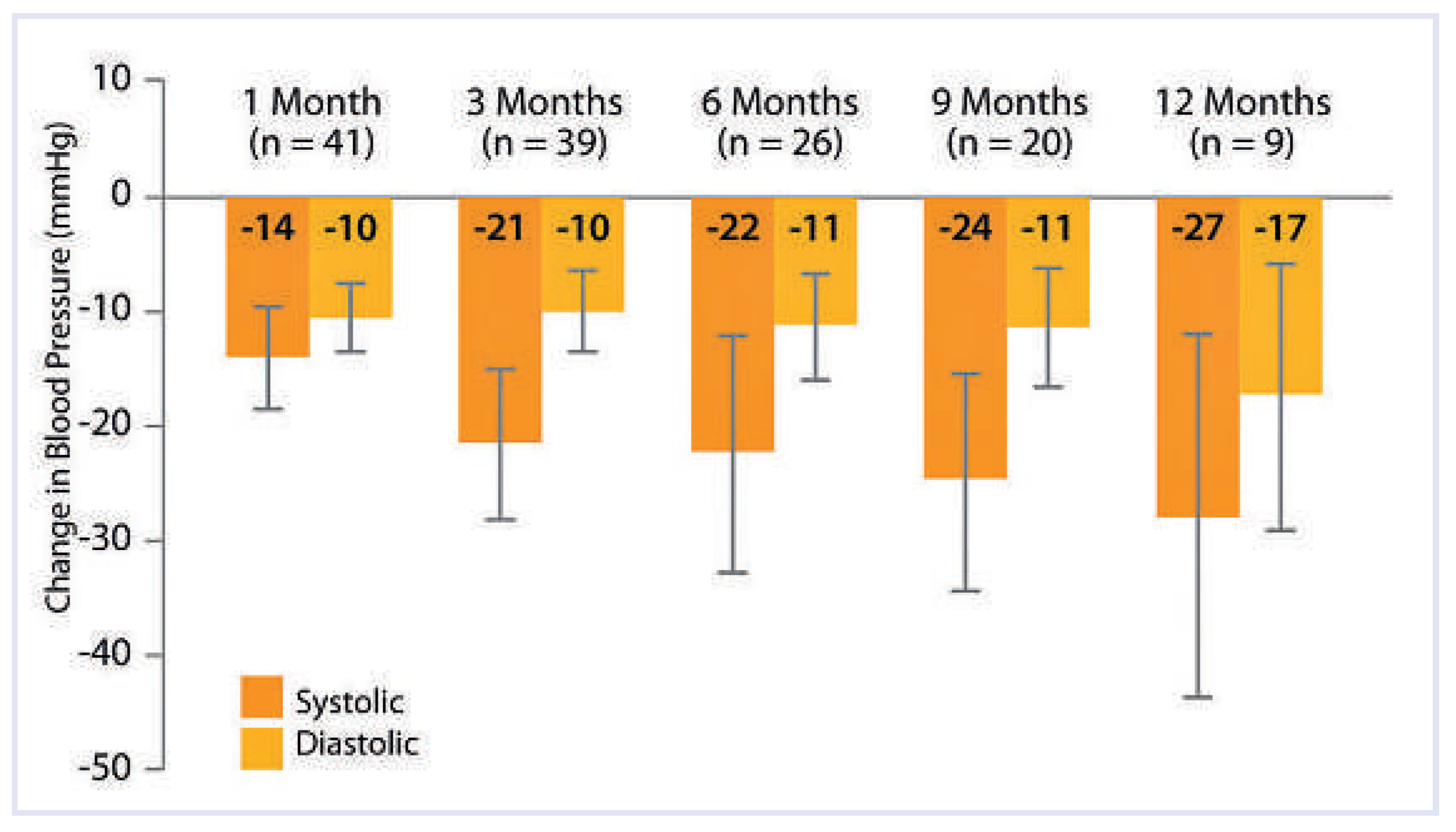
In Symplicity HTN-1 blood pressure at enrollment was 177/101 mm Hg. Patients were treated with a mean of 4.7 hypertensive medications, including 43 (96%) receiving an ACE inhibitor, an angiotensin II receptor blocker, or both, 34 (76%) receiving beta blockers, 31 (69%) receiving calcium-channel blockers, and 8 (18%) receiving direct vasodilators. Ninety-six percent of the patients treated received diuretics. The blood pressure findings were dramatically reduced after the procedure: –14/–10, –21/–10, –22/–11, –24/–11, and –27/–17 mm Hg at 1, 3, 6, 9, and 12 months, respectively (
Figure 2 – based on office blood pressure measures). In five non-treated patients (non-suitable renal anatomy), the mean change in office blood pressure was +3/–2, +2/+3, +14/+9, and +26/+17 mm Hg at 1, 3, 6, and 9 months, respectively. One intra-procedural renal artery dissection occurred before radiofrequency energy delivery, probably related to the presence of an underlying atherosclerotic plaque at the renal artery ostium, which was dissected when inserting the catheter [
12].
The Symplicity HTN-2 trial enrolled 106 resistant-hypertensive patients randomised to renal denervation or usual medical treatment [
13]. At baseline the mean office blood pressure was 178/97 mm Hg on a mean of 5.2 antihypertensive drugs in patients assigned to renal denervation. In the control group the average blood pressure was 178/98 mm Hg on a mean of 5.3 antihypertensive drugs. The primary end-point was office blood pressure at six months. Again, the investigators could observe a significant decrease of systolic and diastolic blood pressure, –32/–12 mm Hg versus +1/0 mm Hg in the control group. Of note, the 24 h ambulatory blood pressure reduction in a subgroup of participants was smaller (11/7 mm Hg), suggesting potentially that renal denervation acts preferentially on the white coat effect. In the Symplicity HTN-2 trial, no serious adverse events related to the procedure were reported. At six months, no vascular abnormalities (e.g., vessel stenosis or vessel dilatation) were observed on the radiofrequency treatment sites [
12].
Other promising fields for renal denervation
Sympathetic nervous system activity is known to be increased in glucose homoeostasis diseases and other conditions like the obstructive sleep apnoea syndrome. The effect of renal denervation was assessed in a sub- study of the Symplicity HTN-2 trial in 37 patients and 13 controls [
13]. Three months after the procedure, the authors observed a significant decrease in fasting glucose, insulin, and C-peptide levels. Oral glucose tolerance and the sensitivity to insulin measured by the HOMA-IR (homeostasis model assessment-insulin resistance) were both significantly improved compared to the control group where no significant changes were observed.
Another sub-study of the Symplicity HTN-2 trial was performed on ten patients presenting with resistant hypertension, obstructive sleep apnoea, and metabolic syndrome. After a follow-up of six months, a significant reduction in the office blood pressure by 34/ 13 mm Hg, together with an improvement of the glucose homoeostasis, as well as a reduction in severity of the obstructive sleep apnoea, assessed by a decrease in median apnoea-hypopnoea index from 16.3 to 4.5/hour, were observed [
14].
Discussion
The findings from these first studies suggest that renal nerve ablation via a catheter-based intervention using radiofrequency energy appears to be safe and associated with a significant improvement in the blood pressure control in patients suffering from resistant hypertension. These effects appear to be mediated by the denervation of both afferent and efferent renal nerves. However, there are still many open questions to be answered and national and European societies have published guidelines for patient selection and also the intervention itself [
12].
First, in most of the published studies, office blood pressure measurements instead of ambulatory blood pressure measurements monitoring were performed. It may be argued that the current data cannot exclude a differential effect on white coat hypertension or resistance, rather than on blood pressure in general. Pseudoresistance has to be excluded in every patient evaluated for renal denervation and each patient has to be evaluated for the common causes of secondary hypertension [
12].
Second, diuretics were underutilised in both Symplicity trials, and especially diuretics acting on the collecting tubules as spironolactone. Consequently some of the patients included may not have fulfilled the definition of refractory hypertension, which includes the use of a fully titrated diuretic [
16], possibly also in combination.
Third, there are no data reporting therapeutic adherence in the Symplicity studies. Indeed, studies have shown a high level of blood pressure control when a well standardised treatment is administered with close monitoring of the medical adherence [
16].
Fourth, only few patients had a reduction in the number of antihypertensive drugs at follow-up, suggesting that the effect on the pill burden may be limited.
Fifth, data addressing the long-term morphological follow-up of the renal arteries and kidneys after radiofrequency renal denervation are still missing, a shortcoming that all recent technologies have to overcome. Currently, it cannot be excluded that unexpected late complications may occur (e.g., late stenosis or aneurysms of the renal arteries) even if, so far, these kinds of complications have been rarely reported, up to the two-year follow-up [
16]. In animal studies, radiofrequency ablation has shown to induce irregularities and fibrosis of the renal arterial wall leading to the development of stenosis or aneurysm [
19].
Sixth, the long-term consequences of renal denervation on kidney function are still unknown in humans. While a transplanted kidney is a good example of a fully denervated kidney, it is well known that a renal graft does not have the same response as native kidneys in conditions such as hypovolaemia, pyelonephritis, diuretic use, and others. There are many other factors influenced by renal transplantation and it is difficult to assess the contribution of renal denervation on the mentioned observations. Careful studies are needed to better define the potential effect of renal denervation.
Finally the procedure should be performed in very experienced hypertension excellence centres where the patients can be followed by a hypertension specialist [
13].
Also of concern is that no patients with eGFR less than 45 mL/min/1.73 m2 were included in the current studies. The average GFR in the studies was 83 mL/ min/1.73 m2 ± 20 mL/min, and among the patients who completed the 2-year follow up, only ten had an evaluation of the eGFR. In these patients, the loss of GFR was remarkably high (i.e., –16 mL/min per 1.73 m2 at 24 months) which is at least twice more than that observed in the major studies evaluating the renal protective effects of antihypertensive treatment. The authors have attributed a deleterious role of diuretics (5 of 10 patients had newly added diuretics), which is possible but the deleterious effect appears to be more marked than in other studies where diuretics were also used. Does it mean that denervated kidneys are more sensitive to diuretics? In another study, including 15 patients with resistant hypertension and stage 3–4 CKD (mean estimated GFR, 31 mL/min per 1.73 m2), bilateral renal denervation did not induce changes in eGFR after the procedure. Night-time ambulatory BP decreased significantly, restoring a more physiologic dipping pattern, and a favourable short-term safety profile [
15]. Similarly, we have no data on the evolution of microalbuminuria in these patients as compared to other studies in hypertension.
All these unanswered questions suggest that the
procedure, although very promising, has still to be considered experimental and has to be performed preferentially in the context of clinical studies and registries. Every patient should be evaluated by a hypertension specialist in a hypertension excellence centre [
13]
, and a multidisciplinary team including an endovascular specialist performing the procedure, a hypertension specialist, and a nephrologist should evaluate the indication for each individual patient. Currently in Switzerland there is no reimbursement for the procedure by the insurance companies, which may further hamper the widespread use of this promising technology. A large single-blinded study is underway to address some of the outstanding questions (Simplicity 3). Ultimately it would also be desirable to conduct studies that investigate the impact of renal denervation on morbidity and mortality instead of blood pressure levels only.
Conclusions
The catheter-based radiofrequency renal denervation is a promising technique, giving hope to patients with a refractory hypertension and to patients intolerant to antihypertensive drugs. Moreover, the novel approach may indeed provide a safe and effective treatment alternative not only for resistant hypertension, but also for some of the adverse consequences linked to the sympathetic overactivity in disorders of glucose homoeostasis and OSA. We hope that the ongoing trials (e.g., Symplicity HTN-3) will soon confirm the safety and the efficacy of the procedure in resistant hypertensives. For the moment, the technique should be used only in selected patients with true resistant hypertension evaluated in a hypertension excellence centre.