Antihypertensive Effect of Aliskiren with/Without Thiazide Diuretic as Related to BMI and Metabolic Syndrome in General Practice
Abstract
Introduction
- The use of aliskiren (A) and aliskiren/hydrochlorothiazide (AHCT) leads to equally effective blood pressure lowering in patients with MetS compared to patients without MetS.
- The use of A and AHCT leads to equally effective target blood pressure attainment in patients with MetS compared to patients without MetS.
- 3.
- A and AHCT lead to similar blood pressure target attainment in patients with BMI ≥30 kg/ m2 and BMI <30 kg/m2.
- 4.
- Treatment approaches and substances prescribed for patients with/without MetS differ in primary care.
Methods
Results
Patient characteristics
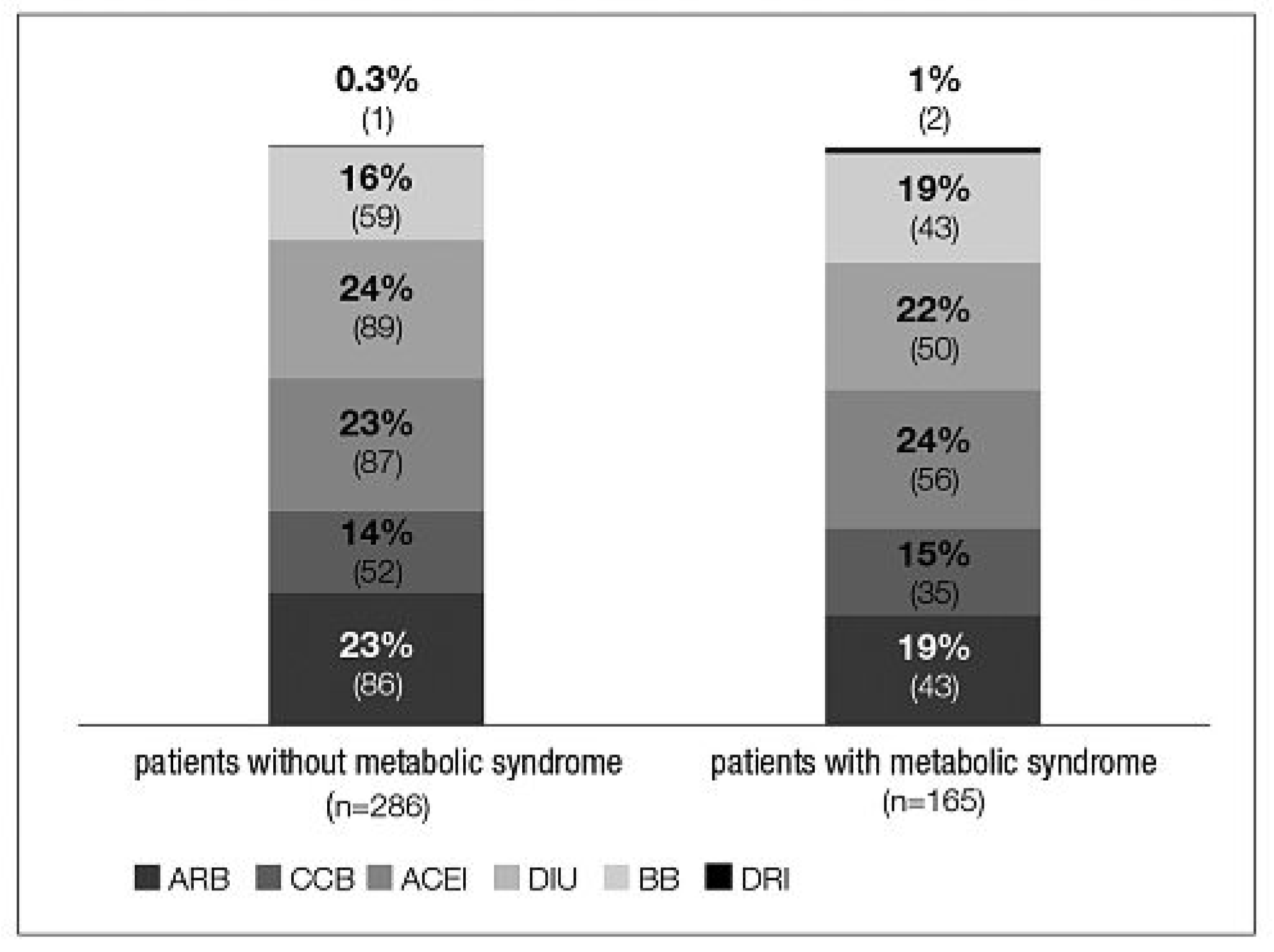
Prior treatment
Adaptation of treatment
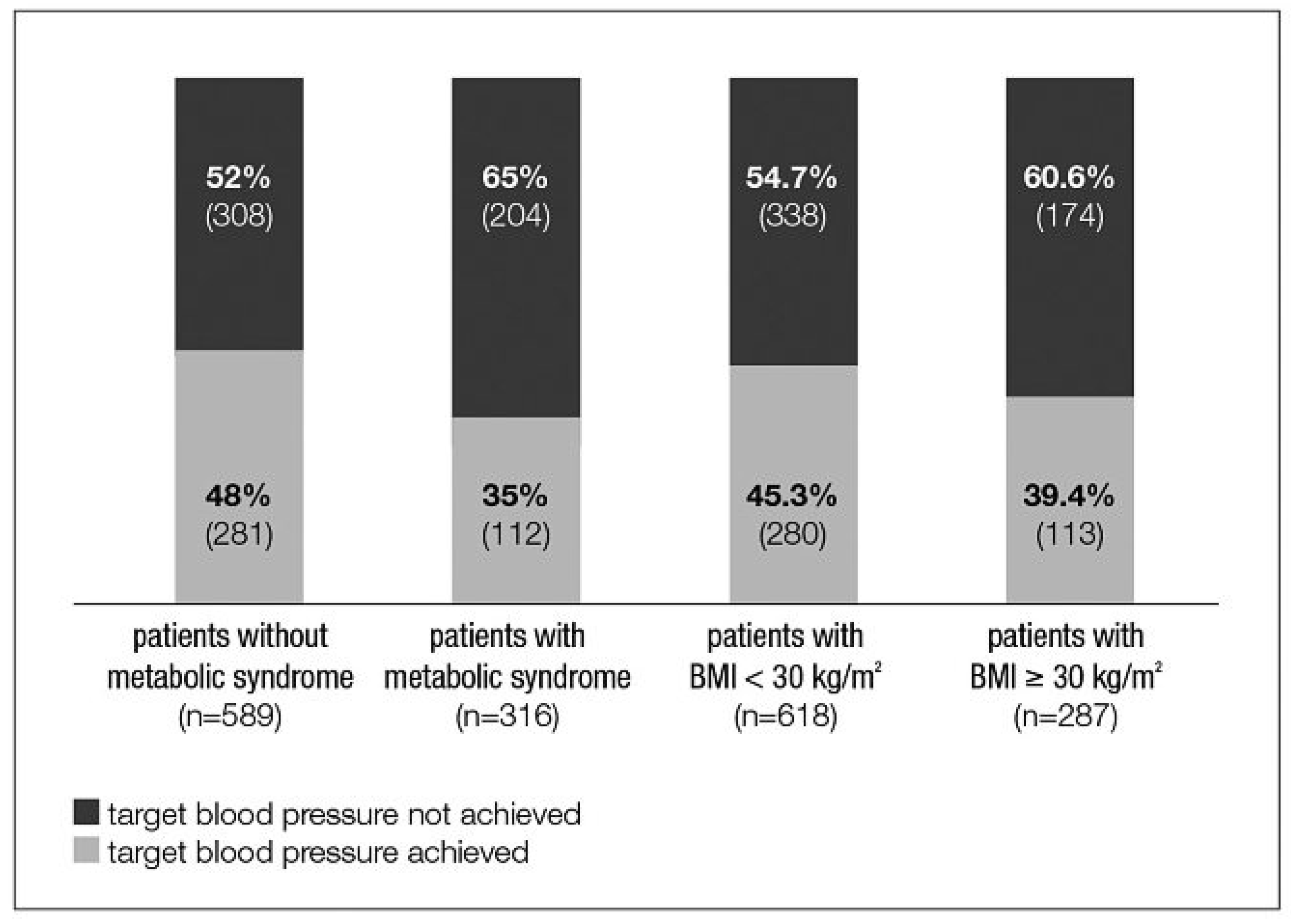
Change in blood pressure and target blood pressure attainment
Influence of BMI on BP reduction and BP control
Adverse events
Discussion
Concluding remarks
Limitations
Funding/Potential Competing Interests
References
- Ratto, E.; Leoncini, G.; Viazzi, F.; Vaccaro, V.; Parodi, A.; Falqui, V.; et al. Metabolic syndrome and cardiovascular risk in primary hypertension. J Am Soc Nephrol. 2006, 17, S120–S122. [Google Scholar] [CrossRef]
- Engeli, S.; Schling, P.; Gorzelniak, K.; Boschmann, M.; Janke, J.; Ailhaud, G.; et al. The adipose-tissue renin–angiotensin–aldosterone system: Role in the metabolic syndrome? Int J Biochem Cell Biol. 2003, 35, 807–825. [Google Scholar] [CrossRef]
- Prasad, A.; Quyyumi, A.A. Renin-angiotensin system and angiotensin receptor blockers in the metabolic syndrome. Circulation 2004, 110, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, G.; Pirro, M.; Vaudo, G.; Gemelli, F.; Marchesi, S.; Porcellati, C.; et al. Prognostic value of the metabolic syndrome in essential hypertension. J Am Coll Cardiol. 2004, 43, 1817–1822. [Google Scholar] [CrossRef]
- Cuspidi, C.; Meani, S.; Fusi, V.; Severgnini, B.; Valerio, C.; Catini, E.; et al. Metabolic syndrome and target organ damage in untreated essential hypertensives. J Hypertens. 2004, 22, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Kang, B.P.; Cheung, S.; Opawumi, D.; Meggs, L.G. Angiotensin II promotes glucose induced activation of cardiac protein kinase C isozymes and phosphorylation of troponin I. Diabetes 2001, 50, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Röling, J.; Strehlow, K.; Schnabel, P.; Böhm, M. Insulin induces upregulation of vascular AT1 receptor gene expression by posttranscriptional mechanisms. Circulation 1998, 98, 2453–2460. [Google Scholar] [CrossRef]
- NCEP. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Janke, J.; Engeli, S.; Gorzelniak, K.; Luft, F.C.; Sharma, A.M. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes 2002, 51, 1699–1707. [Google Scholar] [CrossRef]
- Redona, J.; Cifkovab, R.; Laurent, S.; Nilsson, P.; Narkiewicze, K.; Erdinef, S.; et al. On behalf of the Scientific Council of the European Society of Hypertension. The metabolic syndrome in hypertension: European society of hypertension position statement. J Hypertens. 2008, 26, 1891–1900. [Google Scholar] [CrossRef]
- Guidelines Swiss Society of Hypertension. 2009. http://www.swisshypertension.ch/docs/guidelines_2009_d_leaflet.pdf.
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007. [CrossRef] [PubMed]
- Hansson, L.; Lindholm, L.H.; Niskanen, L.; Lanke, J.; Hedner, T.; Niklason, A.; et al. Effect of angiotensin converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: The Captopril Prevention Project (CAPPP) randomized trial. Lancet 1999, 353, 611–616. [Google Scholar] [CrossRef]
- Scheen, A.J. NAVIGATOR: A trial of prevention of cardiovascular complications and type 2 diabetes with valsartan and/or nateglinide. Rev Med Liege 2010, 65, 217–223. [Google Scholar] [PubMed]
- Krone, W.; Hanefeld, M.; Meyer, H.-F.; Jung, T.; Bartlett, M.; Yeh, C.-M.; et al. Comparative efficacy and safety of aliskiren and irbesartan in patients with hypertension and metabolic syndrome. J Hum Hypertens. 2010, Epub 2001 Apr 8. [CrossRef] [PubMed]
- Duggan, S.T.; Chwieduk, C.M.; Curran, M.P. Aliskiren: A review of its use as monotherapy and as combination therapy in the management of hypertension. Drugs 2010, 70, 2011–2049. [Google Scholar] [CrossRef]
- Arbeitsgruppe Lipide und Atherosklerose (AGLA) der Schweizerischen Gesellschaft für Kardiologie (SGK). Kardiovaskuläre Risikofaktoren und Biomarker. Pocket guide 2010.
- Dusserre, E.; Moulin, P.; Vidal, H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose-tissues. Biochimica et Biophysica Acta 2000, 1500, 88–96. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Quignard-Boulangé, A. Role of adipose tissue reninangiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011, 79, 162–168, Epub 2010 Oct 13. [Google Scholar] [CrossRef]
- Tuck, M.L.; Sowers, J.; Dornfeld, L.; Kledzik, G.; Maxwell, M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981, 304, 930–933. [Google Scholar] [CrossRef]
- Takahashi, N.; Li, F.; Hua, K.; et al. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab. 2007, 6, 506–512. [Google Scholar] [CrossRef]
- Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000, 342, 145–153. [Google Scholar] [CrossRef]
- Prospective Studies Collaboration; Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; et al. Body-mass index and cause-specific mortality in 900000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096, Epub 2009 Mar 18. [Google Scholar] [PubMed]
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002, 288, 2981–2997. [Google Scholar] [CrossRef]
- Schmieder, R.E.; Philipp, T.; Guerediaga, J.; Gorostidi, M.; Bush, C.; Keefe, D.L. Aliskiren-based therapy lowers blood pressure more effectively than hydrochlorothiazide-based therapy in obese patients with hypertension: Sub-analysis of a 52-week, randomized, double blind trial. J Hypertens. 2009, 27, 1493–1501. [Google Scholar] [CrossRef]
- Jordan, J.; Engeli, S.; Boy, S.W.; Le Breton, S.; Keefe, D.L. Direct renin inhibition with aliskiren in obese patients with arterial hypertension. Hypertension. 2007, 49, 1047–1055. [Google Scholar] [CrossRef]
- Prescott, M.F.; Boye, S.W.; Le Breton, S.; Keefe, D.L.; Jordan, J. Antihypertensive efficacy, safety and tolerability of the orally active direct renin inhibitor aliskiren added to hydrochlorothiazide (HCTZ) in patients with grade 3 obesity and hypertension. Int J Obes. 2007, 31, S99 T2:PO.88. [Google Scholar]

| Metabolic syndrome* was defined as | Men | Both | Women |
|---|---|---|---|
| Waist circumference | >102 cm | >88 cm | |
| Elevated triglycerides | ≥1.7 mmol/l | ||
| Low HDL | <1.0 mmol/l | <1.3 mmol/l | |
| Elevated BP | ≥130/≥80 mm Hg (and/or) | ||
| Impaired fasting glucose or Diabetes mellitus | ≥5.6 mmol/l |
| Patients without metabolic syndrome | Patients with metabolic syndromea | |
|---|---|---|
| Age | 60.6 ± 12.2 yrs | 59.6 ± 11.3 yrs |
| Weight | 78.6 ± 13.7 kg | 89.1 ± 19.4 kgb |
| Height | 170.5 ± 8.5 cm | 169.3 ± 9.1 cm |
| BMI | 27.0 ± 4.2 kg/m2 | 31.0 ± 5.9 kg/m2 |
| Male | 353 (60%) | 156 (49.4%)c |
| New hypertension | 284 (48.2%) | 135 (42.7%) |
| msSBP | 159.6 ± 15.5 mm Hg | 161.0 ± 16.6 mm Hg |
| msDBP | 94.4 ± 10.0 mm Hg | 95.6 ± 9.2 mm Hg |
| Patients with target BP | 16 (2.7%) | 8 (2.5%) |
| Heart rate | 76.6 ± 9.9 bpm | 78.2 ± 10.1 bpmd |
| Patients with prior antihypertensive therapy | 286 (49%) | 165 (52%) |
| 1 drug | 151 (26%) | 78 (25%) |
| 2 drugs | 31 (5%) | 26 (8%) |
| ≥3 drugs | 41 (7%) | 27 (9%) |
| Not stated | 63 (11%) | 34 (11%) |
© 2011 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Schaefer, H.-H.; Sudano, I.; Häberer, D.; Noll, G. Antihypertensive Effect of Aliskiren with/Without Thiazide Diuretic as Related to BMI and Metabolic Syndrome in General Practice. Cardiovasc. Med. 2011, 14, 258. https://doi.org/10.4414/cvm.2011.01612
Schaefer H-H, Sudano I, Häberer D, Noll G. Antihypertensive Effect of Aliskiren with/Without Thiazide Diuretic as Related to BMI and Metabolic Syndrome in General Practice. Cardiovascular Medicine. 2011; 14(9):258. https://doi.org/10.4414/cvm.2011.01612
Chicago/Turabian StyleSchaefer, Hans-Hendrik, Isabella Sudano, Doreen Häberer, and Georg Noll. 2011. "Antihypertensive Effect of Aliskiren with/Without Thiazide Diuretic as Related to BMI and Metabolic Syndrome in General Practice" Cardiovascular Medicine 14, no. 9: 258. https://doi.org/10.4414/cvm.2011.01612
APA StyleSchaefer, H.-H., Sudano, I., Häberer, D., & Noll, G. (2011). Antihypertensive Effect of Aliskiren with/Without Thiazide Diuretic as Related to BMI and Metabolic Syndrome in General Practice. Cardiovascular Medicine, 14(9), 258. https://doi.org/10.4414/cvm.2011.01612




