Surgical Treatment of Cardiac Tumours—An Overview and Presentation of Interesting Cases
Abstract
Introduction
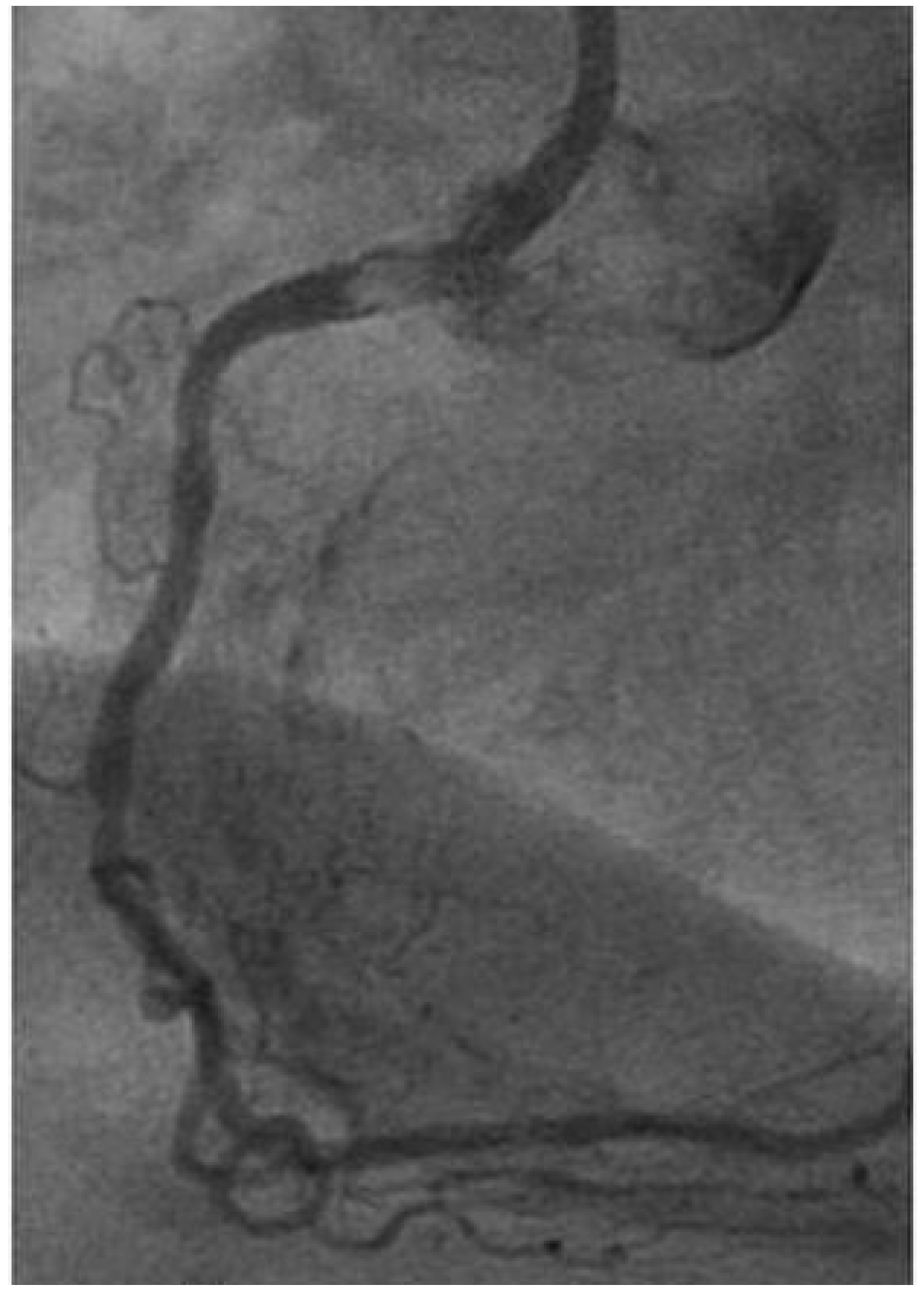
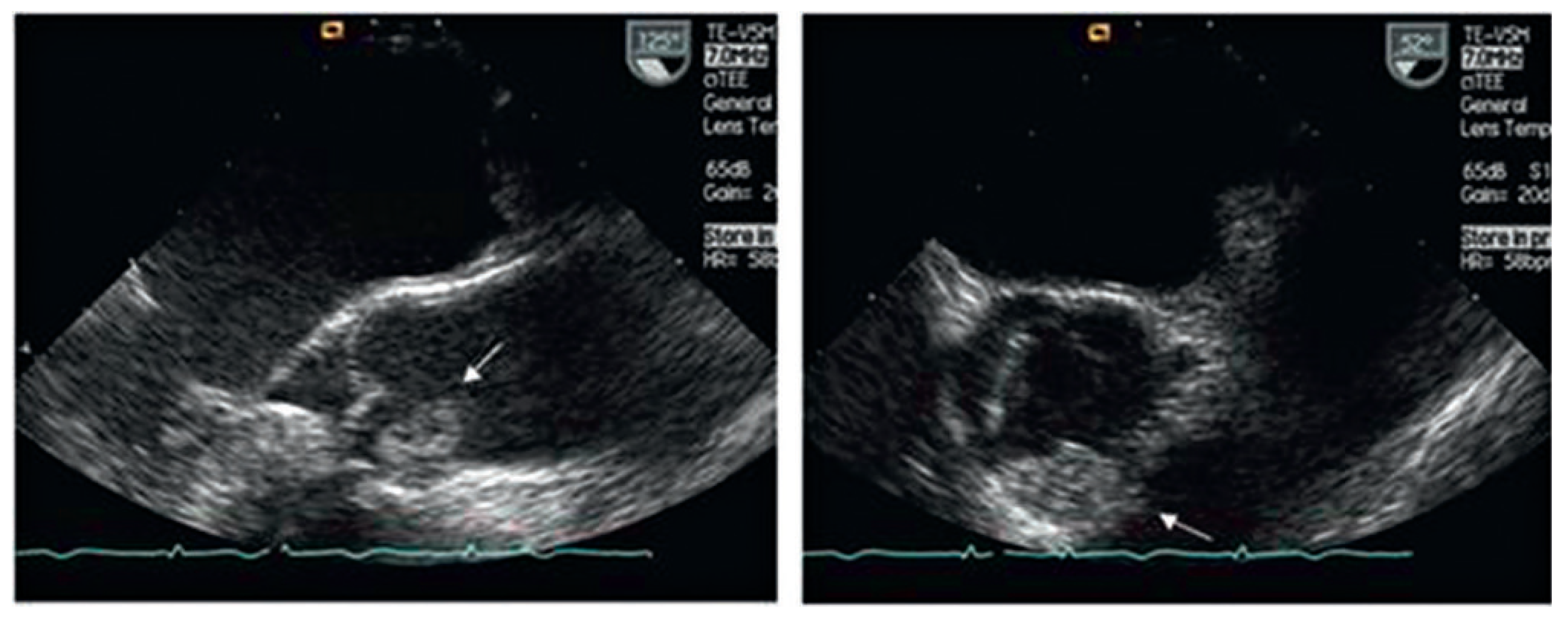
Imaging and diagnosis
Benign cardiac tumours
Cardiac myxoma
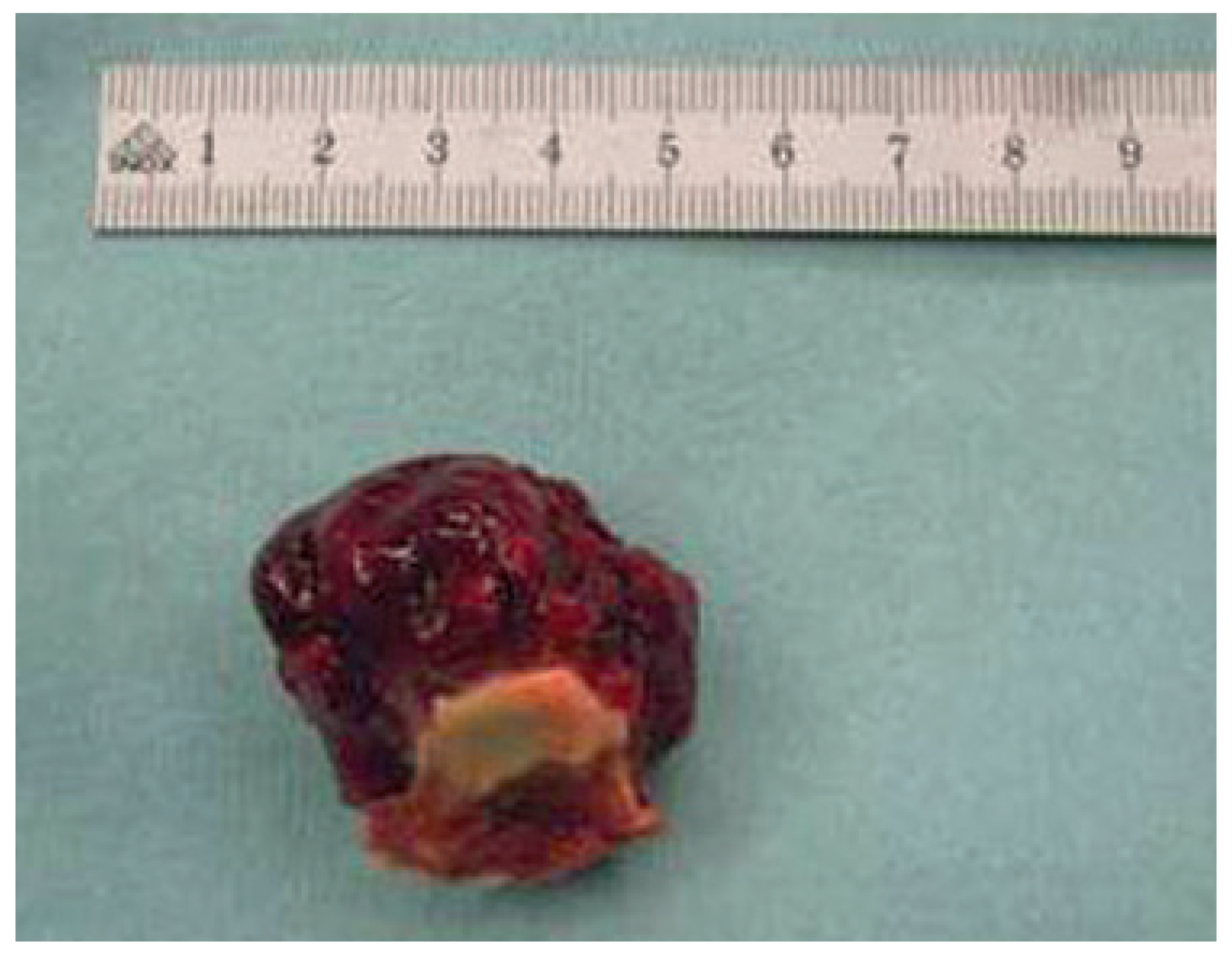
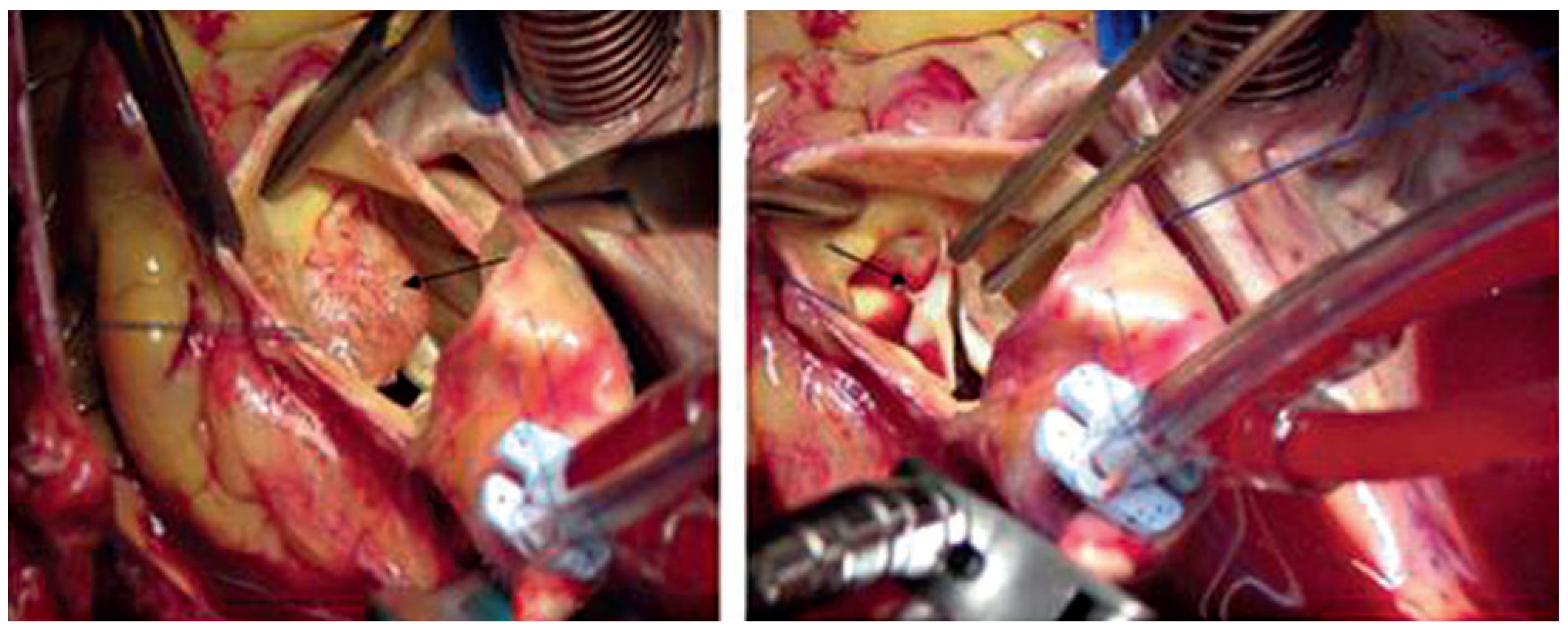
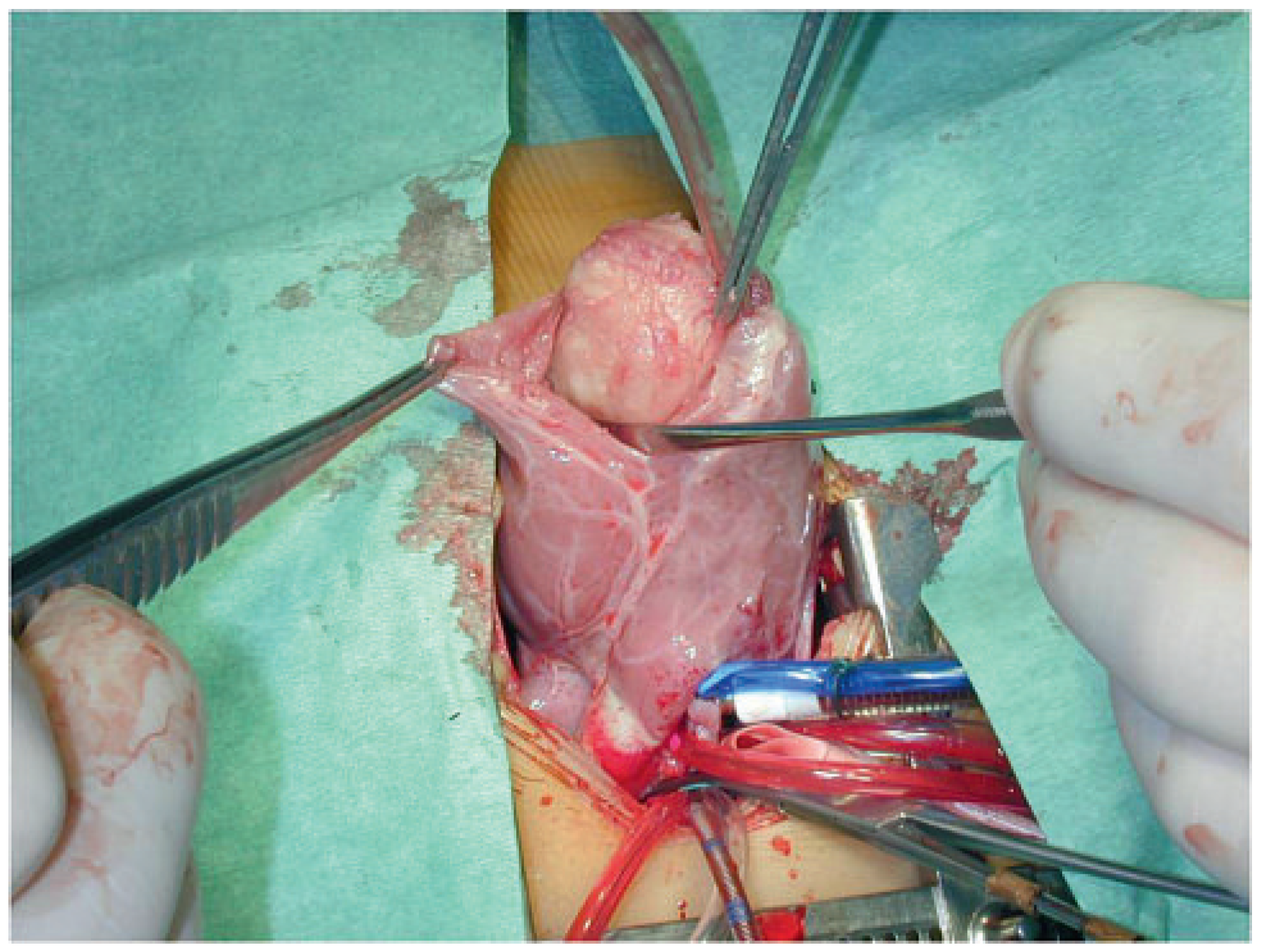
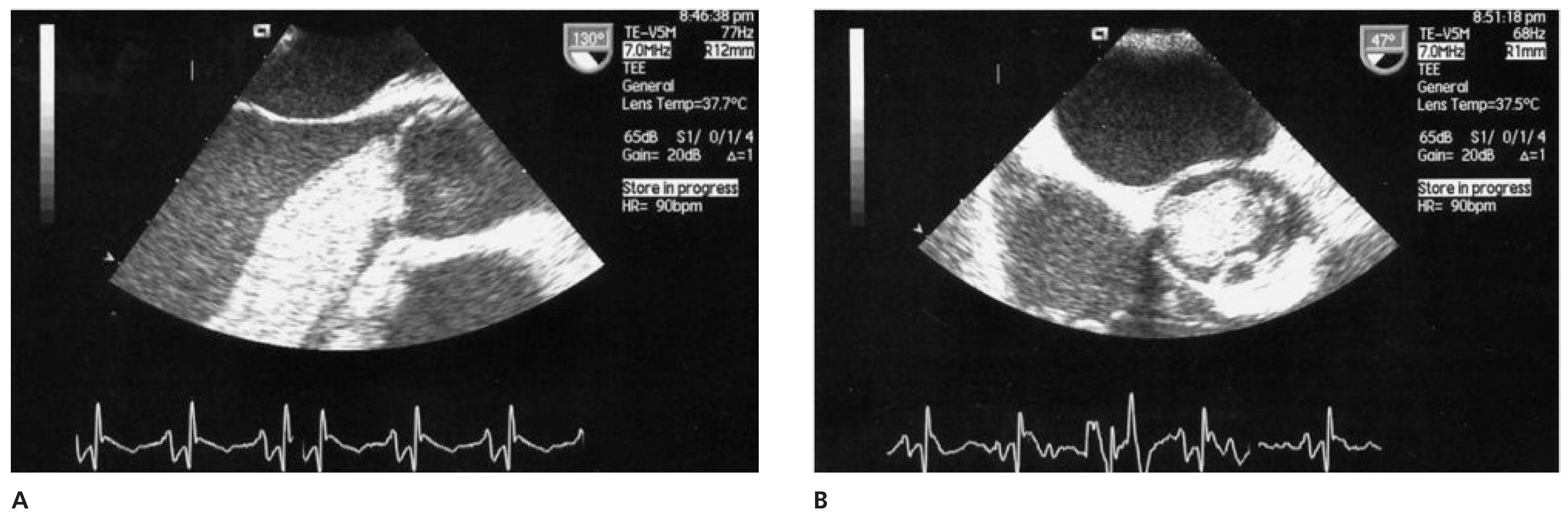
Case report 1: benign myxoma
Case report 2: malignant myxoma
Papillary fibroelastoma
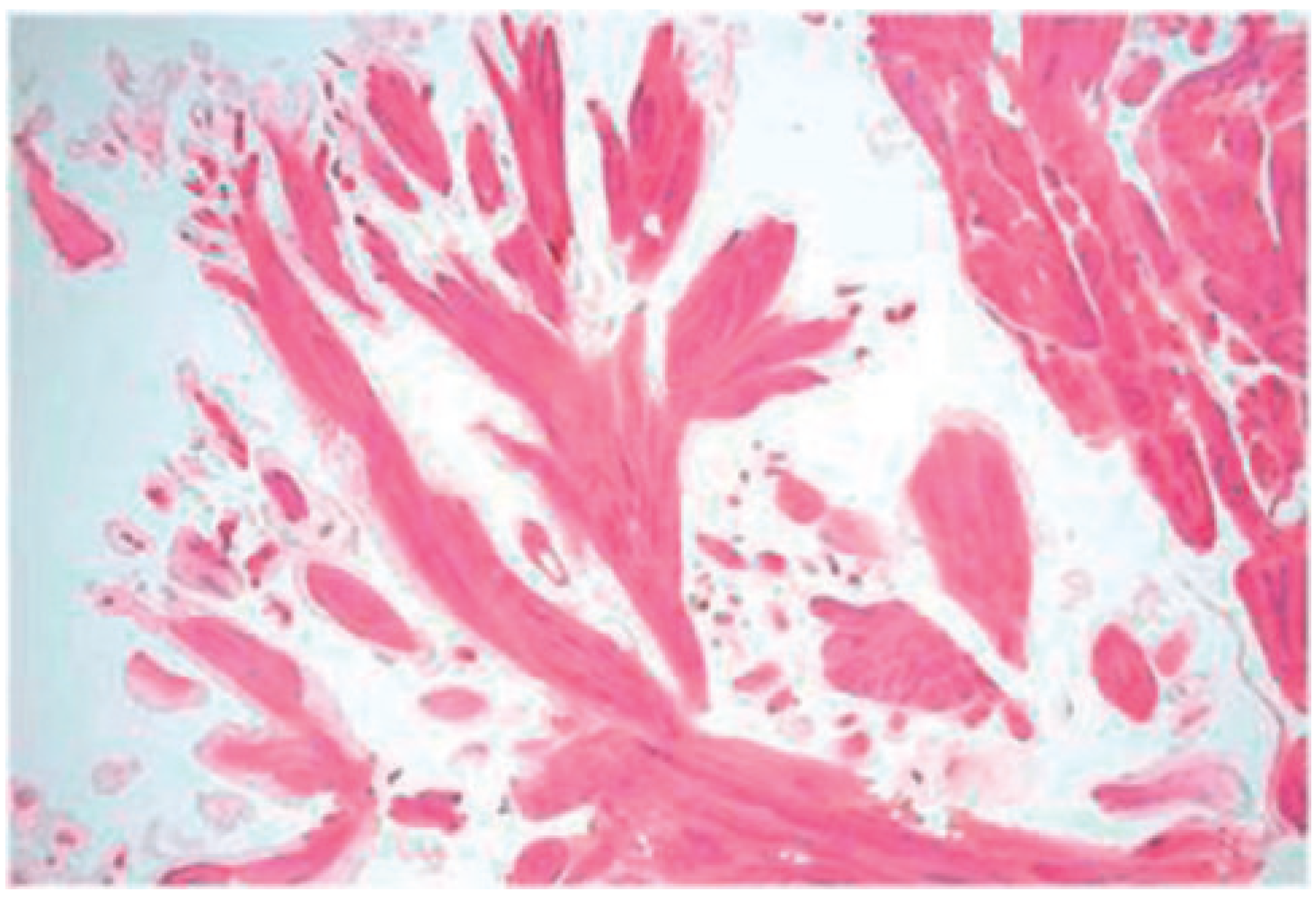
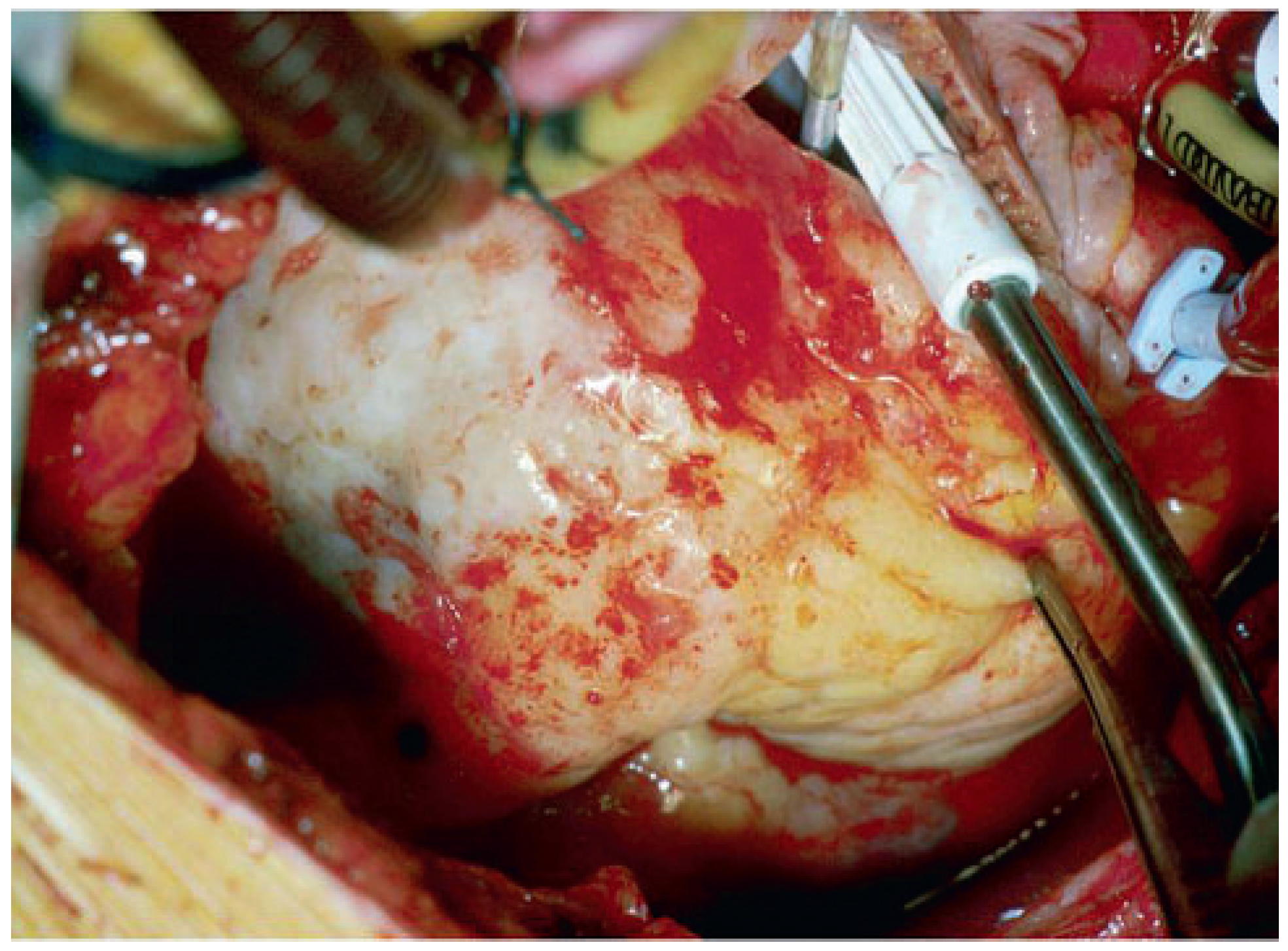
Case report 3: fibroelastoma
Rhabdomyoma
Fibroma
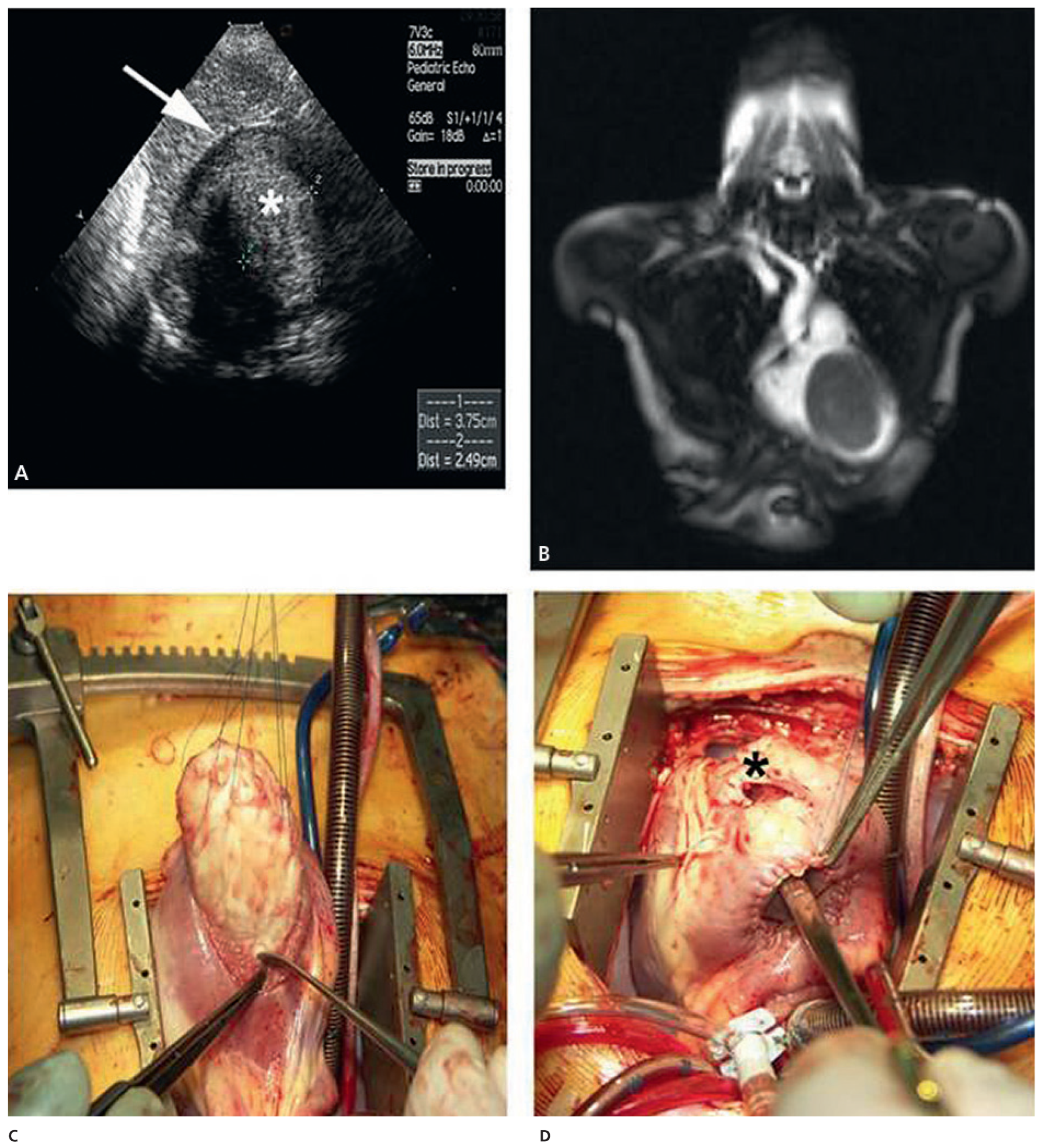
Case report 4: cardiac fibroma
Other rare cardiac tumours and tumour-like conditions
Malignant cardiac tumours
Angiosarcoma
Case report 5: Poorly differentiated angiosarcoma
Intracardiac extension of infradiaphragmatic malignant tumours
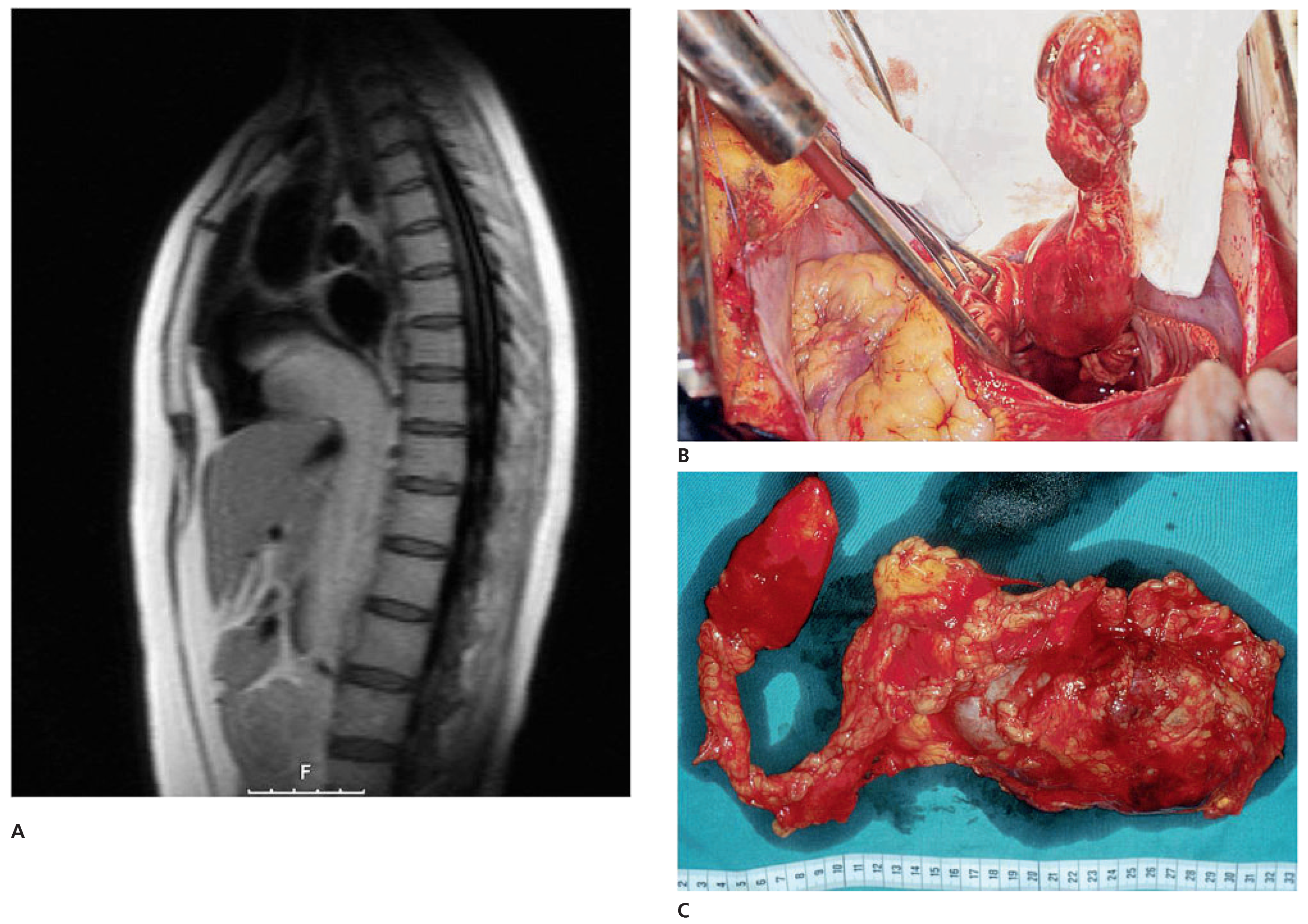
Case report 6: endometrial stromal sarcoma with extension into the right heart
Metastatic heart disease
Carcinoid heart disease
Own experience
Conclusion
Funding/Potential Competing Interests
References
- Guiraudon, C. Cardiac tumours. In Cardiology 2nd Edition; Crawford, M., DiMarco, J.P., Paulus, W.J., Eds.; Mosby Edinburgh: London, UK; New York, NY, USA, 2004; pp. 1507–1514. [Google Scholar]
- Bruce, C.J. Cardiac tumours: Diagnosis and management. Heart. 2011, 97, 151–160. [Google Scholar] [CrossRef]
- Butany, J.; Nair, V.; Naseemuddin, A.; Nair, G.M.; Catton, C.; Yau, T. Cardiac tumours: Diagnosis and management. Lancet Oncol. 2005, 6, 219–228. [Google Scholar] [CrossRef]
- Salcedo, E.E.; Cohen, G.I.; White, R.D.; Davison, M.B. Cardiac tumours: Diagnosis and management. Curr Probl Cardiol 1992, 2, 75–138. [Google Scholar]
- Ekmektzoglou, K.A.; Samelis, G.F.; Xanthos, T. Heart and tumours: Location, metastases, clinical manifestations, diagnostic approaches and therapeutic considerations. J Cardiovasc Med. 2008, 9, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Reeder, G.S.; Khandheria, B.K.; Seward, J.B.; Tajik, A.J. Transesophageal echocardiography and cardiac masses. Mayo Clin Proc. 1991, 66, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.J. Evaluation of cardiac masses: The role of cardiovascular magnetic resonance. Methodist DeBakey Cardiovasc J. 2010, 6, 4–11. [Google Scholar] [CrossRef]
- DeVille, J.B.; Corley, D.; Jin, B.S.; de Castro, C.M.; Hall, R.J.; Wilansky, S. Assessment of intracardiac masses by transoesophageal echocardiography. Tex Heart Inst J. 1995, 22, 134–137. [Google Scholar]
- Tolstrup, K.; Shiota, T.; Gurudevan, S.; Luthringer, D.; Luo, H.; Siegel, R.J. Left atrial myxomas: Correlation of two-dimensional and live three-dimensional transesophageal echocardiography with the clinical and pathological findings. J Am Soc Echocardiogr. 2011. Feb 28 [Epub ahead of print]. [Google Scholar] [CrossRef]
- Fussen, S.; DeBoeck, B.W.; Zellweger, M.J.; Bremerich, J.; Goetschalkx, K.; Zuber, M.; et al. Cardiovascular magnetic resonance imaging for diagnosis and clinical management of suspected cardiac masses and tumours. Eur Heart J. 2011, 32, 1151–1160. [Google Scholar] [CrossRef]
- Hoey, E.T.; Mankad, K.; Puppala, S.; Gopalan, D.; Sivananthan, M.U. MRI and CT appearances of cardiac tumours in adults. Clin Radiol. 2009, 64, 1214–1230. [Google Scholar] [CrossRef]
- Jain, D.; Maleszewski, J.; Halushka, M.K. Benign cardiac tumours and tumorlike conditions. Ann Diagn Path. 2010, 14, 215–230. [Google Scholar] [CrossRef]
- Miller, D.V.; Edwards, W.D. Cardiovascular tumorlike conditions. Semin Diagn Pathol. 2008, 25, 54–64. [Google Scholar] [CrossRef]
- Oliveira, R.G.; Branco, L.; Dias, L.; Timotea, A.T.; Patricio, L.; Agapito, A.; et al. Mitral valve myxomas: An unusual entity. Eur J Echocardiogr. 2008, 9, 181–183. [Google Scholar] [CrossRef][Green Version]
- Erdös, G.; Reineke, D.; Basciani, R.; Carrel, T.; Eberle, B. Left atrial myxoma attached to the anterior mitral leaflet with symptoms suggestive of infective endocarditis. Eur J Echocardiography. 2010, 11, E8. [Google Scholar] [CrossRef]
- Tönz, M.; Laske, A.; Carrel, T.; da Silva, A.; Real, F.; Turina, M. Convulsions, hemiplegia, and central retinal occlusion due to a left atrial myxoma in a child. Eur J Pediatr. 1992, 151, 652–654. [Google Scholar] [CrossRef]
- Schaff, H.V.; Mullany, C.J. Surgery for cardiac myomas. Semin Thorac Cardiovasc Surg. 2000, 12, 77–88. [Google Scholar] [CrossRef]
- Bernet, F.; Stulz, P.; Carrel, T. Long-term remission after combined resection, chemotherapy and irradiation of a metastatic left atrial myxoma. Ann Thorac Surg. 1998, 66, 1791–1792. [Google Scholar] [CrossRef] [PubMed]
- Reynen, K. Cardiac myxomas. New Engl J Med. 1995, 333, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Gegouskov, V.; Kadner, A.; Englberger, L.; et al. Papillary Fibroelastoma of the Heart. Heart Surg Forum. 2008, 11, E333–E339. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, H.; Sekii, H. Surgical Resection of Cardiac Papillary Fibroelastoma in the Left Ventricular Outflow Tract. Ann Thorac Cardiovasc Surg. 2008, 14, 393–395. [Google Scholar]
- Hort, W.; Horskotte, D. Fibroelastoma and Lambl’s excrescences: Localization, morphology and pathogenesis, differential diagnosis and infection. J Heart Valve Dis. 2006, 15, 591–593. [Google Scholar]
- Erdös, G.; Stalder, M.; Basciani, R.; Gugger, M.; Carrel, T.; Eberle, B. An Uncommon Cause of Coronary Artery Ostial Obstruction: Papillary Fibroelastoma. Echocardiography. 2010, 27, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Yerebakan, C.; Liebold, A.; Steinhoff, G.; Skrabal, C.A. Papillary fibroelastoma of the aortic wall with partial occlusion of the right coronary ostium. Ann Thorac Surg. 2009, 87, 1953–1954. [Google Scholar] [CrossRef] [PubMed]
- Uzun, O.; Wilson, D.G.; Vujanic, S.; Parson, J.M.; De Giovanni, J.V. Cardiac tumours in children. Orphanet J Rare Dis. 2007, 2, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Günther, T.; Schreiber, C.; Noebauer, C.; Eiken, A.; Lange, R. Treatment strategies for paediatric patients with primary cardiac and pericardial tumours: A 30-year review. Pediatr Cardiol. 2008, 29, 1071–1076. [Google Scholar] [CrossRef]
- Delgueldre, S.C.; Chockalingam, P.; Mivelaz, Y.; DiBernardo, S.; Pfammatter, J.P.; Barrea, C.; et al. Considerations form prenatal counselling of patients with cardiac rhabdomyomas based on their cardiac and neurologic outcomes. Cardiol in the Young. 2010, 20, 18–24. [Google Scholar] [CrossRef]
- Vidaillet, H.J. Cardiac tumours associated with hereditary syndromes. Am J Cardiol. 1988, 61, 1355–1359. [Google Scholar] [CrossRef]
- Centofanti, P.; di Rosa, E.; Deorsola, L.; Dato, G.M.; Patanè, F.; La Torre, M. Primary cardiac tumours: Early and late results of surgical treatment in 91 patients. Ann Thorac Surg. 1999, 68, 1236–1241. [Google Scholar] [CrossRef]
- Ceitham, E.L.; Midgley, F.M.; Pery, L.W.; Dullum, M.K. Intramural ventricular fibroma in infancy: Survival after partial excision in two patients. Ann Thorac Surg. 1990, 50, 471. [Google Scholar] [CrossRef]
- Fuidl, M.; Kadner, A.; Loup, O.; Carrel, T. Kardiales Fibrom: Ursache einer unklaren Gedeihstörung bei einem Säugling. Schweiz Med Forum. 2009, 14, 286–287. [Google Scholar]
- Cina, S.J.; Smialek, J.E.; Burke, A.P.; Virmani, R.; Hutchins, G.M. Primary cardiac tumours causing sudden death: A review of the literature. Am J Forensic Med Pathol. 1996, 17, 271–281. [Google Scholar] [CrossRef]
- Burke, A.P.; Johns, J.P.; Virmani, R. Hemangiomas of the heart: A clinicopathological study of ten cases. Am J Cardiovasc Pathol. 1990, 3, 283–290. [Google Scholar]
- Kipfer, B.; Englberger, L.; Stauffer, E.; Carrel, T. Rare presentation of cardiac haemangioma. Ann Thorac Surg. 2000, 70, 977–979. [Google Scholar] [CrossRef]
- Xanthos, T.; Giannakopoulos, N.; Papadimitriou, L. Lipomatous hypertrophy of the interatrial septum. A pathological and clinical approach. Int J Cardiol. 2007, 121, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.; Tazelaar, H.D.; Edwards, W.D. Calcified amorphous tumour of the heart. Hum Pathol. 1997, 28, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Forman, M.B.; Virmani, R.; Robertson, R.M.; Stone, W.J. Mitral anulus calcification in chronic heart failure. Chest. 1984, 85, 367–371. [Google Scholar] [CrossRef]
- Lewin, M.; Nazarian, S.; Marine, J.E. Fatal outcome of a calcified amorphous tumour of the heart. Cardiovasc Pathol. 2006, 15, 299–302. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, S.K.; Lee, C.W.; Song, J.K.; Park, J.S.; Choo, S.J. Giant left atrial ball thrombus in a patient with chronic non valvular atrial fibrillation. Ann Thorac Surg. 2008, 85, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.A.; Summerson, C. Intracardiac thrombus in antiphospholipoid antibody syndrome. J Am Soc Echocardiogr. 2000, 13, 873–875. [Google Scholar] [CrossRef]
- Chartier, L.; Bera, J.; Delomez, M.; Asseman, P.; Beregi, J.P.; Bauchard, J.J.; et al. Free-floating thrombi in the right heart: Diagnosis, management, and prognosis-indexes in 38 consecutive patients. Circulation. 1999, 99, 2779–2783. [Google Scholar] [CrossRef]
- Neragi-Miandoab, S.; Kim, J.; Vlahakes, G.J. Malignant tumours of the heart: A review of tumour type, diagnosis and therapy. Clin Oncol. R Coll Radiol 2007, 19, 748–756. [Google Scholar] [CrossRef]
- Luk, A.; Nwachukwu, H.; Lim, K.D.; Cusimano, R.J.; Butany, J. Cardiac angiosarcoma: A case report and review of the literature. Cardiovasc Pathol. 2010, 19, e69–e74. [Google Scholar] [CrossRef]
- Vujin, B.; Benc, D.; Srdic, S.; Bikicki, M.; Vuckovic, D.; Dodic, S. Rhabdomyosarcoma of the heart. Herz. 2006, 31, 798–800. [Google Scholar] [CrossRef]
- Meng, Q.; Lai, H.; Lima, J.; Tong, W.; Qian, Y.; Lai, S. Echocardiographic and pathologic characteristics of primary cardiac tumours: A study of 149 cases. Int J Cardiol. 2002, 84, 69–75. [Google Scholar] [CrossRef]
- Hugo, C.; Lederlin, M.; Bégueret, H.; Guisset, O.; Corneloup, O.; Laurent, F. Primary pulmonary angiosarcoma: CT-pathology correlation. J Radiol. 2011, 92, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Tueller, C.; Fischer Biner, R.; Minder, S.; Gugger, M.; Stoupis, C.; et al. FDGPET in diagnostic work-up of pulmonary artery sarcoma. Eur Respir J. 2010, 35, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Llombart-Cussac, A.; Pivot, X.; Contesso, G.; Rhor-Alvarado, A.; Delord, J.P.; Spielmann, M.; et al. Adjuvant chemotherapy for primary cardiac sarcomas: The IGR experience. Br J Cancer. 1998, 78, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Grandmougin, D.; Fayad, G.; Decoene, C.; Pol, A.; Warembourg, H. Total orthotopic heart transplantation for primary cardiac rhabdomyosarcoma: Factors influencing long-term survival. Ann Thorac Surg. 2001, 71, 1438–1441. [Google Scholar] [CrossRef]
- Crespo, M.G. Heart transplantation for cardiac angiosarcoma: Should the indication be questioned? J Heart Lung Transplant. 1993, 12, 527–530. [Google Scholar]
- Aravot, D.J.; Banner, N.R.; Madden, B. Primary cardiac tumours. Is there a place for heart transplantation? Eur J Cardio-thorac Surg. 1989, 3, 521–524. [Google Scholar] [CrossRef]
- Carrel, T.; Jenni, R.; Schmid, E.R.; Vorburger, C.; Largiader, F.; Turina, M. Successful resection of y hypernephroma extending into the right ventricle: Utilization of extracorporeal circulation in general surgery. Schweiz Med Wschr. 1991, 121, 1460–1464. [Google Scholar]
- Chatterjee, T.; Müller, M.; Carrel, T.; Kaufmann, U.; Meier, B. Renal cell carcinoma with tumour thrombus extending through the inferior vena cava into the right cardiac cavities. Circulation. 1997, 96, 2729–2730. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, J.C.; Soltero, E.R.; Dinney, C.P.; Walsh, G.L.; Schrump, D.S.; Swanson, D.A.; et al. Surgical management of renal cell carcinoma with inferior vena cava tumour thrombus. Ann Thorac Surg. 1997, 63, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, P.; Weimann, R.; Barras, J.P.; Carrel, T.; Candinas, D. Low-grade endometrial stromal sarcoma with inferior vena cava tumour thrombus and intracardiac extension: Radical resection may improve recurrence free survival. Surg Oncol. 2009, 18, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Carrel, T.; Linka, A.; Turina, M. Tricuspid valve obstruction caused by plasmocytoma metastasis. Ann Thorac Surg. 1992, 54, 352–354. [Google Scholar] [CrossRef]
- Bohlman, M.; Eckstein, F.; Allemann, Y.; Carrel, T. Successful resection of a chorio-carcinoma metastasis from the left atrium with obstruction of the pulmonary veins. Gynecol Oncol. 2002, 84, 157–160. [Google Scholar]
- Bohlmann, M.; Eckstein, F.; Carrel, T. Amelanotic cardiac melanoma: Dismal prognosis despite extensive intracardiac debulking. Br J Dermatol. 2002, 146, 912–915. [Google Scholar] [CrossRef]
- Bussani, R.; De-Giorgio, F.; Abbate, A.; Silvestri, F. Cardiac metastases. J Clin Pathol. 2007, 60, 27–34. [Google Scholar] [CrossRef]
- Catton, C. The management of malignant cardiac tumours: Clinical considerations. Semin Diagn Pathol. 2008, 25, 69–75. [Google Scholar] [CrossRef]
- Bernheim, A.M.; Connolly, H.M.; Hobday, T.J.; Abel, M.D.; Pellikka, P.A. Carcinoid heart disease. Prog Cardiovasc Dis. 2007, 49, 439–451. [Google Scholar] [CrossRef]
- Castillo, J.G.; Filsoufi, F.; Adams, D.H.; Raikhelkar, J.; Zaku, B.; Fischer, G.W. Management of patients undergoing multivalvular surgery for carcinoid heart disease: The role of the anesthesiologist. Br J Anaesth. 2008, 101, 618–626. [Google Scholar] [CrossRef]
- Raja, S.G.; Bhattacharyya, S.; Davar, J.; Dreyfus, G.D. Surgery for carcinoid heart disease: Current outcomes. Concerns and controversies. Future Cardiol. 2010, 6, 647–655. [Google Scholar] [CrossRef]
- Arghami, A.; Connolly, H.M.; Abel, M.D.; Schaff, H.V. Quadruple valve replacement in patients with carcinoid heart syndrome. J Thorac Cardiovasc Surg. 2010, 140, 1432–1434. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Raja, S.G.; Toumpanakis, C.; Caplin, M.E.; Dreyfus, G.D.; Davar, J. Outcome, risks and complications of cardiac surgery for carcinoid heart disease. Eur J Cardio-thorac Surg. 2010 Dec 16 [Epub ahead of print]. [CrossRef]

| Benign cardiac tumours Myxoma | n 95 | ||
| Papillary fibroelastoma | 14 | ||
| Fibroma (children) | 4 | ||
| Rhabdomyoma | 1 | ||
| Malignant tumours | n | Deaths within 1 yr | |
| Primary malignant cardiac | Sarcoma (not otherwise specified) | 2 | 1 |
| tumours | Angiosarcoma (SVC, IVC, PA) | 6 | 1 |
| Leiomyosarcoma | 3 | 2 | |
| Infra-diaphragmatic tumours | Wilms tumour | 7 | 1 |
| Renal cell carcinoma | 12 | – | |
| Endometrial stromal cell sarcoma | 1 | – | |
| Metastatic heart tumour | Metastatic lung carcinoma: debulking RV | 1 | 1 |
| Metastatic thyroid carcinoma: debulking RA, RV | 1 | 1 | |
| Non Hodgkin Lymphoma: debulking RV, Pericardium | 1 | 1 | |
| Synovialom RV: resection + tricuspid valve replacement | 1 | ||
| Plasmocytoma RV/RA: partial resection | 1 | 1 | |
| Chorio-Carcinoma: resection pulmonary veins, LA | 1 | – | |
| Melanoma RV: palliative resection (debulking) | 1 | 1 | |
| Hamartoma LA: curative resection + MVR | 1 | – | |
| Haemangiosarcoma RV/RA: palliative resection | – | ||
| Lymphangiosarcoma LV: palliative resection | 1 | 1 | |
| Carcinoid heart disease (TVR + PVR) | 9 | 1 | |
| SVC = superior vena cava; LV = left ventricle; VC = inferior vena cava; LA = left atrium; PA = pulmonary artery; MVR = mitral valve replacement; RV = right ventricle; TVR = tricuspid valve replacement; RA = right atrium; PVR = pulmonary valve replacement. | |||
© 2011 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Carrel, T.; Erdös, G.; Eberle, B.; Englberger, L.; Pfammatter, J.-P.; Schmidli, J.; Kadner, A.; Stalder, M. Surgical Treatment of Cardiac Tumours—An Overview and Presentation of Interesting Cases. Cardiovasc. Med. 2011, 14, 242. https://doi.org/10.4414/cvm.2011.01611
Carrel T, Erdös G, Eberle B, Englberger L, Pfammatter J-P, Schmidli J, Kadner A, Stalder M. Surgical Treatment of Cardiac Tumours—An Overview and Presentation of Interesting Cases. Cardiovascular Medicine. 2011; 14(9):242. https://doi.org/10.4414/cvm.2011.01611
Chicago/Turabian StyleCarrel, Thierry, Gabor Erdös, Balthasar Eberle, Lars Englberger, Jean-Pierre Pfammatter, Jürg Schmidli, Alexander Kadner, and Mario Stalder. 2011. "Surgical Treatment of Cardiac Tumours—An Overview and Presentation of Interesting Cases" Cardiovascular Medicine 14, no. 9: 242. https://doi.org/10.4414/cvm.2011.01611
APA StyleCarrel, T., Erdös, G., Eberle, B., Englberger, L., Pfammatter, J.-P., Schmidli, J., Kadner, A., & Stalder, M. (2011). Surgical Treatment of Cardiac Tumours—An Overview and Presentation of Interesting Cases. Cardiovascular Medicine, 14(9), 242. https://doi.org/10.4414/cvm.2011.01611




