The Use of Shear Rate-Diameter Dose-Response Curves to Assess Endothelial Function
Summary
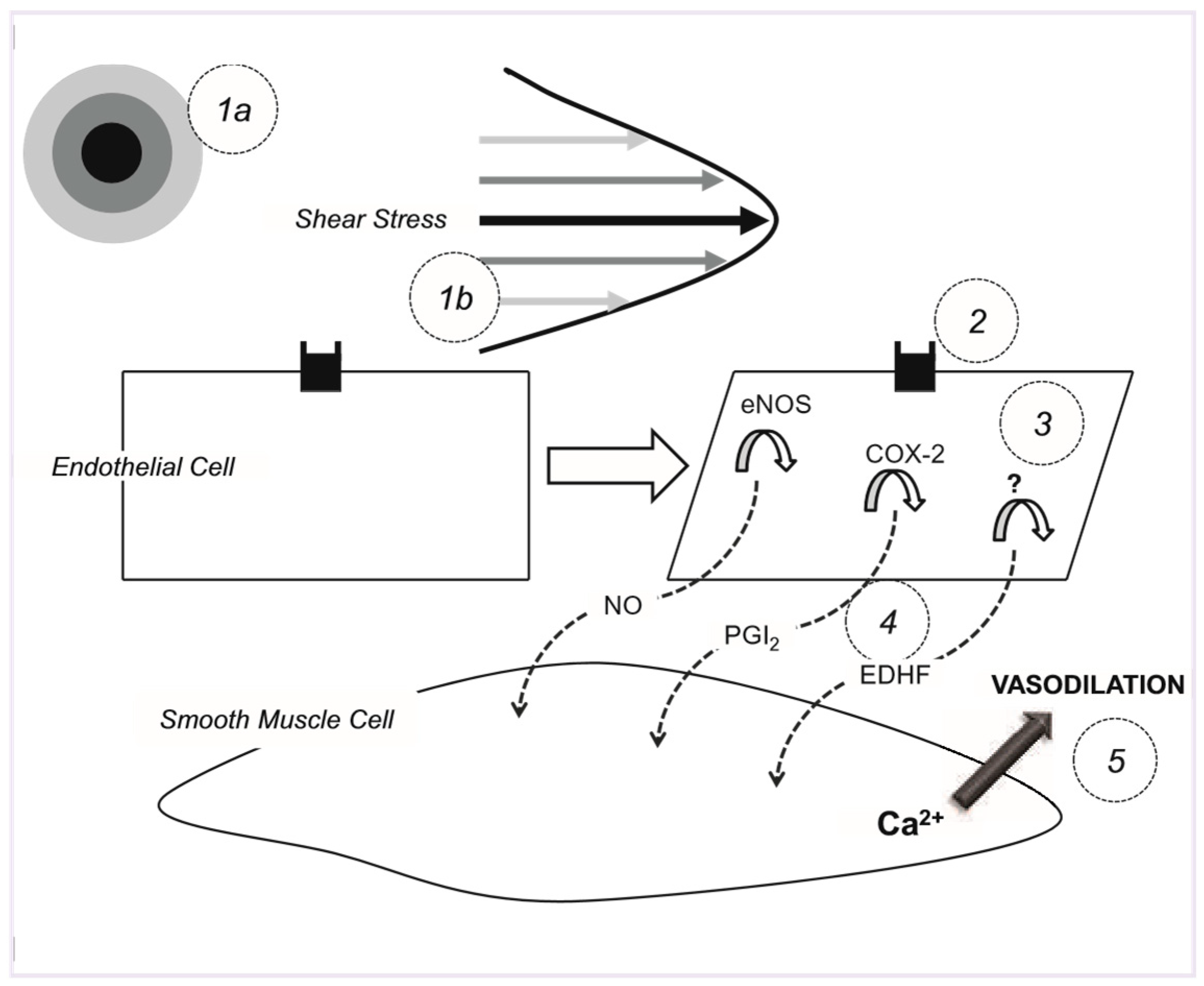
Introduction
Hypothesis
Manipulating the shear stimulus using ischaemia

Shear stress calculation
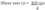
- where d is the internal arterial diameter, v is time averaged mean blood velocity, and n represents the shape of the velocity profile. (For a fully developed parabolic profile, n is 2.) Poiseuille’s law assumes that: 1) The fluid (blood) is Newtonian. 2) Blood flows through a rigid tube. 3) The velocity profile is parabolic. And 4) Whole blood viscosity represents viscosity at the vessel wall and is linearly proportional to shear rate. First, although blood is non-Newtonian, the effect of the nonNewtonian behavior does not appear to be pronounced in large arteries [17]. Second, blood vessels are distensible, meaning that wall shear rate may be ~30% less in a distensible artery as compared with a rigid tube [18]. Third, in arteries, the velocity profile will generally not develop to a full parabola as a consequence of flow unsteadiness and short vessel entrance lengths. However, in the brachial artery, under resting conditions, the underestimation is less pronounced—likely due to a more parabolic velocity profile in this artery, i.e., n (velocity profile) is closer to 2 [19]. Though, this may only be true for resting conditions; occurrence of flow turbulence is possible during reactive hyperaemia [20]. Third, blood viscosity exhibits low intra-subject variability [21], particularly among a healthy, homogeneous group. Shear rate has been used as a surrogate measure of shear stress in a number of previous studies [21,22,23,24,25].
Calculating the appropriate shear stimulus
Manipulating the shear stimulus using hand warming and handgrip exercise
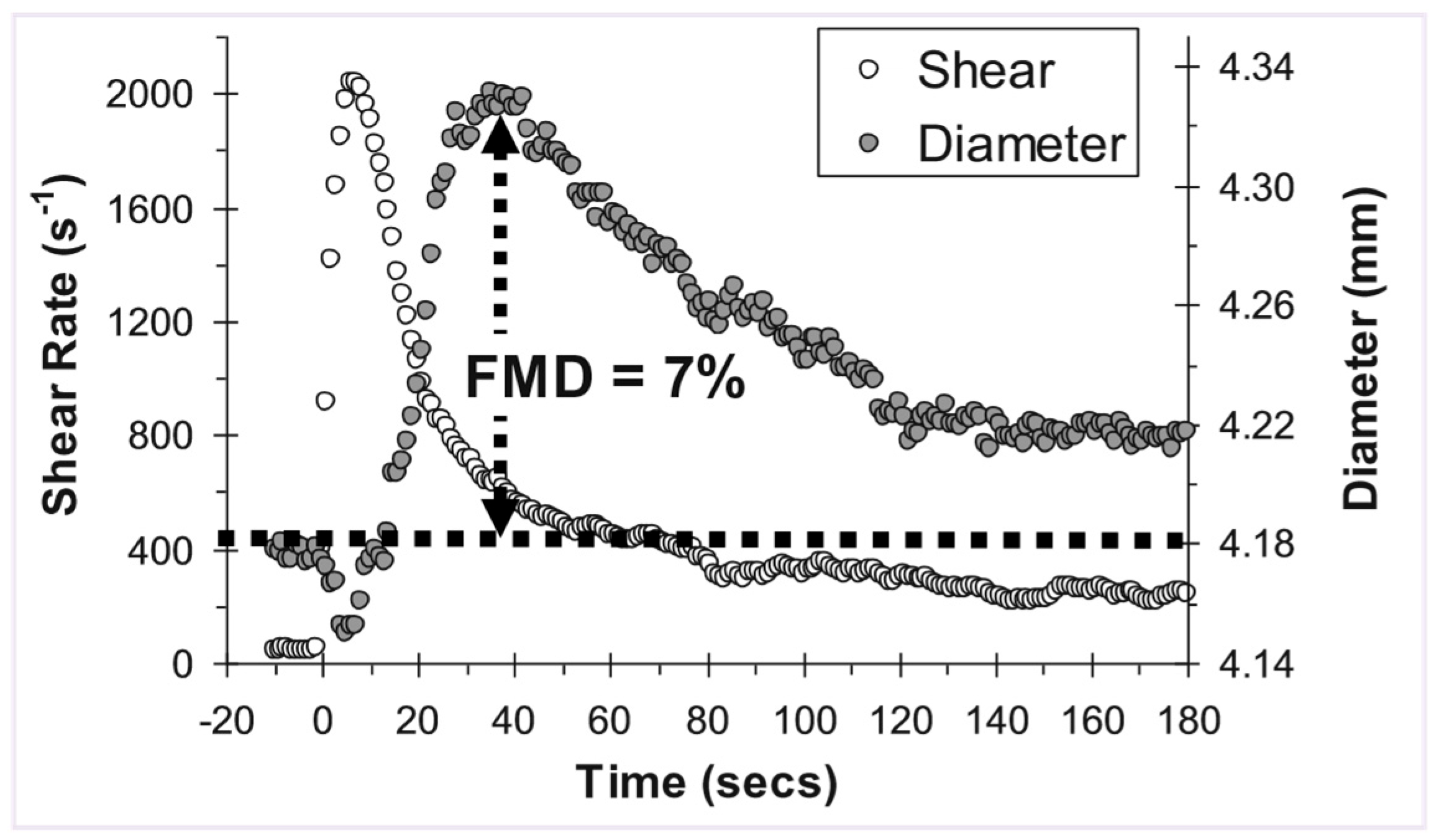
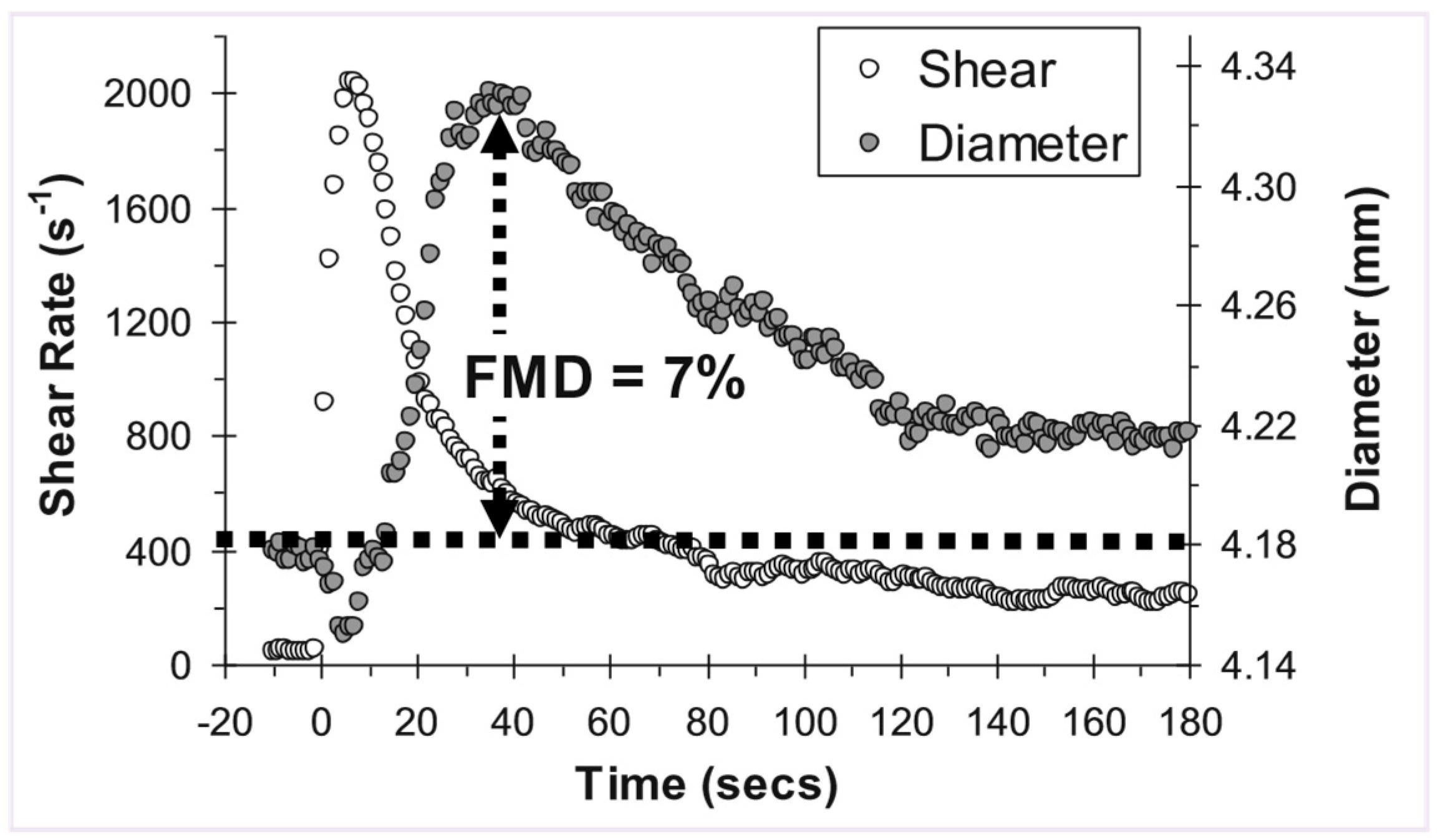
Expressing dose-response outcomes
Statistical analysis
Advantages
Limitations
Conclusions
Funding
Conflicts of Interest
References
- Celermajer, D.S.; et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992, 340, 1111–5. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980, 299, 373–6. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993, 2002–12. [Google Scholar]
- Schroeder, S.; et al. Noninvasive determination of endothelium-media-ted vasodilation as a screening test for coronary artery disease: pilot study to assess the predictive value in comparison with angina pectoris, exercise electrocardiography, and myocardial perfusion imaging. Am Heart J. 1999, 138, 731–9. [Google Scholar] [CrossRef]
- De Roos, N.M.; et al. Within-subject variability of flow-mediated vasodi-lation of the brachial artery in healthy men and women: implications for experimental studies. Ultrasound Med Biol. 2003, 29, 401–6. [Google Scholar] [CrossRef]
- Vickers, A.J. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001, 1, 6. [Google Scholar] [CrossRef]
- Tu, Y.K.; et al. Statistical power for analyses of changes in randomized controlled trials. J Dent Res. 2005, 84, 283–7. [Google Scholar] [CrossRef] [PubMed]
- Twisk, J.; Proper, K. Evaluation of the results of a randomized controlled trial: how to define changes between baseline and follow-up. J Clin Epidemiol. 2004, 57, 223–8.
- Koller, A.; Sun, D.; Kaley, G. Role of shear stress and endothelial prosta-glandins in flow and viscosity-induced dilation of arterioles in vitro. Circ Res. 1993, 72, 1276–84. [Google Scholar] [CrossRef]
- Mitchell, G.F.; et al. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004, 44, 134–9. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J. Regression to the mean. A threat to exercise science? Sports Med. 2003, 33, 575–84. [Google Scholar] [CrossRef] [PubMed]
- Black, C.D.; Vickerson, B.; McCully, K.K. Noninvasive assessment of vascular function in the posterior tibial artery of healthy humans. Dyn Med. 2003, 2, 1. [Google Scholar] [CrossRef]
- Naylor, L.H.; et al. Measuring Peripheral Resistance And Conduit Arterial Structure In Humans Using Doppler Ultrasound. J Appl Physiol. 2005, 98, 2311–5. [Google Scholar] [CrossRef]
- Olive, J.L.; McCully, K.K.; Dudley, G.A. Blood flow response in individuals with incomplete spinal cord injuries. Spinal Cord. 2002, 40, 640–6. [Google Scholar] [CrossRef]
- Harris, R.A.; et al. Variability of flow-mediated dilation measurements with repetitive reactive hyperemia. Vasc Med. 2006, 11, 1–6. [Google Scholar] [CrossRef]
- Pyke, K.E.; Jazuli, F. Impact of repeated increases in shear stress via reactive hyperemia and handgrip exercise: no evidence of systematic changes in brachial artery FMD. Am J Physiol Heart Circ Physiol. 2011, 300, H1078–89. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.; et al. Effects of hematocrit and plasma proteins on human blood rheology at low shear rates. J Appl Physiol. 1966, 21, 81–7. [Google Scholar] [CrossRef]
- Duncan, D.D.; et al. The effect of compliance on wall shear in casts of a human aortic bifurcation. J Biomech Eng. 1990, 112, 183–8. [Google Scholar] [CrossRef]
- Dammers, R.; et al. Shear stress depends on vascular territory: comparison between common carotid and brachial artery. J Appl Physiol. 2003, 94, 485–9. [Google Scholar] [CrossRef]
- Stoner, L.; Young, J.M.; Sabatier, M.J. Examination of possible flow turbulence during flow-mediated dilation testing Open J. Med. Imaging. 2011, 1, 1–8. [Google Scholar]
- Gnasso, A.; et al. Association between intima-media thickness and wall shear stress in common carotid arteries in healthy male subjects. Circulation. 1996, 94, 3257–62. [Google Scholar] [CrossRef] [PubMed]
- Joannides, R.; et al. Evaluation of the determinants of flow-mediated radial artery vasodilatation in humans. Clin Exp Hypertens. 1997, 19, 813–26. [Google Scholar] [CrossRef]
- Betik, A.C.; Luckham, V.B.; Hughson, R.L. Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. Am J Physiol Heart Circ Physiol. 2004, 286, H442–8. [Google Scholar] [CrossRef] [PubMed]
- Pyke, K.E.; Dwyer, E.M.; Tschakovsky, M.E. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol. 2004, 97, 499–508. [Google Scholar] [CrossRef]
- Padilla, J.; et al. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovasc Ultrasound. 2008, 6, 44. [Google Scholar] [CrossRef]
- Stoner, L.; et al. Relationship between blood velocity and conduit artery diameter and the effects of smoking on vascular responsiveness. J Appl Physiol. 2004, 96, 2139–45. [Google Scholar] [CrossRef]
- Pyke, K.E.; Tschakovsky, M.E. Peak vs. total reactive hypereamia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007, 102, 1510–9. [Google Scholar] [CrossRef]
- Stoner, L.; et al. Occasional cigarette smoking chronically affects arterial function. Ultrasound Med Biol. 2008, 34, 1885–92. [Google Scholar] [CrossRef]
- Mullen, M.J.; et al. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001, 88, 145–51. [Google Scholar] [CrossRef] [PubMed]
- Joannides, R.; et al. Influence of vascular dimension on gender difference in flow-dependent dilatation of peripheral conduit arteries. Am J Physiol Heart Circ Physiol. 2002, 282, H1262–9. [Google Scholar] [CrossRef]
- Johnson, J.M.; et al. Effect of local warming on forearm reactive hyperemia. Clin Physiol. 1986, 6, 337–46. [Google Scholar] [CrossRef]
- Taylor, W.F.; et al. Effect of high local temperature on reflex cutaneous vasodilation. 1984, 57, 191–6. [Google Scholar] [CrossRef]
- Kellogg, D.L., Jr.; et al. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999, 86, 1185–90. [Google Scholar] [CrossRef] [PubMed]
- Shastry, S.; et al. Effects of nitiric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol. 1998, 830–4. [Google Scholar] [CrossRef]
- Kellogg, D.L., Jr.; et al. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998, 85, 824–9. [Google Scholar] [CrossRef]
- Crandall, C.G.; MacLean, D.A. Cutaneous interstitial nitric oxide concentration does not increase during heat stress in humans. J Appl. Physiol. 2001, 90, 1020–4. [Google Scholar] [CrossRef] [PubMed]
- Savage, M.V.; Brengelmann, G.L. Reproducibility of the vascular response to heating in human skin. J Appl Physiol. 1994, 76, 1759–63. [Google Scholar] [CrossRef]
- Stoner, L.; McCully, K. Peak and time integrated-shear rates independently predict flow-mediated dilation. J. Clin. Ultrasound. In Press. [CrossRef] [PubMed]
- Frangos, J.A.; et al. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985, 227, 1477–9. [Google Scholar] [CrossRef]
- Kuchan, M.J.; Jo, H.; Frangos, J.A. Role of G proteins in shear stress-mediated nitric oxide production by endothelial cells. Am J Physiol. 1994, 267, C753–758. [Google Scholar] [CrossRef]
- Frangos, J.A.; Huang, T.Y.; Clark, C.B. Steady shear and step changes in shear stimulate endothelium via independent mechanisms—superposition of transient and sustained nitric oxide production. Biochem Biophys Res Commun. 1996, 224, 660–5. [Google Scholar] [CrossRef]
- Macarthur, H.; et al. Selective inhibition of agonist-induced but not shear stress-dependent release of endothelial autacoids by thapsigargin. Br J Pharmacol. 1993, 108, 100–5. [Google Scholar] [CrossRef]
- Doshi, S.N.; et al. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci. (Lond) 2001, 101, 629–35. [Google Scholar] [CrossRef]
- Lie, M.; Sejersted, O.M.; Kiil, F. Local regulation of vascular cross section during changes in femoral arterial blood flow in dogs. Circ Res. 1970, 27, 727–37. [Google Scholar] [CrossRef] [PubMed]
- Hilton, S.M. A peripheral arterial conducting mechanism underlying dilatation of the femoral artery and concerned in functional vasodilatation in skeletal muscle. J Physiol. 1959, 149, 93–111. [Google Scholar] [CrossRef]
- Harris, L.M.; et al. Vascular reactivity in patients with peripheral vascular disease. Am J Cardiol. 1995, 76, 207–12. [Google Scholar] [CrossRef]
- Stoner, L.; et al. Upper vs. lower extremity arterial function after spinal cord injury. J Spinal Cord Med. 2006, 29, 138–46. [Google Scholar] [CrossRef]
- Sabatier, M.J.; et al. Doppler ultrasound assessment of posterior tibial artery size in humans. J Clin Ultrasound. 2006, 34, 223–30. [Google Scholar] [CrossRef]
- Raudenbush, S.W.; Bryk, A.S. Hierarchical Linear Models: Applications and Data Analysis Methods (Advanced Quantitative Techniques in the Social Sciences); SAGE Publications: Thousand Oaks, CA, USA, 2001. [Google Scholar]
- Stoner, L.; et al. Electrical stimulation-evoked resistance exercise therapy improves arterial health after chronic spinal cord injury. Spinal Cord. 2007, 45, 49–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stoner, L.; McCully, K. Velocity acceleration as a determinant of flow-mediated dilation. Ultrasound in Medicine and Biology In Press. [CrossRef] [PubMed]
- Busse, R.; et al. EDHF: Bringing the concepts together. Trends Pharmacol Sci. 2002, 23, 374–80. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.M.; et al. Local cholinergic mechanisms mediate nitric oxidedependent flow-induced vasorelaxation in vitro. Am J Physiol. 1996, 270 Pt 2, H442–6. [Google Scholar]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a metaanalysis. Int J Cardiovasc Imaging. 2010, 26, 631–40. [Google Scholar] [CrossRef]
- Green, D.J.; et al. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011, 57, 363–9. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Stoner, L.; Sabatier, M.J. The Use of Shear Rate-Diameter Dose-Response Curves to Assess Endothelial Function. Cardiovasc. Med. 2011, 14, 339. https://doi.org/10.4414/cvm.2011.01630
Stoner L, Sabatier MJ. The Use of Shear Rate-Diameter Dose-Response Curves to Assess Endothelial Function. Cardiovascular Medicine. 2011; 14(12):339. https://doi.org/10.4414/cvm.2011.01630
Chicago/Turabian StyleStoner, Lee, and Manning J. Sabatier. 2011. "The Use of Shear Rate-Diameter Dose-Response Curves to Assess Endothelial Function" Cardiovascular Medicine 14, no. 12: 339. https://doi.org/10.4414/cvm.2011.01630
APA StyleStoner, L., & Sabatier, M. J. (2011). The Use of Shear Rate-Diameter Dose-Response Curves to Assess Endothelial Function. Cardiovascular Medicine, 14(12), 339. https://doi.org/10.4414/cvm.2011.01630



