Summary
We report the case of a 47-year old man who presented with severe cardiogenic shock and signs of ST-elevation myocardial infarction. Urgent coronary angiography showed thrombotic occlusion of the mid left anterior descending artery (LAD) and the circumflex artery, as well as a subtotal thrombotic occlusion of the right coronary artery without signs of relevant atherosclerotic coronary disease. The patient was treated with thrombus aspiration in all coronary vessels and PTCA of the LAD with good result. Despite thorough investigation of major nonatherosclerotic causes of myocardial infarction, which are further discussed in our case report, the case remains unsolved. Until today the patient is free of symptoms under long-term dual antiplatelet-inhibitor therapy.
Case report
A 47-year-old man without known cardiovascular risk factors was admitted to the emergency department complaining of ongoing left sided chest pain, radiating into both arms with sudden onset 1.5 h prior to the admission. During the last three days he suffered from headaches and symptoms of a common cold. The patient had no past medical history and was not taking any medication or drugs.
The ECG at admission showed normal sinus rhythm and ST-segment changes in the infero-lateral and the antero-septal leads (Figure 1). The initial troponin and CK-values were normal. Clinically the patient was in cardiogenic shock. Urgent invasive assessment revealed a severely depressed LV-function (EF 30%) with apical, anteroseptal and posterolateral akinesia. There was a total thrombotic occlusion of the mid LAD and the distal circumflex artery (Figure 2).
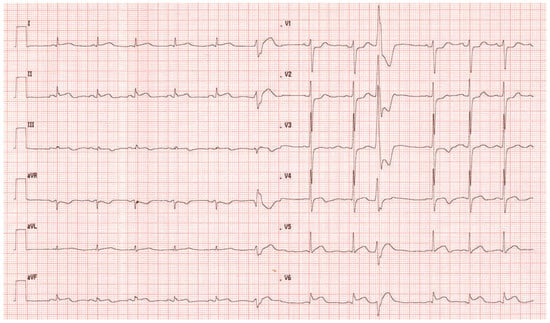
Figure 1.
ECG at admission. Normal sinus rhythm and ST-segment changes in the infero-lateral and the antero-septal leads.
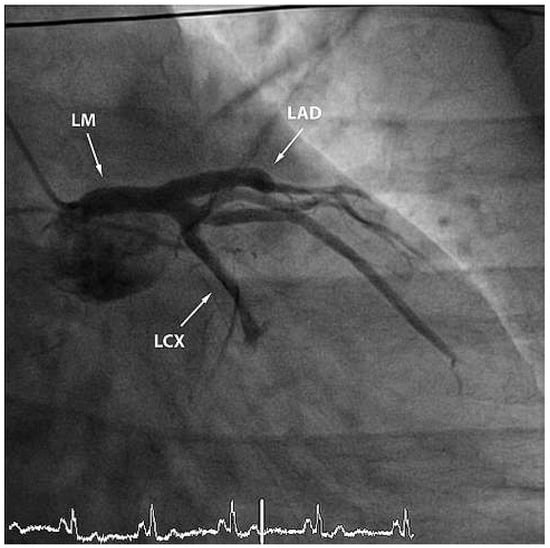
Figure 2.
RAO view showing occlusion of the mid-LAD and of the left circumflex artery. LM = left main coronary artery; LAD = left anterior descending artery; LCX = left circumflex artery.
Furthermore, the right coronary artery showed a subtotal thromNo botic occlusion (Figure 3). Prior to the local treatment, a bolus of heparine 5000 IU and Abciximab were administered due to the heavy thrombus load. Thrombus aspiration was performed using an aspiration-catheter (Export AP, Medtronic) in all three coronary vessels. Due to persistent thrombotic material in the mid LAD, two low-pressure balloon inflations were done with a good end result (Figure 4 and Figure 5). There were no periprocedural complications. A treatment with life-long aspirin, one-year prasugrel, Betablocker and ACE-Inhibitor was initiated. The next day, a transesophageal echocardiography demonstrated improved LV-function (EF 50%) without evidence for an intracardiac thrombus or a patent foramen ovale (PFO).
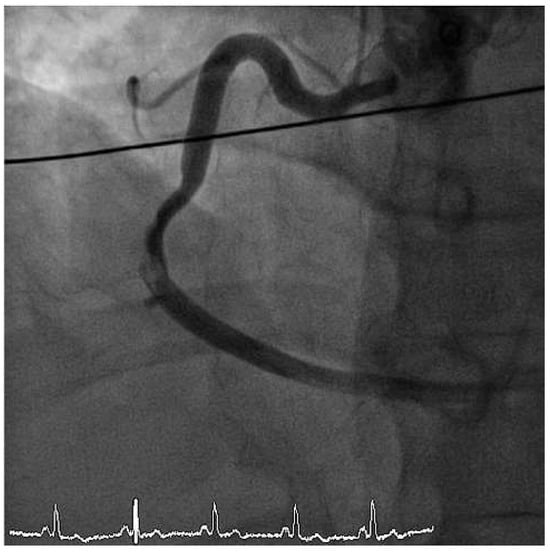
Figure 3.
LAO cranial view showing right coronary artery with subtotal thrombotic occlusion.
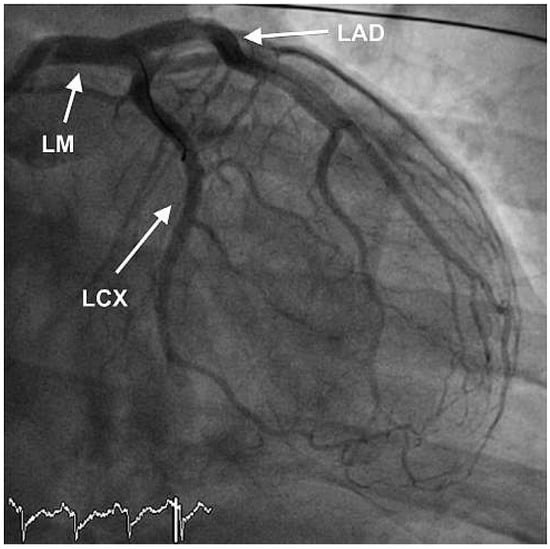
Figure 4.
RAO view showing good end result after thrombus aspiration in LAD and RCX and PTCA of the LAD.

Figure 5.
LAO cranial view showing successful thrombus aspiration in the right coronary artery.
A complementary laboratory analysis revealed hypercholesterolemia (cholesterol 5.6 mmol/l, LDL-cholesterol 4.5 mmol/l). Homocystein-level was normal. Thrombophilia workup showed elevated fibrinogen (5.4 g/l, normal < 3.75) as well as slightly elevated factor VIII (196%, normal < 164) and plasma von Willebrand factor (vWF) (166%, normal <150%). Protein Cand S-activity, as well as Antithrombin III-activity were within normal limits. Factor V Leiden mutation and Prothrombin mutation G20210A were negative. Immunological investigations (ANA, ANCA, rheumatoid factor, dsDNA-antibodies, anti-β2-glycoprotein Iantibodies, Anticardiolipin-antibodies, JAK2, BCRLABL, Hepatitis B/C, HIV, CMV, Parvovirus B19 and Lues) were, however, completely unremarkable. A drug screening was not performed, because the patient denied convincingly any drug consumption.
Under therapy with aspirin and prasugrel, the patient remained symptom-free and no other thrombotic event occurred during the 10-month follow-up.
Discussion
Atherosclerotic disease is the primary cause of myocardial infarction. Others are nonatherosclerotic coronary diseases such as coronary anomalies, inflammatory diseases or spontaneous dissections, coronary embolism, drug abuse and hypercoagulability states [1], all of them are rare.
Nonatherosclerotic coronary artery pathology accounts only for 3% of sudden cardiac death caused by myocardial infarction [2]. Nearly half of them are due to congenital anomalies of coronary arteries. Most of them are associated with an anomalous origin of coronary arteries. Vasculitis (12%), fibromuscular dysplasia (4%) or acute dissections of coronary arteries (16%) are other rare causes of nonatherosclerotic coronary artery disease [2]. The latter occurs primarily in women during the peripartum period and has a high mortality rate [3].
Coronary embolism is another uncommon cause of acute myocardial infarction, mainly because of the anatomy of the coronary artery. It has been speculated that the take-off of the coronary arteries from the aorta immediately beyond the aortic valve and at a right angle attribute to the infrequency of coronary embolism. Additionally the fact that coronary perfusion mainly occurs during diastole might be another reason [4]. Coronary embolism is classified into three types according to pathological mechanism: direct, paradoxical and iatrogenic embolism. Direct embolism to the coronary arteries occurs most commonly in patients with aortic valve endocarditis and from thrombotic material on diseased or prosthetic aortic or mitral valves [4].
The diagnosis of paradoxical embolism is often difficult to prove because of the high prevalence of PFO in individuals without embolic events. The mechanism of paradoxical embolism was first described by Cohnheim already in 1877 [5]. A paradoxical embolism is clinically presumed if there is evidence of an arterial embolism in the absence of a source in the left heart, the source of embolism being identified in the venous system and if a communication between the venous and arterial circulation is found. However rare, the case of direct visualisation by TEE of an entrapped thrombus in the PFO and of a separate lodged thrombus in the left main coronary ostium has been published [6]. The third type of coronary embolism is iatrogenic, which probably is the today’s most common form of coronary embolism. It may occur during percutanous coronary interventions and cardiac surgery. In particular, iatrogenic coronary air embolism is a complication that is associated with high morbidity and even mortality [7].
The leading drug that causes myocardial infarction in young patients is cocaine abuse. In the age group between 18 and 45 years, 25% of nonfatal myocardial infarctions are attributable to frequent cocaine abuse [8]. Most of them occur in the absence of significant atherosclerotic stenosis within the epicardial coronary arteries [9]. Three mechanisms have been proposed for cocaine-induced ischaemia: increased myocardial oxygen demand that results from the sympathomimetic actions of cocaine, coronary artery vasoconstriction or spasm and coronary artery thrombosis [10].
Other rare causes for a myocardial infarction are coagulation disorders. Various coagulation anomalies are associated with an elevated cardiovascular risk. Myeloproliferative disorders particularly polycythemia vera or essential thrombocythemia are well known atypical risk factors for coronary thrombosis [11], as well as the antiphospholipid syndrome, Factor V Leiden mutation and Prothrombin mutation G20210A [12]. These disorders should be screened in all young patients (male and female younger than 55 and 60 years, respectively) with myocardial infarction not explained by coronary atherosclerosis or other apparent cause [11]. The significance of other coagulation factors is less well established. In a large meta-analysis of over 150 000 healthy middle-aged adults, a moderately strong association was found between elevated plasma fibrinogen level and the risk of coronary heart disease and stroke [13]. Conflicting data exist on the relation between elevated plasma vWF concentrations and an increased risk of cardiovascular disease [14,15]. However, patients with diabetes mellitus and elevated vWF levels were clearly associated with a higher risk of cardiovascular diseases [16].
Elevated levels of coagulation factors VIII, IX and XI increase the risk of myocardial infarction in men. In particular, the constellation of high levels of factor XI and low factor XII activities showed the highest risk [17].
In our case, most of the above-described causes of myocardial infarction could be ruled out, but there remain open questions. An embolic event without signs of an intracardiac source or a PFO and no other clinical manifestation of an embolic event in other organs is highly unlikely. The fact, that thrombotic occlusion of all three coronary arteries occurred simultaneously rather implies a “local” process such as vascular inflammation. Despite the negative laboratory workup and the absence of clinical signs of a classic vasculitis, this diagnosis cannot be ruled out, particularly without histological analysis.
The elevation of a few of the coagulation factors is a sign of an elevated risk for a thrombotic event. Whether this constellation explains the clinical situation is difficult to prove.
Our patient did not undergo stent implantation since there was no angiographic evidence for intimal rupture. This was not further confirmed by intravascular ultrasound or OCT due to the unfavourable riskbenefit ratio in the hemodynamically compromised patient with the risk to harm a potentially diseased arterial wall, as it is known from spontaneous coronary dissections. Furthermore, routine stenting may have increased the risk of rethrombosis in the present setting due to additional foreign material.
Conclusion
Our case is exceptional considering the patient’s age and the thrombotic occlusion of all three native coronary arteries at the same time without signs of relevant coronary atherosclerosis. A number of open questions remain and the long-term prognosis as well as the treatment is unclear. However, the patient was free from further thromboembolic events under dual antiplatelet therapy.
Although atherosclerotic disease remains the principal cause of myocardial infarction, other rare differential diagnosis must always be considered.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mirza, A. Myocardial infarction resulting from nonatherosclerotic coro-nary artery diseases. Am J Emerg Med. 2003, 21, 578–84. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.F.; Sheppard, M.N. Non-atherosclerotic coronary artery disease as-sociated with sudden cardiac death. Heart. 2010, 96, 1119–25. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, R.; Singh, G.; Fesniak, H. Spontaneous coronary artery dissec-tion: The clinical spectrum. Angiology. 2002, 53, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.O. Coronary embolism. Int J Cardiol. 2009, 136, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cohnheim, J. Thrombose und Embolie. In Vorlesungen Über Allgemeine Pathologie; Hirschwald: Berlin, Germany, 1877; p. 134. [Google Scholar]
- Meier-Ewert, H.K.; Labib, S.B.; Schick, E.C.; Gossman, D.E.; Stix, M.S.; Wil-liamson, C.A. Paradoxical Embolism in the Left Main Coronary Artery: Diagnosis by Transesophageal Echocardiography. Mayo Clin Proc. 2003, 78, 103–6. [Google Scholar] [CrossRef] [PubMed]
- Dib, J.; Boyle, A.J.; Chan, M.; Resar, J.R. Coronary air embolism: A case report and review of the literature. Catheter Cardiovasc Interv. 2006, 68, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Suri, M.F.; Guterman, L.R.; Hopkins, L.N. Cocaine use and the likelihood of nonfatal myocardial infarction and stroke: Data from the Third National Health and Nutrition Examination Survey. Circulation. 2001, 103, 502–6. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.E.; Hoffman, R.S. Cocaine-induced myocardial infarction: An analysis and review of the literature. J Emerg Med. 1992, 10, 169–77. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.G.; Rezkalla, S.; Kloner, R.A. Cardiovascular effects of cocaine. Circulation. 2010, 122, 2558–69. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, F.; Becker, R.C. Atherothrombotic disorders: New insights from hematology. Circulation. 2005, 111, 1855–63. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.S.; Branch, D.W.; Rauch, J. The antiphospholipid syndrome. N Engl J Med. 2002, 346, 752–63. [Google Scholar] [CrossRef] [PubMed]
- Fibrinogen Studies Collaboration Danesh, J.; Lewington, S.; Thompson, S.G.; Lowe, G.D.; Collins, R.; Kostis, J.B. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: An individual participant meta-analysis. JAMA. 2005, 294, 1799–809. [Google Scholar] [PubMed]
- Whincup, P.H.; Danesh, J.; Walker, M.; Lennon, L.; Thomson, A.; Appleby, P.; et al. Von Willebrand factor and coronary heart disease: Prospective study and meta-analysis. Eur Heart J. 2002, 23, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Patterson, C.; Yarnell, J.; Rumley, A.; Ben-Shlomo, Y.; Lowe, G. Which hemostatic markers add to the predictive value of conventional risk factors for coronary heart disease and ischemic stroke? Circulation. 2005, 112, 3080–7. [Google Scholar] [CrossRef] [PubMed]
- Frankel, D.S.; Meigs, J.B.; Massaro, J.M.; Wilson, P.W.; O’Donnell, C.J.; D’Agostino, R.B.; et al. Von Willebrand factor, type 2 diabetes mellitus, and risk of cardiovascular disease: The framingham offspring study. Circulation. 2008, 118, 2533–9. [Google Scholar] [CrossRef] [PubMed]
- Doggen, C.J.; Rosendaal, F.R.; Meijers, J.C. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: Opposite and synergistic effects of factors XI and XII. Blood. 2006, 108, 4045. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.