Percutaneous Implantation of an ASD Occluder with Intracardiac Ultrasound
Conflicts of Interest
References
- Hein, R.; et al. Atrial and ventricular septal defects can safely be closed by percutaneous intervention. J Interv Cardiol. 2005, 18(6), 515–522. [Google Scholar] [CrossRef] [PubMed]
- Ben Zekry, S.; et al. Percutaneous closure of atrial septal defect. JACC Cardiovasc Imaging. 2008, 1(4), 515–517. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; et al. Experience with transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: a single centre study in 236 consecutive patients. Heart. 2003, 89(2), 199–204. [Google Scholar] [CrossRef] [PubMed]
- Koenig, P.; Cao, Q.L. Echocardiographic guidance of transcatheter closure of atrial septal defects: is intracardiac echocardiography better than transesophageal echocardiography? Pediatr Cardiol. 2005, 26(2), 135–139. [Google Scholar] [CrossRef] [PubMed]
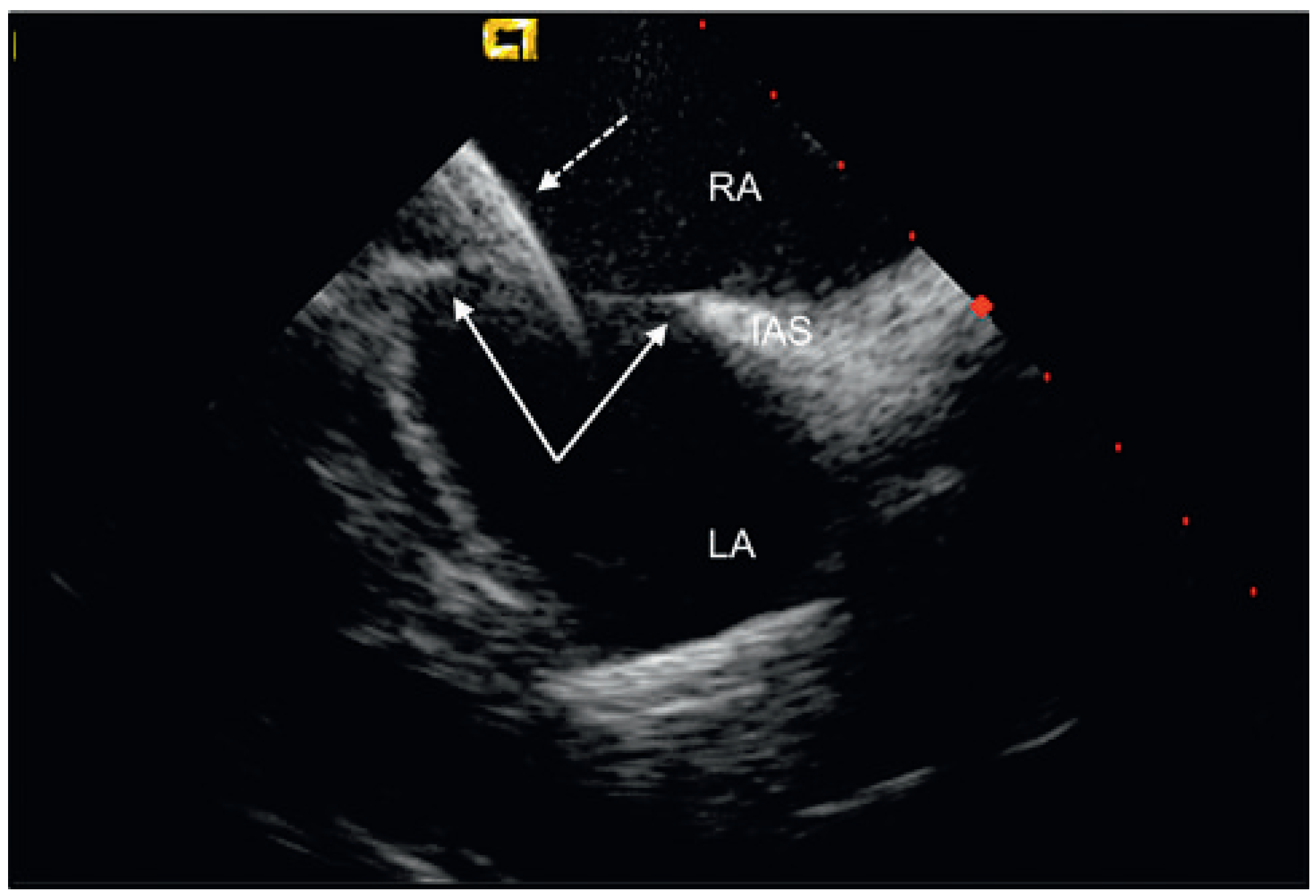

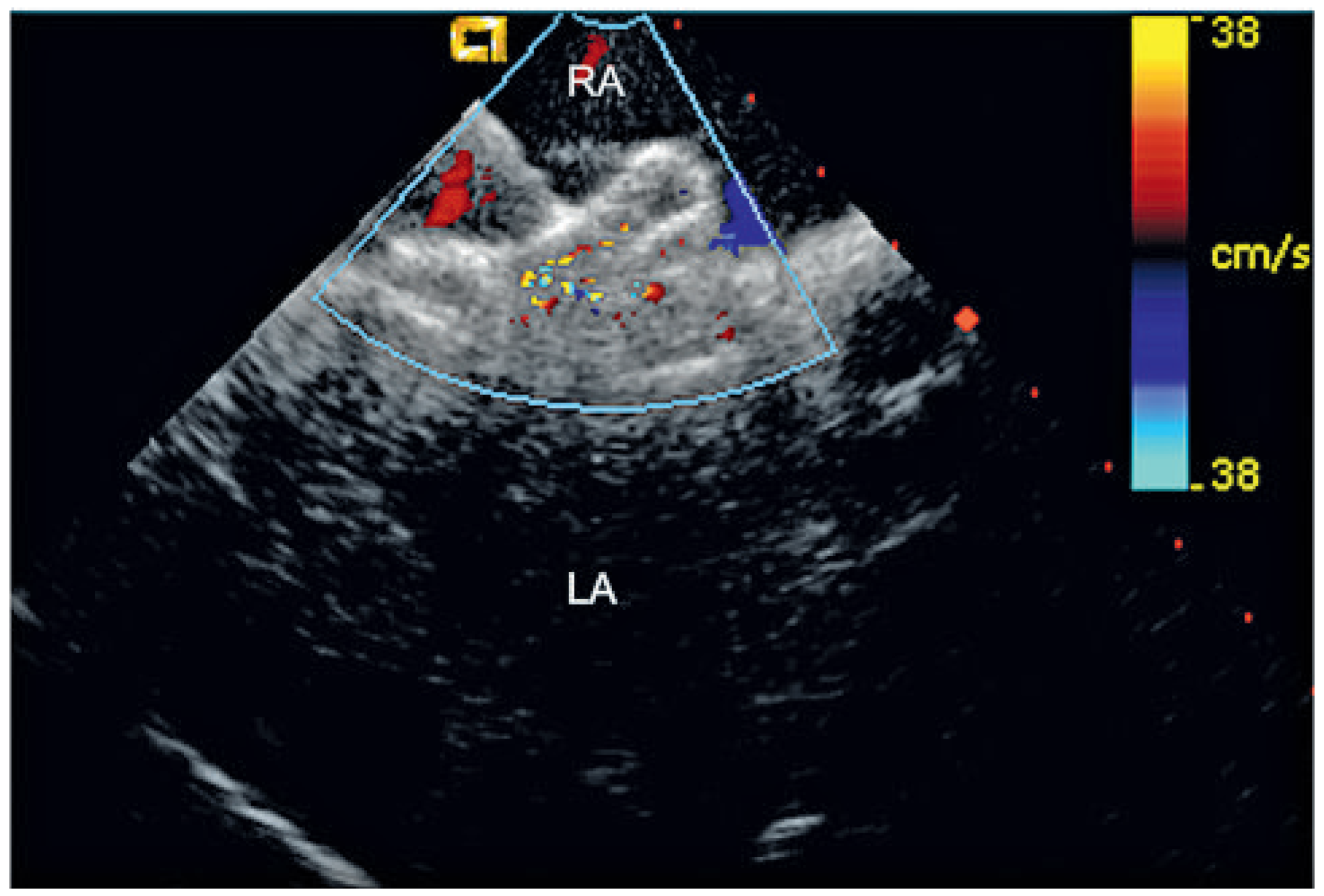
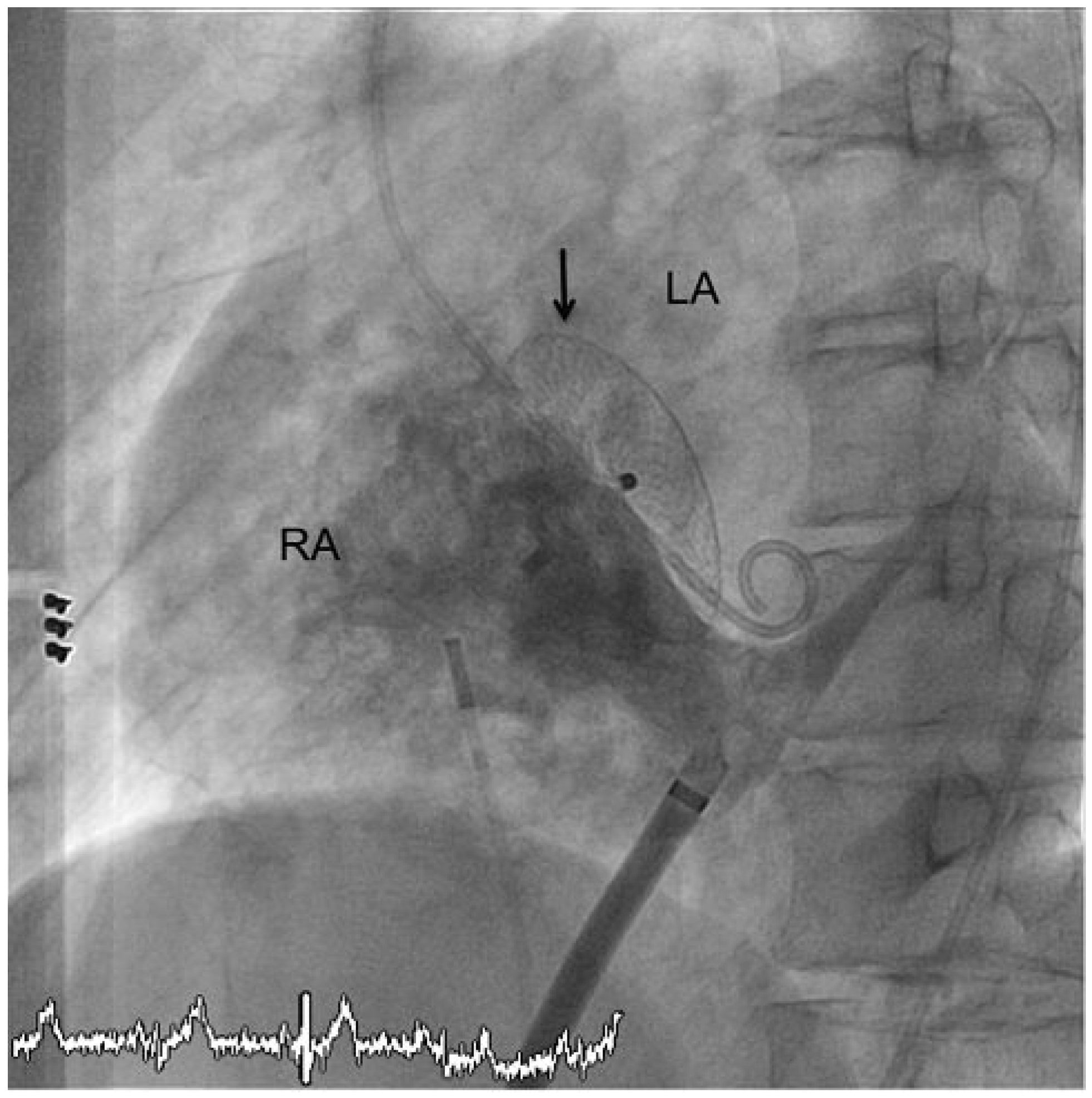
© 2010 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Enseleit, F.; Kretschmar, O.; Lüscher, T.F. Percutaneous Implantation of an ASD Occluder with Intracardiac Ultrasound. Cardiovasc. Med. 2010, 13, 290. https://doi.org/10.4414/cvm.2010.01522
Enseleit F, Kretschmar O, Lüscher TF. Percutaneous Implantation of an ASD Occluder with Intracardiac Ultrasound. Cardiovascular Medicine. 2010; 13(9):290. https://doi.org/10.4414/cvm.2010.01522
Chicago/Turabian StyleEnseleit, Frank, Oliver Kretschmar, and Thomas F. Lüscher. 2010. "Percutaneous Implantation of an ASD Occluder with Intracardiac Ultrasound" Cardiovascular Medicine 13, no. 9: 290. https://doi.org/10.4414/cvm.2010.01522
APA StyleEnseleit, F., Kretschmar, O., & Lüscher, T. F. (2010). Percutaneous Implantation of an ASD Occluder with Intracardiac Ultrasound. Cardiovascular Medicine, 13(9), 290. https://doi.org/10.4414/cvm.2010.01522



