Introduction
Elevated low-density lipoprotein (LDL) cholesterol is a key risk factor for atherosclerotic coronary heart disease. The discovery of statins as the most effective LDL lowering agents has prompted numerous clinical trials showing considerable clinical benefits due to LDL lowering [
1,
2]. These studies also showed that lower LDL cholesterol levels correlate to a lower incidence of coronary events. In the recently published JUPITER trial [
3], lowering of mean LDL cholesterol levels of 3.4 mmol/L, corresponding to moderate cardiovascular risk, by 50% to very low levels with a mean of 1.5 mmol/L was associated with a relative risk reduction in cardiovascular mortality by 44% (absolute risk reduction: 0.9%) and of total mortality by 20% (absolute risk reduction: 0.55%) [
4]. This data most impressively shows that low LDL cholesterol levels are associated with a low incidence of coronary heart disease. Since doubling statin doses only lowers LDL cholesterol by an additional 6% [
5], high statin doses are necessary for optimal LDL cholesterol lowering. Furthermore, a greater incidence of adverse effects occurs at higher statin doses.
However, even when lowering LDL cholesterol to very low levels, a considerable residual risk remains [
6]. This has prompted attempts to not only lower LDL cholesterol, but also to increase HDL cholesterol, especially in individuals with low HDL.
Both lowering LDL and increasing HDL calls for a combination of drugs. In this article, new possibilities to increase the effects of statins in LDL lowering, and to lower LDL and to concomitantly increase HDL, and their clinical function will be described.
The Cholesterol Absorption Inhibitor Ezetimibe and Its Combination with Statins
Ezetimibe has been shown to lower LDL cholesterol levels by about 18%, which equates to a statin dose doubled 3 times. Ezetimibe alone, in cases of statin incompatibility or in combination with statins, is a new interesting principle for LDL cholesterol lowering, especially with regard to factors that influence the response to statin therapy and that can be overcome with this treatment option.
There are a number of extrinsic factors which can modify the LDL cholesterol response to statin therapy (e.g., patient compliance, interaction with other drugs, undesired effects). However, even when these extrinsic factors are taken into account, there remain intrinsic differences in LDL cholesterol response to statin therapy.
First, statins inhibit cholesterol synthesis by inhibition of HMG CoA reductase, a rate determining enzyme in the endogenous cholesterol synthesis. This is partially compensated by an increased absorption rate of cholesterol in the intestine. Vice versa, ezetimibe inhibits cholesterol absorption of exogenous and biliary cholesterol in the intestine, leading to an increased synthesis of cholesterol in the liver. The combination of both drugs has a complementary effect on both mechanisms (
Figure 1).
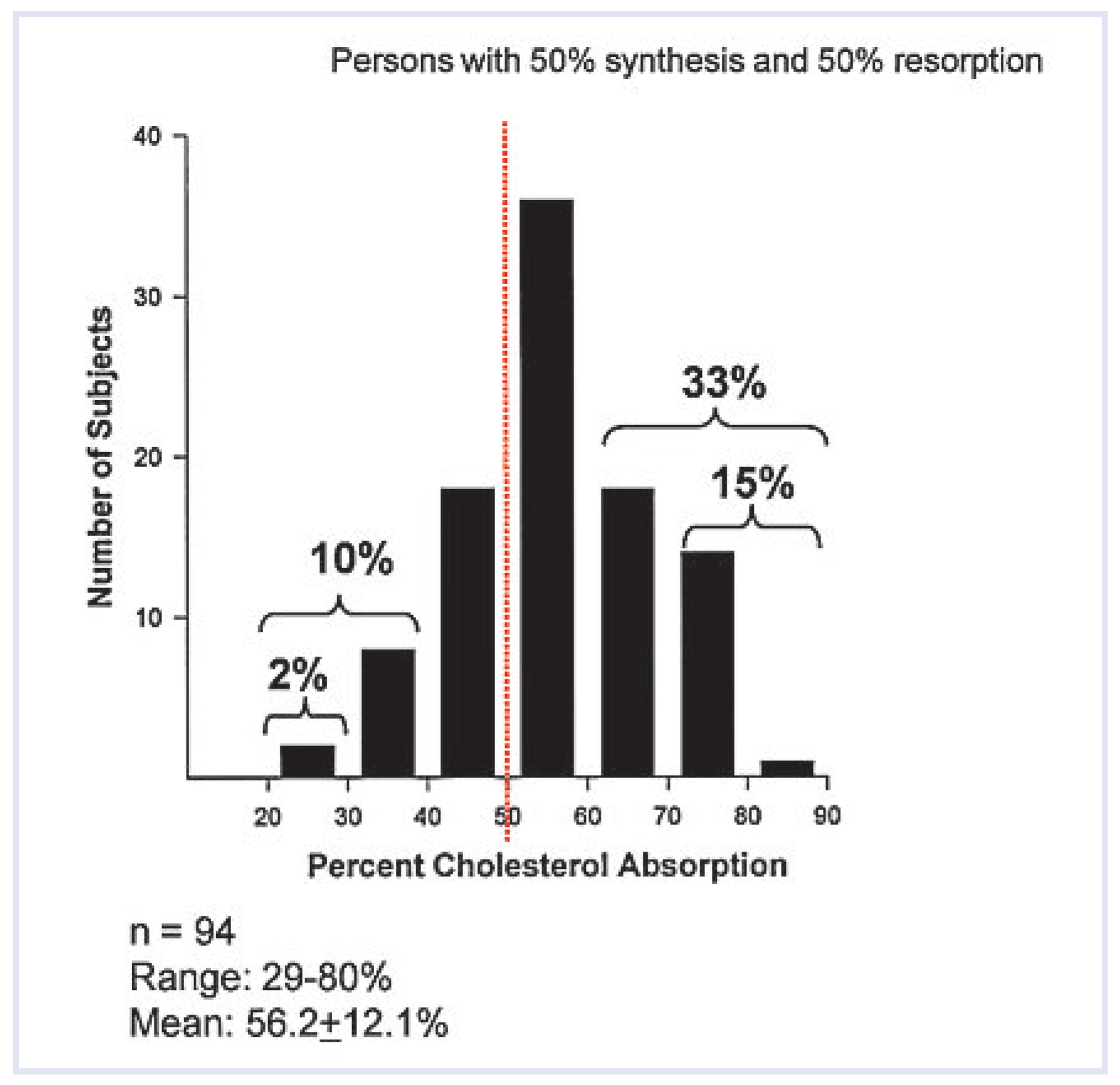
Individual variability exists with regard to the proportion of cholesterol absorption versus cholesterol synthesis. Some individuals are avid “absorbers” whereas others are “synthesisers” (
Figure 2). High absorbers often do not respond to statin therapy, whereas in high synthesisers the contrary occurs. The large biological variation in individual cholesterol absorption and synthesis is reflected by the individual responses to ezetimibe (−70% to 0% decrease in LDL values [
9]) and to statins (e.g., −58% to −3% decrease in LDL cholesterol for 10 mg atorvastatin [
10] and −80% to + 15% in LDL cholesterol change for rosuvastatin 40 mg [
11]).
The variation in cholesterol absorption also transfers into clinical outcome. In the 4S study, high cholesterol absorbers treated with simvastatin did not show an improved clinical outcome (
Figure 3).
Several clinical outcome studies, such as ENHANCE [
13], SEAS [
14] and SANDS [
15], have been done with ezetimibe/simvastatin. The data are summarised as follows:
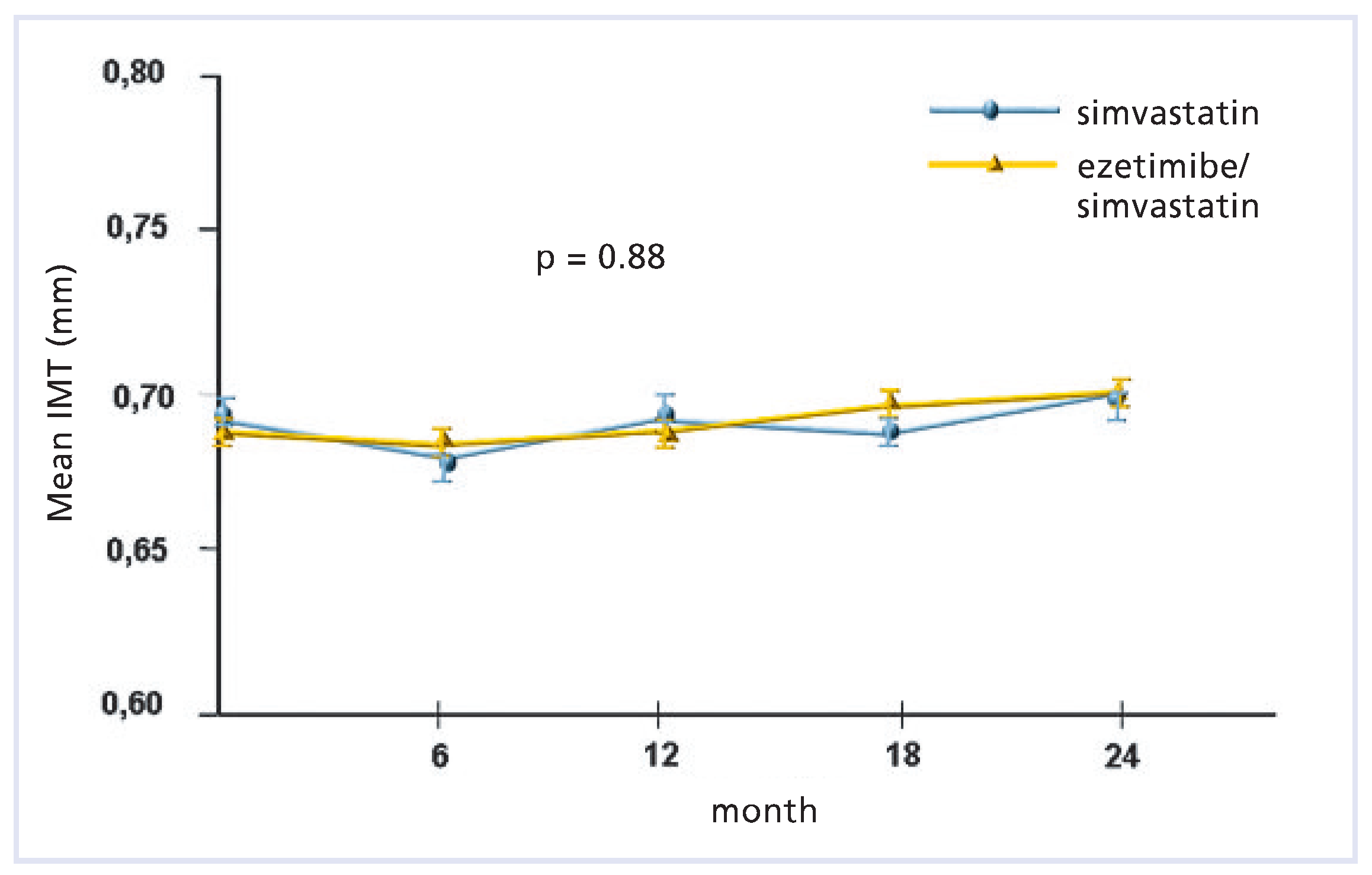
ENHANCE (Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression) [13]
This was a double-blind, randomised, 24-month trial comparing the effects of daily therapy with 80 mg of simvastatin plus placebo versus 80 mg of simvastatin plus 10 mg ezetimibe in 720 patients with heterozygous familial hypercholesterolemia.
Data of the primary outcome (mean change in the carotid-artery intima-media thickness) is illustrated below (
Figure 4) according to Kastelein et al. [
13]). Secondary outcomes (consisting of other variables regarding the intima/media thickness of the carotid and femoral arteries) did not differ significantly between the two groups. At the end of the study, the mean (±SD) LDL cholesterol level was 4.98 ± 1.56 mmol/L in the simvastatin group and 3.65 ± 1.36 mmol/L in the combined-therapy group (a between-group difference of 16.5%,
p < 0.01).
SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) [14]
SEAS was a randomised, double-blind trial involving 1873 patients with mild-to-moderate, asymptomatic aortic stenosis. The patients received either 40 mg of simvastatin plus 10 mg of ezetimibe or a placebo daily. Compared with the placebo, LDL cholesterol was reduced by 61% (2.0 mmol/L).The primary outcome was a composite of major cardiovascular events, including death from cardiovascular causes, aortic-valve replacement, non fatal myocardial infarction, hospitalisation for unstable angina pectoris, heart failure, coronary-artery bypass grafting, percutaneous coronary intervention and non-haemorrhagic stroke. Secondary outcomes were events related to aortic-valve stenosis and ischaemic cardiovascular events. Data on the composite endpoints are shown in
Figure 5.
During a median follow-up of 52.2 months, the primary outcome occurred in 333 patients (35.3%) in the simvastatin/ezetimibe group and in 355 patients (38.2%) in the placebo group (hazard ratio in the simvastatin/ezetimibe group: 0.96; 95% confidence interval [CI]: 0.83 to 1.12; p = 0.59). Aortic-valve replacement was performed in 267 patients (28.3%) in the simvastatin/ezetimibe group and in 278 patients (29.9%) in the placebo group (hazard ratio: 1.00; 95% CI: 0.84 to 1.18; p = 0.97).
Compared with the placebo, cardiovascular disease events were reduced by 4.4% from 20.1% to 15.7% in the simvastatin/ezetimibe group (p = 0.02). Fewer patients had ischemic cardiovascular events in the simvastatin/ezetimibe group (148 patients) than in the placebo group (187 patients) (hazard ratio: 0.78; 95% CI: 0.63 to 0.97; p = 0.02). In conclusion, the treatment with simvastatin/ezetimibe reduced the incidence of ischaemic cardiovascular events but not events related to aortic-valve stenosis.
SANDS (Stop Atherosclerosis in Native Diabetics Study) [15]
This secondary analysis from the SANDS trial examined the effects of lowering LDL cholesterol with statins alone versus statins plus ezetimibe on common carotid artery intima/media thickness (CIMT) In this trial, change in CIMT over 36 months was compared within an aggressive group (target LDL cholesterol ≤ 70 mg/dl) over 36 months in diabetic individuals >40 years of age. The CIMT changes in both aggressive subgroups were compared with changes in the standard subgroups (target LDL cholesterol ≤ 100 mg/dl).
Mean (95% CIs) LDL cholesterol was reduced by 31 (23 to 37) mg/dl and 32 (27 to 38) mg/dl in the aggressive group receiving statins plus ezetimibe or statins alone, respectively, compared with changes of 1 (−3 to 6) mg/dl in the standard group (p < 0.0001) versus both aggressive subgroups. Within the aggressive group, mean CIMT at 36 months regressed from baseline similarly in the ezetimibe (−0.025 [−0.05 to 0.003] mm) and non ezetimibe subgroups (−0.012 [−0.03 to 0.008] mm) but progressed in the standard treatment arm (0.039 [0.02 to 0.06] mm, intergroup p < 0.0001). Aggressive lowering of LDL-cholesterol with ezetimibe/simvastatin or statin alone resulted in similar CIMT-regression in both groups (p = 0.02).
The data on treatment with ezetimibe, although not entirely conclusive, suggests that lipid lowering with statin plus ezetimibe has comparable clinical effects to treatment with statins alone at a high dosage. In ENHANCE, the study population was already optimally treated at the beginning of the study and the IMT was probably too low to see any affects by the treatment. SEAS showed that ezetimibe plus statins did not improve outcome in patients with aortic stenosis. However in the subgroup with ischaemic events, a significant reduction of ischaemic cardiovascular events was observed for the treatment group. Finally SANDS showed the expected results, indicating that aggressive lipid lowering either with statins at high dosage or with a combination of low dose statins with ezetimibe gives similar results with respect to CIMT.
Residual Cardiovascular Risk
Lipid management has typically focused on the control of LDL cholesterol. Statin therapy has been the cornerstone of cardiovascular risk management due to the large number of patients at different levels of risk who have shown significant cardiovascular event reduction. Statins have been shown to reduce coronary heart disease mortality by approximately
1⁄
3. One of the likely reasons for this may be that optimal LDL cholesterol levels were not reached in the statin trials. However, even with very low levels of LDL cholesterol a considerable residual risk for cardiovascular events exists (
Table 1).To further reduce the residual coronary heart disease (CHD) event risk, the regulation of other lipid parameters beyond LDL cholesterol alone may be of benefit, the most important additional risk factor being HDL cholesterol. The Framingham Heart Study was one of the first epidemiologic studies to demonstrate an inverse relationship between HDL cholesterol and increased CHD. Evidence suggests that the prevalence of CHD increases by 25% to 30% for every 0.26 mmol decrease in HDL cholesterol (<1.0 mmol/L) and that more than 50% of coronary events occur in individuals with HDL cholesterol <1.0 mmol/L. A
post hoc analysis of the TNT trial (Treating to New Targets) showed that HDL cholesterol was predictive in patients receiving statin therapy. Even among patients with very low LDL cholesterol (<1.8 mmol/L), those in the highest quintile of HDL cholesterol had a significantly reduced risk of major cardiovascular events compared to those in the lowest quintile (
p = 0.03) [
16].
Contrary to LDL lowering treatment, strategies to increase HDL have so far proven to be less efficacious. Lifestyle modification represents first-line therapy for men and women with low HDL cholesterol. Increases can be achieved by weight loss (HDL cholesterol increases 1 mg/dl per weight loss of 3 kg), diets rich in monounsaturated and polyunsaturated fatty acids (up to 5% increase), smoking cessation (5–10% increase), moderate alcohol consumption (5–15% increase) and physical exercise (up to 15% increase). Due to these modest effects, often lifestyle modifications combined with pharmacotherapy is necessary for optimal treatment of low HDL cholesterol. HDL cholesterol increasing medications include niacin and fibrates. Statins have little effects, and ezetimibe and bile acid sequestrants have no effect on HDL cholesterol. Niacin is the most effective drug for raising HDL cholesterol. Niacin is a an old drug, which has proven its efficacy in regulating HDL cholesterol, LDL cholesterol, triglyceride and Lp(a) levels. In addition, niacin decreases the atherogenic small dense LDL particles. Early studies with niacin showed plaque regression as well as reduction in clinical endpoints [
17,
18]. A meta-analysis has indicated that niacin alone or in combination with statins also has important effects on plaque regression as well as on clinical events in patients with CHD [
19].
Tredaptive® (Nicotinic Acid/Laropiprant): A New Lipid-Modifying Therapy for the Treatment of LDL Cholesterol, HDL Cholesterol and Triglycerides
Statins have been shown to reduce coronary heart disease mortality by approximately
1⁄
3 (
Table 1). To further reduce the residual coronary heart disease event risk in dyslipidaemic patients, the regulation of other lipid parameters beyond LDL cholesterol alone may have benefits; the most important additional risk factor being HDL cholesterol. Niacin (nicotinic acid) lowers LDL cholesterol, triglycerides, and lipoprotein(a), and it is the most effective agent approved for increasing HDL cholesterol levels. Although niacin has been used for the treatment of cardiovascular disease for over 50 years and is the most effective treatment currently available for raising levels of HDL cholesterol, its use is limited by side effects such as flushing [
20], which occurs in nearly all patients taking niacin. This results in a high rate of patient noncompliance and discontinuation of treatment. Laropiprant is a selective antagonist of the prostaglandin D2 receptor subtype 1 (DP1) that can mediate niacin-induced vasodilatation by inhibition of the vasodilatory effects associated with prostaglandin D2 [
21]. This effect is especially pronounced when laropiprant is coadministered with extended release niacin. The PDG2-mediated pathway is independent of the pathway underlying the beneficial lipid-modifying effects of niacin [
22]. Coadministration of laropiprant 30, 100, and 300 mg with extended-release (ER) niacin was found to significantly lower flushing symptom scores (by approximately 50% or more) and also significantly reduced skin blood flow measured by Laser Doppler perfusion imaging. Laropiprant was shown to be effective, after multiple doses, in reducing symptoms of flushing and attenuating the increased skin blood flow induced by ER niacin. In conclusion, the DP1 receptor antagonist laropiprant was effective in suppressing both subjective and objective manifestations of niacin-induced vasodilation [
23]. The effects of niacin-induced flushing severely limits its use for the treatment of dyslipidaemia. The introduction of Tredaptive
® represents a viable strategy for the suppression of niacin-induced flushing, which in turn—despite its tablet size—should enhance patient acceptance and adherence to niacin regimens [
24,
25].
The combination of increasing HDL cholesterol by niacin and lowering LDL cholesterol by statins may be much more efficacious than lowering LDL cholesterol alone. Preliminary studies treating both LDL cholesterol and HDL cholesterol with a combination of statins and niacin have already indicated that a much greater clinical benefit may be expected from such procedures [
26,
27], which prompted much greater relative risk reductions in coronary events.
The most recently published ARBITER 6-HALTS (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies) study compared the effects of further lowering of LDL by ezetimibe or increasing HDL by niacin in statin-treated patients [
28]. A total of 363 patients who had either atherosclerotic coronary or vascular heart disease, or a coronary heart disease risk equivalent including diabetes, who were on longterm statin therapy and in whom LDL cholesterol was below 2.6 mmol/L and HDL cholesterol below 1.3 mmol/L for men or 1.4 mmol/L for women, were randomly assigned to receive extended-release niacin (target does, 2000 mg/d) or ezetimibe (10 mg/d). The primary endpoint was the between-group difference in the change from baseline in the mean carotid intima-media thickness after 14 months. The trial was terminated early (more than 40% of the patients did not undergo the measurement at 14 months of the carotid intima-media thickness) on the basis of efficacy, according to a prespecified analysis conducted after 208 patients had completed the trial. Over the 14-month study period, the mean HDL cholesterol level in the niacin group was increased by 18.4% (
p < 0.001), and the mean LDL cholesterol level in the ezetimibe group decreased by 19.2% (to 1.7 mmol/L) (
p < 0.001). Niacin significantly reduced LDL cholesterol and triglycerides, and ezetimibe reduced HDL cholesterol and triglycerides. The change in mean carotid intima-media thickness over 14 months was greater in the niacin group (
p = 0.003), leading to a significant reduction in both mean (
p = 0.001) and maximal carotid intima-media thickness (≤0.001) for all comparisons. Paradoxically, greater reductions in LDL cholesterol level in association with ezetimibe were significantly associated with an increase in the carotid intima/media thickness (R = −0.31;
p < 0.001). The incidence of major cardiovascular events was lower in the niacin group than in the ezetimibe group (1% vs. 5%;
p = 0.04 by the chisquare test). Whether this result was due to the effect of niacin on Lp(a), LDL cholesterol, remnant lipoproteins, triglycerides or HDL cholesterol remains unknown.
The study has some limitations; for example the premature termination of this study, the use of the carotid intima-media thickness as a surrogate marker for coronary atherosclerosis is controversial, the association of a greater reduction in LDL cholesterol with an increase in the carotid intima-media thickness is not supported by the analysis of the whole 111 patients in the ezetimibe group, which did not show a significant difference from baseline. Nevertheless, the data support the concept that in patients at high cardiovascular risk, treated with a statin to LDL cholesterol target levels, the combination with a drug to raise HDL cholesterol (niacin) might be more effective in preventing cardiovascular events than the combination with a drug to lower LDL cholesterol (ezetimibe).
Ongoing coronary heart disease outcome studies, such as AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Nealth Outcomes) and HPS2-Thrive (Heart Protection Study 2: Treatment of HDL to Reduce the Incidence of Vascular Events), will provide better insight regarding the benefits of niacin in general, and the safety and efficacy of Tredaptive® specifically.