Management of Acute Heart Failure
Summary
Introduction
Clinical Presentation
Pathophysiology
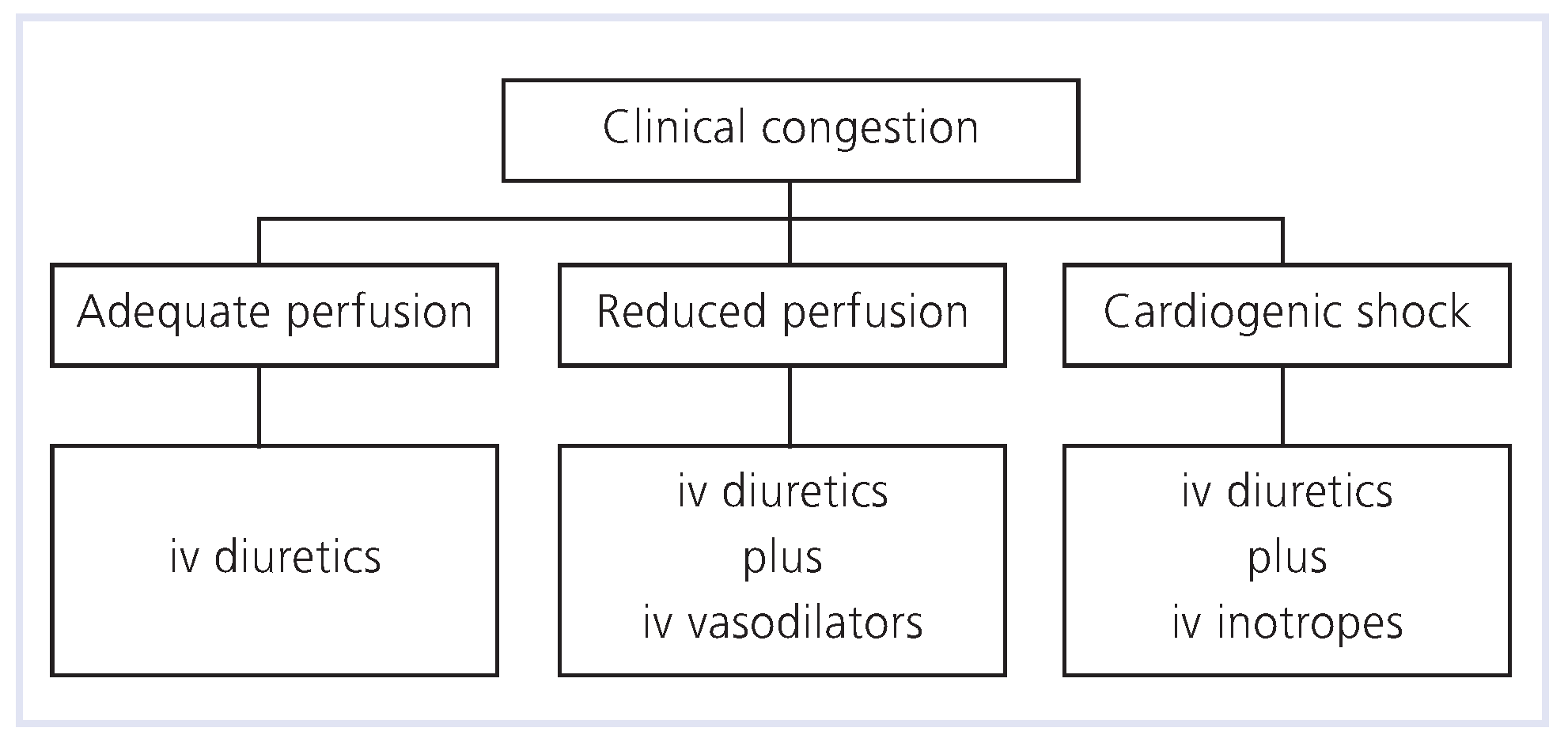
Pharmacologic Therapy
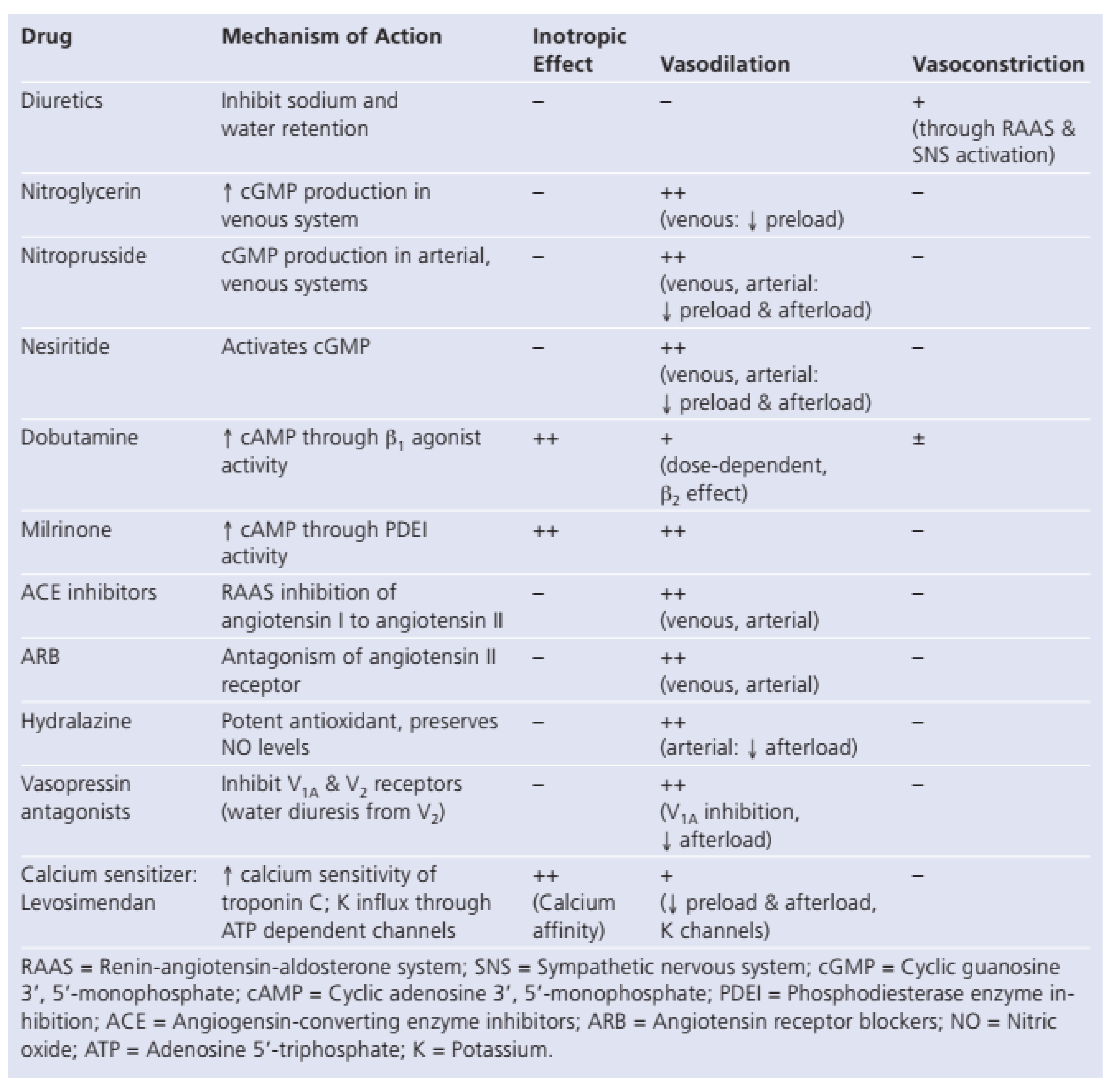
- Diuretics
- Vasodilators
- Inotrope
- Ultrafiltration
- OralVasodilators:ACEInhibitors,AngiotensinReceptorBlockers,HydralazineandNitrates
- Beta-Blockers
- AldosteroneAntagonists
- Emerging Therapies
Conclusion
References
- Rosamond, W.; Flegal, K.; Friday, G.; et al. Heart disease and stroke statistics – 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007, 115, e69–e171.
- Croft, J.B.; Giles, W.H.; Pollard, R.A.; Keenan, N.L.; Casper, M.L.; Anda, R.F. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med. 1999, 159, 505–510. [Google Scholar] [CrossRef]
- Adams, K.F.; Fonarow, G.C.; Emerman, C.L.; LeJemtel, T.H.; Costanzo, M.R.; Abraham, W.T.; et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100, 000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005, 149, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Mensah, G.A.; Croft, J.B.; Keenan, N.L. Heart failure-related hospitalization in the U.S., 1979–2004. J Am Coll Cardiol. 2008, 52, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Tunstall-Pedoe, H.; Kuulasmaa, K.; Mähönen, M.; Tolonen, H.; Ruokokoski, E.; Amouyel, P. Contribution of trends in survival and coronary event rates to changes in coronary heart disease mortality: 10–year results from 37 WHO MONICA Project populations. Lancet 1999, 353, 1547–1557. [Google Scholar] [CrossRef]
- Tang, W.H.; Francis, G.S. Trends and treatment of heart failure developing after acute myocardial infarction. Am Heart Hosp J. 2003, 1, 216–218. [Google Scholar] [CrossRef]
- Dec, G.W. Management of acute decompensated heart failure. Curr Probl Cardiol. 2008, 32, 321–366. [Google Scholar] [CrossRef] [PubMed]
- Nohria, A.; Lewis, E.; Stevenson, L.W. Medical management of advanced heart failure. JAMA 2002, 287, 628–640. [Google Scholar] [CrossRef]
- Nohria, A.; Tsant, S.W.; Fang, J.C.; et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003, 41, 1797–1804. [Google Scholar] [CrossRef]
- ESCAPE Investigators and Study Coordinators. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness. The ESCAPE trial. JAMA 2005, 294, 1625–1633. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Heywood, J.T. The confounding issue of comorbid renal insufficiency. Am J of Med. 2006, 119, S17–S25. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Abraham, W.T. Diuretics for the treatment of acute decompensated heart failure. Heart Fail Rev. 2007, 12, 125–130. [Google Scholar] [CrossRef]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal Syndrome. J Am Coll Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef]
- Kalra, D.K.; Zhu, X.; Ramchandani, M.K.; Lawrie, G.; Reardon, M.J.; Lee-Jackson, D.; et al. Increased myocardial gene expression of tumor necrosis factor a and nitric oxide synthase-2: a potential mechanism for depressed myocardial function in hibernating myocardium in humans. Circulation 2002, 105, 1537–1540. [Google Scholar] [CrossRef]
- ADHERE (Acute Decompensated Heart Failure National Registry). Third quarter 2004 National Benchmark Report. Available online: www.ahereregistry.com.
- Opie, L.H.; Kaplan, N.M.; Pool-Wilson, P. (Eds.) Drugs for the Heart: Diuretics, 5th ed.; W.B. Saunders: Philadelphia, PA, USA, 2001; pp. 84–106. [Google Scholar]
- Dormans, T.P.; van Meyel, J.J.; Gerlag, P.G.; Tan, Y.; Russel, F.G.; Smits, P. Diuretic efficacy of high dose furosemide in severe heart failure: bolus injection versus continuous infusion. J Am Coll Cardiol. 1996, 28, 376–382. [Google Scholar] [CrossRef]
- Pivac, N.; Rumboldt, Z.; Sardelic, S.; et al. Diuretic effects of furosemide infusion versus bolus injection in congestive heart failure. Int J Clin Pharm Res. 1998, 18, 121–128. [Google Scholar]
- Butler, J.; Forman, D.E.; Abraham, W.T.; Gottlieb, S.S.; Loh, E.; Massie, B.M.; et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J. 2004, 147, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.F.; Lindenfeld, J.A.; Arnold, J.M.O.; Baker, D.W.; Barnard, D.H.; Baughman, K.L.; et al. Executive summary: HFSA 2006 Comprehensive Heart Failure Practice Guidelines. J Card Fail 2006, 12, 10–38. [Google Scholar]
- McBride, B.F.; White, C.M. Acute Decompensated Heart Failure: A Contemporary Approach to Pharmacotherapeutic Management. Pharmacotherapy 2003, 23, 997–1020. [Google Scholar] [CrossRef] [PubMed]
- VMAC Publications Committee. Intravenous nesiritide versus nitroglycerin for treatment of decompensated congestive heart failure. A randomized controlled trial. JAMA 2002, 287, 1531–1540. [Google Scholar]
- Silver, M.A.; Horton, D.; Ghali, J.K.; et al. Effect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failure. J Am Coll Cardiol. 2002, 39, 798–803. [Google Scholar] [CrossRef]
- Burger, A.J.; Horton, D.P.; LeJemtel, T.; et al. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated heart failure: the PRECEDENT study. Am Heart J. 2002, 144, 1102–1108. [Google Scholar] [CrossRef]
- Sackner-Bernstein, J.D.; Kowalski, M.; Fox, M.; et al. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA 2005, 293, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T. Nesiritide does not increase 30–day or 6–month mortality risk (abstract 3169). Circulation 2005, 17 (Suppl II), II–676. [Google Scholar]
- Gortney, J.S.; Porter, K.B. Risk of death with nesiritide. JAMA 2005, 294, 897–898. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adams, K.F.; Fonarow, G.C.; the ADHERE Scientific Advisory Committee and Investigators, and the ADHERE Study group; et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications. An analysis form the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005, 46, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W. Benefit-Risk Assessment of Nesiritide in the Treatment of Acute Decompensated Heart Failure. Drug Safety. 2007, 30, 765–781. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Gattis, W.A.; Uretsky, B.F.; et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan international randomized survival trial (FIRST). Am Heart J. 1999, 138 pt 1, 78–86. [Google Scholar] [CrossRef]
- Cuffe, M.S.; Califf, R.M.; Adams, K.F.; Benza, R.; Bourge, R.; Colucci, W.S. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized, controlled trial. JAMA 2002, 287, 1541–1547. [Google Scholar] [CrossRef]
- Felker, G.M.; Benza, R.L.; Chandler, A.B.; for the OPTIME-CHF Investigators; et al. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol. 2003, 41, 997–1003. [Google Scholar] [CrossRef]
- Wertman, B.M.; Gura, V.; Scwarz, E.R. Ultrafiltration for the Management of Acute Decompensated Heart Failure. J Card Fail. 2008, 14, 754–759. [Google Scholar] [CrossRef]
- Jaski, B.E.; Ha, J.; Denys, B.G.; Lamba, S.; Trupp, R.J.; Abraham, W.T. Peripherally inserted veno-venous ultrafiltration for rapid treatment of volume overloaded patients. J Card Fail. 2003, 9, 227–231. [Google Scholar] [CrossRef]
- Pepi, M.; Marenzi, G.C.; Agostoni, P.G.; Doria, E.; Barbier, P.; Muratori, M.; et al. Sustained cardiac diastolic changes elicited by ultrafiltration in patients with moderate congestive heart failure: pathophysiological correlates. Br Heart J. 1993, 70, 135–140. [Google Scholar] [CrossRef]
- Marenzi, G.; Guazzi, M.; Lauri, G.; Perego, G.B.; Sganzerla, P.; Agostoni, P. Body fluid withdrawal with isolated ultrafiltration effects persistent improvement of functional capacity in patients with chronic congestive heart failure. Furosemide does not produce the same result. Cardiologia 1994, 39, 763–772. [Google Scholar] [PubMed]
- Costanzo, M.R.; Guglin, M.E.; Saltzberg, M.T.; Jessup, M.L.; Bart, B.A.; Teerlink, J.R.; UNLOAD Trial Investigators; et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007, 49, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Cotter, G.; Metzkor, E.; Kaluski, E.; et al. Randomized trial of high-dose isosorbide dinitrate plus low dose furosemide versus high-dose furosemide and low dose isosorbide dinitrate in severe pulmonary edema. Lancet 1998, 351, 389–393. [Google Scholar] [CrossRef]
- Taylor, A.L.; Ziesche, S.; Yancy, C.; for the African-American Heart Failure Trial Investigators; et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N. Engl J Med. 2004, 351, 2049–2057. [Google Scholar] [CrossRef]
- Satwani, S.; Dec, G.W.; Narula, J. Beta-adrenergic blockers in heart failure: a review of mechanisms of action and clinical outcomes. J Cardiovasc Pharmacol Therapeut. 2004, 9, 243–255. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; for Randomized Aldactone Evaluation Study Investigators; et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N. Engl J Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [PubMed]
- White, H.; Nicolau, J.; Martinez, F. ; for the EPHESUS Investigators; et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol. 2005, 46, 425–531. [Google Scholar]
- Orlandi, C.; Zimmer, C.A.; Gheorghiade, M. Role of vasopressin antagonists in the management of acute decompensated heart failure. Curr Heart Fail Rep. 2005, 2, 131–139. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Gattis, W.A.; O’Connor, C.M.; for the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure (ACTIV in CHF) Investigators; et al. Effect of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure. JAMA 2004, 291, 1963–1971. [Google Scholar] [CrossRef]
- Konstam, M.A.; Gheorghiade, M.; Burnett, J.C.; Grinfeld, L.; Maggioni, A.P.; Swedberg, K.; et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA 2007, 297, 1319–1331. [Google Scholar] [CrossRef]
- Moiseyyev, V.S.; Poder, P.; Andrejevs, N.; et al. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction: a placebo-controlled, double blind study (RUSSLAN). Eur Heart J. 2002, 2, 1422–1432. [Google Scholar] [CrossRef]
- Fllath, F.; Cleland, J.G.F.; Papp, J.G.Y.; et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 2002, 360, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Zairis, M. The effect of a calcium sensitizer or an inotrope or none in chronic low output decompensated heart failure. J Am Coll Cardiol. 2004, 43, 206A. [Google Scholar] [CrossRef]
- The SURVIVE-W trial: Comparison of dobutamine and levosimendan on survival in acutely decompensated heart failure. Presented at the 2005 American Heart Association Scientific Sessions, Dallas, TX, November 16, 2005.
- Coletta, A.P.; Cleland, J.P. Clinical trials update: highlights of the scientific sessions of the XXIII Congress of the European Society of Cardiology: WARIS II, ESCAMI, PAFAC, RITZ-1, and TIME. Eur J Heart Fail. 2001, 3, 747–750. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teerlink, J.R.; McMurray, J.J.; Bourge, R.C.; for the VERITAS Investigators; et al. Tezosentan in patients with acute heart failure: design of the Value of Endothelin Receptor Inhibition with Tezosentan in Acute heart failure Study (VERITAS). Am Heart J. 2005, 150, 46–53. [Google Scholar] [CrossRef]
- Packer, M. Effects of the endothelin receptor bosentan on the morbidity and mortality in patients with chronic heart failure: results of the ENABLE 1 and 2 trial program. J Am Coll Cardiol. 2002, 39, 101–102. [Google Scholar]
- Cotter, G.; Dittrich, H.C.; Weatherley, B.D.; Bloomfield, D.M.; O’Connor, C.M.; Metra, M.; et al. The PROTECT pilot study: a randomized, placebo-controlled, dose-finding study of the adenosine A1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment. J Card Fail. 2008, 14, 631–640. [Google Scholar] [CrossRef]
- FDAnews Drug Daily Bulletin, June 9, 2009, Vol. 6, No. 111. Available online: http://www.fdanews.com/newsletter/article?articleId=117773&issueId=12728 (accessed on 9 June 2009).
- Gottlieb, S.S.; Brater, D.C.; Thomas, I.; Havranek, E.; Bourge, R.; Goldman, S.; Dyer, F.; Gomez, M.; Bennett, D.; Ticho, B.; Beckman, E.; Abraham, W.T. BG9719 (CVT-124), an adenosine A1 receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation 2002, 105, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Thomas, I.; Banish, D.; Goldman, S.; Havranek, E.; Massie, B.; Zhu, Y.; Ticho, B.; Abraham, W.T. Effects of multiple oral doses of an A1 adenosine antagonist, BG9928, in patients with heart failure: results of a placebo-controlled, dose-escalation study. J Am Coll Cardiol 2007, 50, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, V.; Lüss, H.; Nitsche, K.; Forssmann, K.; Maronde, E.; Fricke, K.; Forssmann, W.G.; Meyer, M. Effects of the renal natriuretic peptide urodilatin (ularitide) in patients with decompensated chronic heart failure: a double-blind, placebo-controlled, ascending-dose trial. Am Heart J. 2005, 150, 1239. [Google Scholar] [CrossRef] [PubMed]
 |
© 2009 by the author. Attribution - Non-Commercial - NoDerivatives 4.0
Share and Cite
Hasan, A.; Abraham, W.T. Management of Acute Heart Failure. Cardiovasc. Med. 2009, 12, 294. https://doi.org/10.4414/cvm.2009.01459
Hasan A, Abraham WT. Management of Acute Heart Failure. Cardiovascular Medicine. 2009; 12(11):294. https://doi.org/10.4414/cvm.2009.01459
Chicago/Turabian StyleHasan, Ayesha, and William T. Abraham. 2009. "Management of Acute Heart Failure" Cardiovascular Medicine 12, no. 11: 294. https://doi.org/10.4414/cvm.2009.01459
APA StyleHasan, A., & Abraham, W. T. (2009). Management of Acute Heart Failure. Cardiovascular Medicine, 12(11), 294. https://doi.org/10.4414/cvm.2009.01459




