Fondaparinux: A New Antithrombotic Treatment Strategy in Venous Thromboembolism and Acute Coronary Syndromes
Summary
Résumé
Introduction
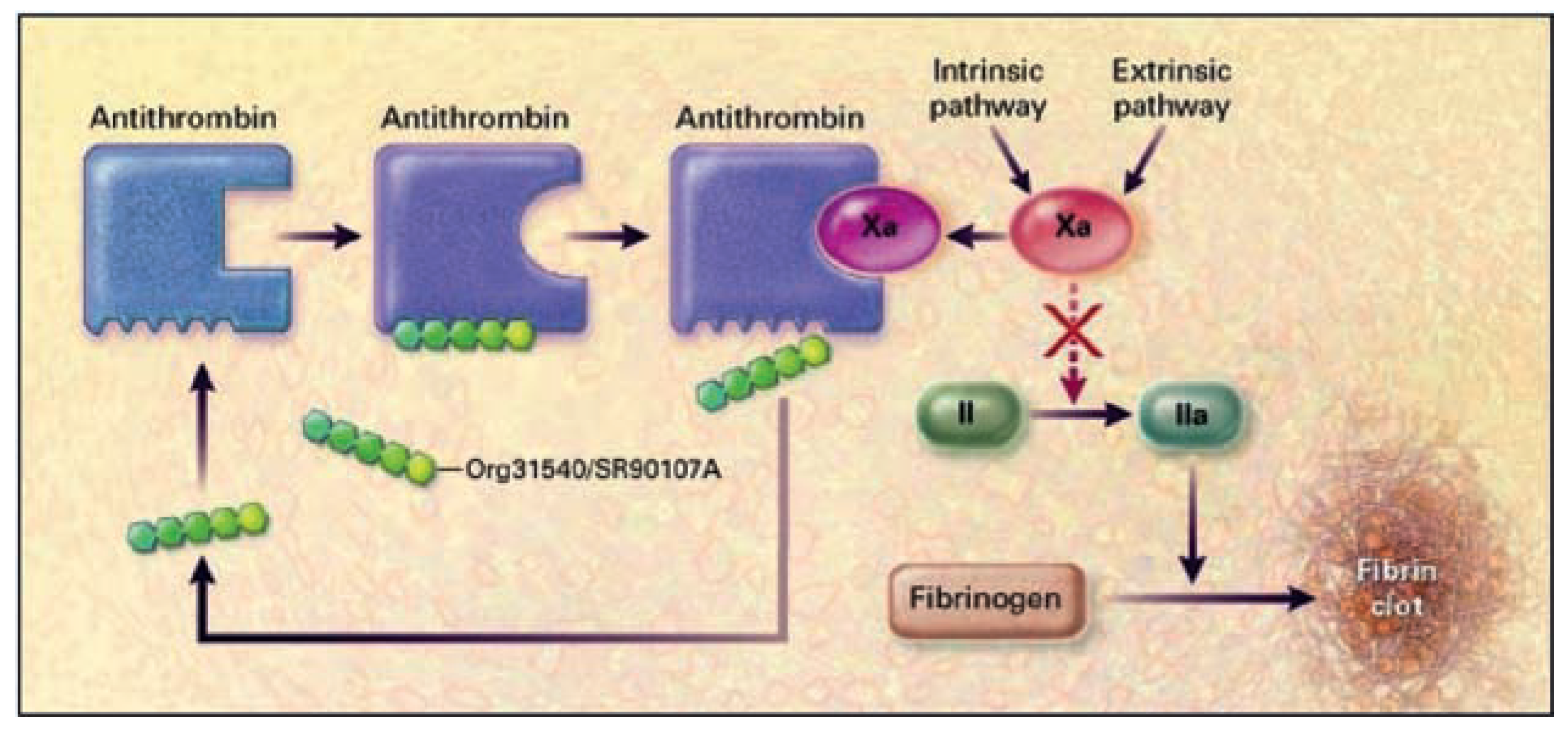
Mechanism of Action and Pharmacokinetics/Pharmacodynamics
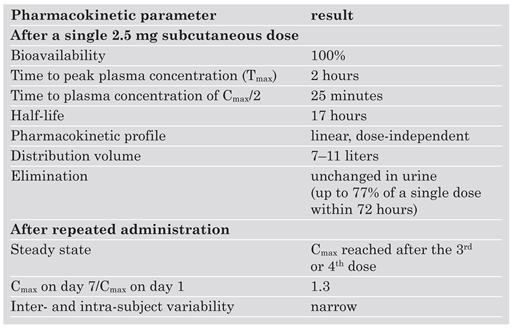 |
Fondaparinux in Venous Thromboembolism
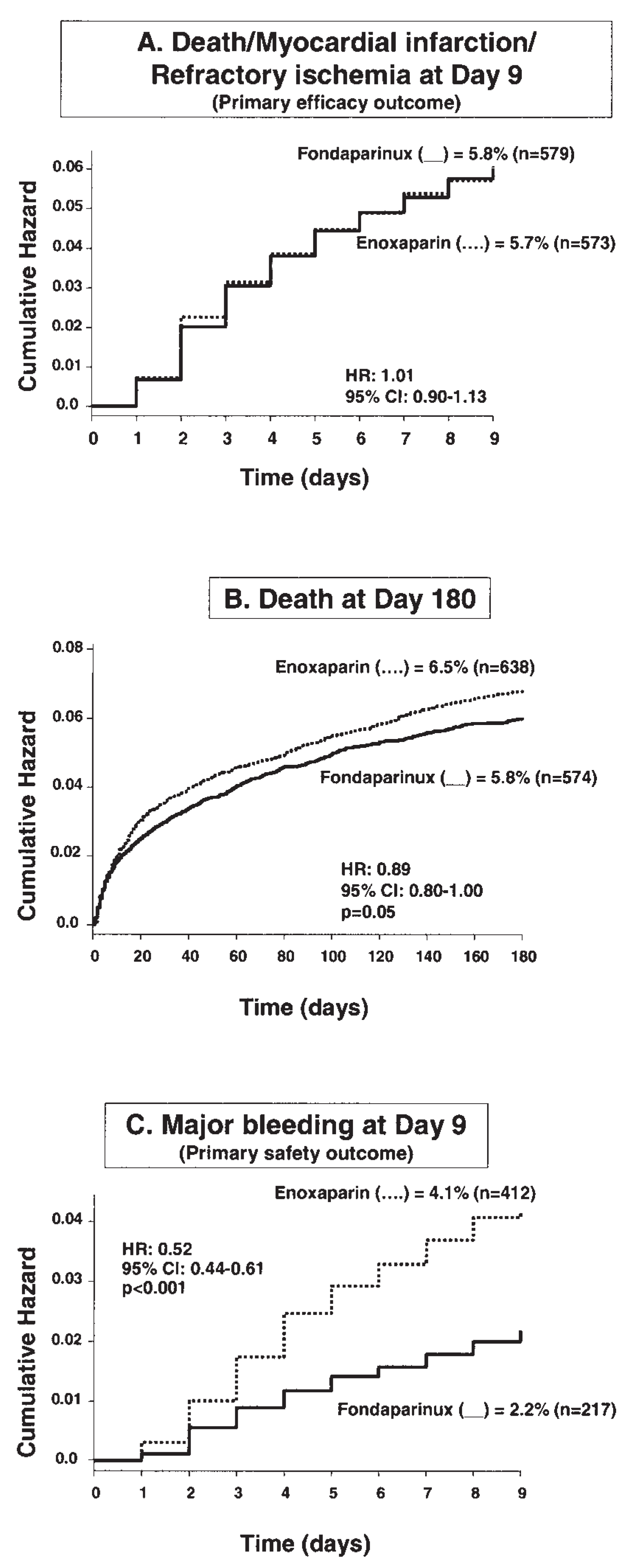
Fondaparinux in Non-ST Elevation Acute Coronary Syndromes (NSTE-ACS)
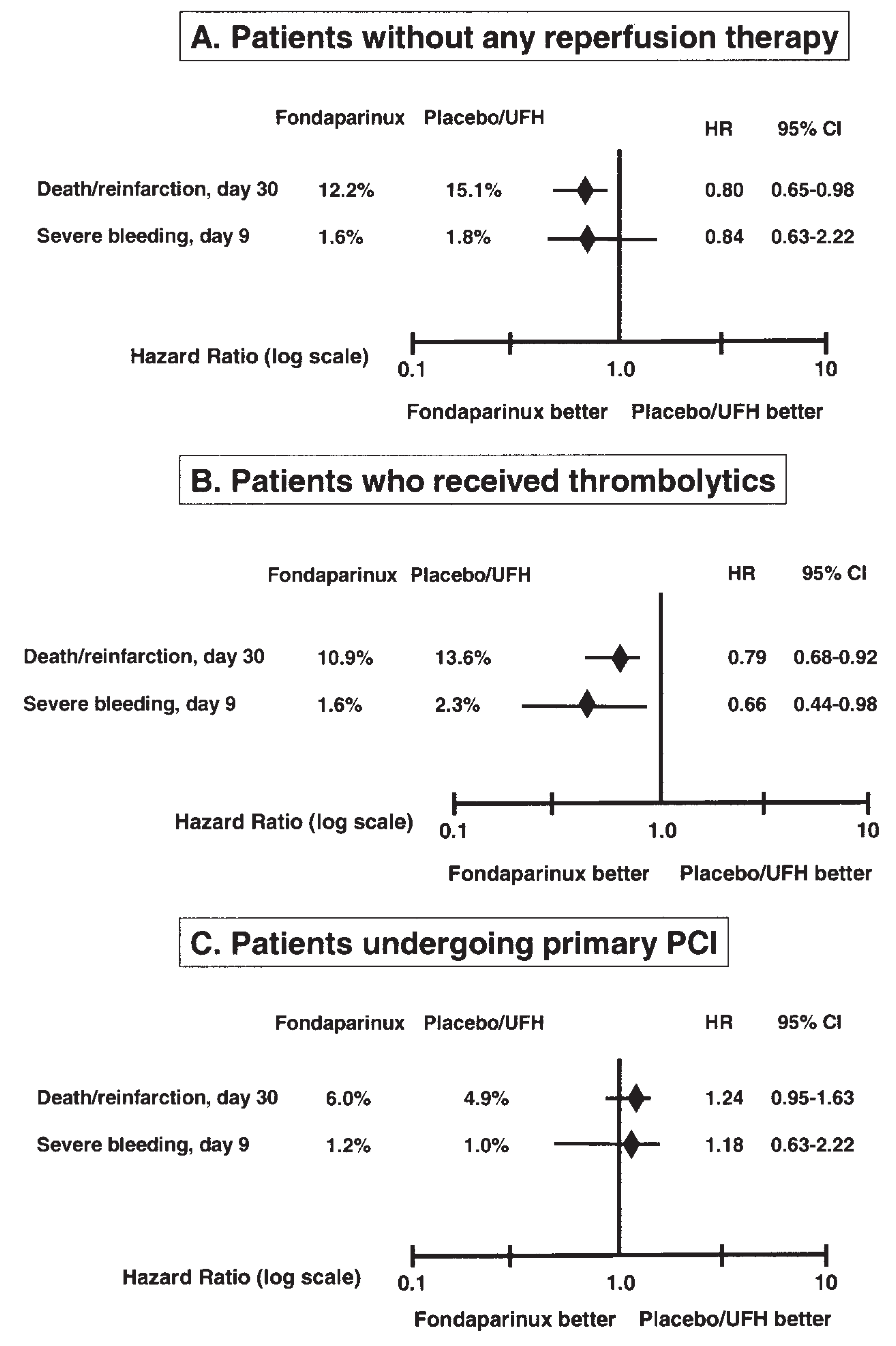
Fondaparinux in ST Elevation Acute Myocardial Infarction (STEMI)
Fondaparinux in Percutaneous Coronary Interventions
Comments
- –
- In the setting of non-ST elevation ACS, fondaparinux can be used in all situations (unstable angina or non-STEMI).
- –
- Fondaparinux cannot be used as standalone anticoagulant in case of PCI, where an additional dose of UFH is required.
- –
- Fondaparinux is recommended in STEMI patients who are not eligible for reperfusion, as well as in patients submitted to thrombolytic treatment.
- –
- Fondaparinux is not recommended in the setting of primary PCI.
Conflicts of Interest
References
- Donat, F.; Duret, J.P.; Santoni, A.; et al. The pharmacokinetics of fondaparinux sodium in healthy volunteers. Clin Pharmacokinet. 2002, 41 (Suppl. 2), 1–9. [Google Scholar] [CrossRef]
- Turpie, A.G. The safety of fondaparinux for the prevention and treatment of venous thromboembolism. Expert Opin Drug Saf. 2005, 4, 707–721. [Google Scholar] [CrossRef]
- Turpie, A.G.; Gallus, A.S.; Hoek, J.A. Asynthetic pentasaccharide for the prevention of deep-vein thrombosis after total hip replacement. N Engl J Med. 2001, 344, 619–625. [Google Scholar] [CrossRef]
- Bauer, K.A.; Eriksson, B.I.; Lassen, M.R.; Turpie, A.G. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med. 2001, 345, 1305–1310. [Google Scholar] [CrossRef]
- Eriksson, B.I.; Bauer, K.A.; Lassen, M.R.; Turpie, A.G. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med. 2001, 345, 1298–1304. [Google Scholar] [CrossRef]
- Lassen, M.R.; Bauer, K.A.; Eriksson, B.I.; Turpie, A.G. Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: a randomised double-blind comparison. Lancet. 2002, 359, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Turpie, A.G.; Bauer, K.A.; Eriksson, B.I.; Lassen, M.R. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studies. Arch Intern Med. 2002, 162, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Turpie, A.G.; Bauer, K.A.; Eriksson, B.I.; Lassen, M.R. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trial. Lancet. 2002, 359, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.I.; Lassen, M.R. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2003, 163, 1337–1342. [Google Scholar] [CrossRef]
- Turpie, A.G.; Bauer, K.A.; Caprini, J.A.; Comp, P.P.; Gent, M.; Muntz, J.E. Fondaparinux combined with intermittent pneumatic compression versus intermittent pneumatic compression alone for prevention of venous thromboembolism after abdominal surgery: a randomized, double-blind comparison. J Thromb Haemost. in press. 2007. [Google Scholar] [CrossRef]
- Agnelli, G.; Bergqvist, D.; Cohen, A.T.; Gallus, A.S.; Gent, M. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg. 2005, 92, 1212–1220. [Google Scholar] [CrossRef]
- Cohen, A.T.; Davidson, B.L.; Gallus, A.S.; et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006, 332, 325–329. [Google Scholar] [CrossRef]
- Buller, H.R.; Davidson, B.L.; Decousus, H.; et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004, 140, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Buller, H.R.; Davidson, B.L.; Decousus, H.; et al. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003, 349, 1695–1702. [Google Scholar] [PubMed]
- Warkentin, T.E.; Maurer, B.T.; Aster, R.H. Heparin-induced thrombocytopenia associated with fondaparinux. N Engl J Med. 2007, 356, 2653–2655. [Google Scholar]
- Efird, L.E.; Kockler, D.R. Fondaparinux for thromboembolic treatment and prophylaxis of heparin-induced thrombocytopenia. Ann Pharmacother. 2006, 40, 1383–1387. [Google Scholar] [CrossRef]
- Simoons, M.L.; Bobbink, I.W.; Boland, J.; et al. A dose-finding study of fondaparinux in patients with non-ST-segment elevation acute coronary syndromes: the Pentasaccharide in Unstable Angina (PENTUA) Study. J Am Coll Cardiol. 2004, 43, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Yusuf, S.; Granger, C.B.; et al. Design and rationale of the MICHELANGELO Organization to Assess Strategies in Acute Ischemic Syndromes (OASIS)-5 trial program evaluating fondaparinux, a synthetic factor Xa inhibitor, in patients with non-ST-segment elevation acute coronary syndromes. Am Heart J. 2005, 150, 1107. [Google Scholar]
- Yusuf, S.; Mehta, S.R.; Chrolavicius, S.; et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006, 354, 1464–1476. [Google Scholar]
- Fox, K.A.; Bassand, J.P.; Mehta, S.R.; et al. Influence of renal function on the efficacy and safety of fondaparinux relative to enoxaparin in non ST-segment elevation acute coronary syndromes. Ann Intern Med. 2007, 147, 304–310. [Google Scholar] [CrossRef]
- Budaj, A.; Eikelboom, J.W.; Wallentin, L.; et al. Bleeding Complications Predict Major Cardiovascular Outcomes in Non ST-Elevation Acute Coronary Syndromes: Results From the OASIS – 5 Trial. J Am Coll Cardiol. 2006, 49 (Suppl. 1), 195A. [Google Scholar]
- Coussement, P.K.; Bassand, J.P.; Convens, C.; et al. A synthetic factor-Xa inhibitor (ORG31540/SR9017A) as an adjunct to fibrinolysis in acute myocardial infarction. The PENTALYSE study. Eur Heart J. 2001, 22, 1716–1724. [Google Scholar] [CrossRef]
- Yusuf, S.; Mehta, S.R.; Chrolavicius, S.; et al. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA. 2006, 295, 1519–1530. [Google Scholar]
- Mehta, S.R.; Steg, P.G.; Granger, C.B.; et al. Randomized, blinded trial comparing fondaparinux with unfractionated heparin in patients undergoing contemporary percutaneous coronary intervention: Arixtra Study in Percutaneous Coronary Intervention: a Randomized Evaluation (ASPIRE) Pilot Trial. Circulation. 2005, 111, 1390–1397. [Google Scholar] [CrossRef]
- Mehta, S.R.; Granger, C.B.; Eikelboom, J.W.; et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol. 2007, 50, 1742–1751. [Google Scholar] [CrossRef]
- Ferguson, J.J.; Califf, R.M.; Antman, E.M.; et al. Enoxaparin vs unfractionated heparin in high-risk patients with non-STsegment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004, 292, 45–54. [Google Scholar]
- Eikelboom, J.W.; Mehta, S.R.; Anand, S.S.; Xie, C.; Fox, K.A.; Yusuf, S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006, 114, 774–782. [Google Scholar] [CrossRef]
- Bassand, J.P.; Hamm, C.W.; Ardissino, D.; et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. The Task Force for the Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of the European Society of Cardiology. Eur Heart J. 2007, 28, 1598–1660. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Adams, C.D.; Antman, E.M.; et al. ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction-Executive Summary A Report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-STElevation Myocardial Infarction) Developed in Collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007, 50, 652–726. [Google Scholar]
- Antman, E.M.; Hand, M.; Armstrong, P.W.; et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008, 51, 210–247. [Google Scholar] [CrossRef] [PubMed]

© 2008 by the author. Attribution Non-Commercial NoDerivatives 4.0.
Share and Cite
Bassand, J.-P. Fondaparinux: A New Antithrombotic Treatment Strategy in Venous Thromboembolism and Acute Coronary Syndromes. Cardiovasc. Med. 2008, 11, 278. https://doi.org/10.4414/cvm.2008.01350
Bassand J-P. Fondaparinux: A New Antithrombotic Treatment Strategy in Venous Thromboembolism and Acute Coronary Syndromes. Cardiovascular Medicine. 2008; 11(9):278. https://doi.org/10.4414/cvm.2008.01350
Chicago/Turabian StyleBassand, Jean-Pierre. 2008. "Fondaparinux: A New Antithrombotic Treatment Strategy in Venous Thromboembolism and Acute Coronary Syndromes" Cardiovascular Medicine 11, no. 9: 278. https://doi.org/10.4414/cvm.2008.01350
APA StyleBassand, J.-P. (2008). Fondaparinux: A New Antithrombotic Treatment Strategy in Venous Thromboembolism and Acute Coronary Syndromes. Cardiovascular Medicine, 11(9), 278. https://doi.org/10.4414/cvm.2008.01350




