Long-Term Follow-Up After Thoracic Radiotherapy: Symptomatic Heart Disease Is an Ominous Sign
Summary
Introduction
Methods
Study group
Echocardiography
ECG analysis
Coronary angiography
Definition of combined heart disease
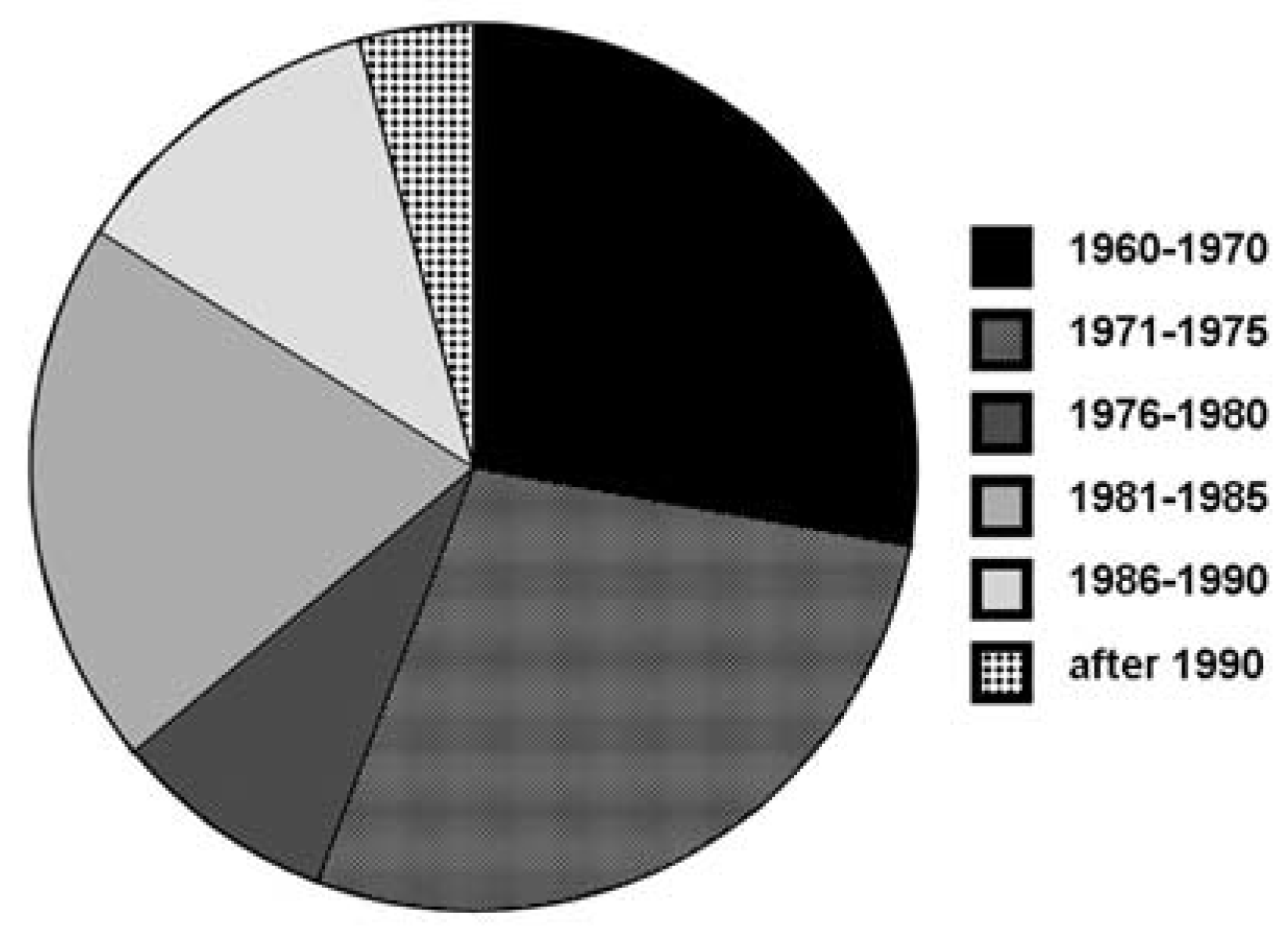
Data on radiation therapy
Heart surgery
Statistical analysis
Results
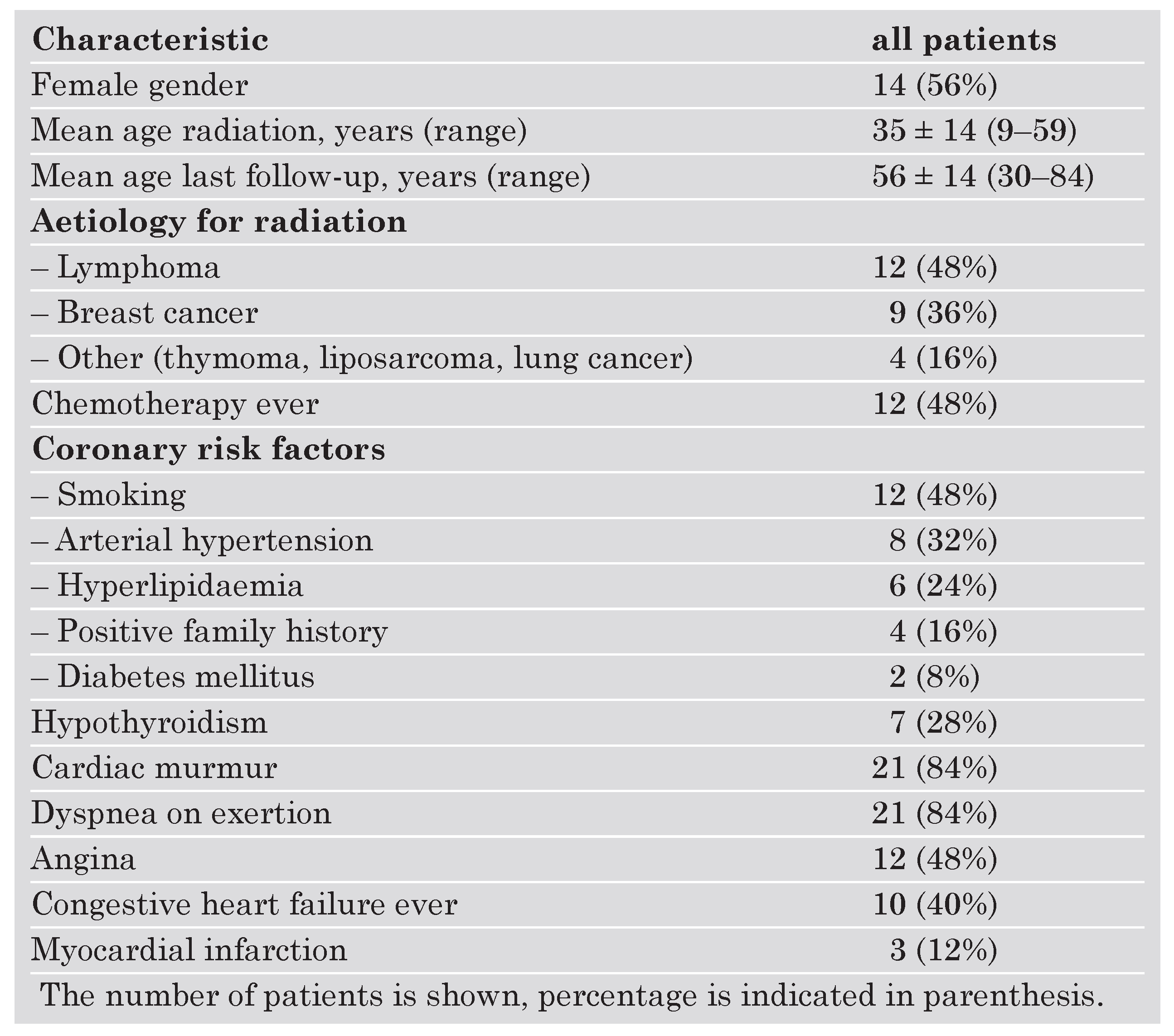
Clinical characteristics
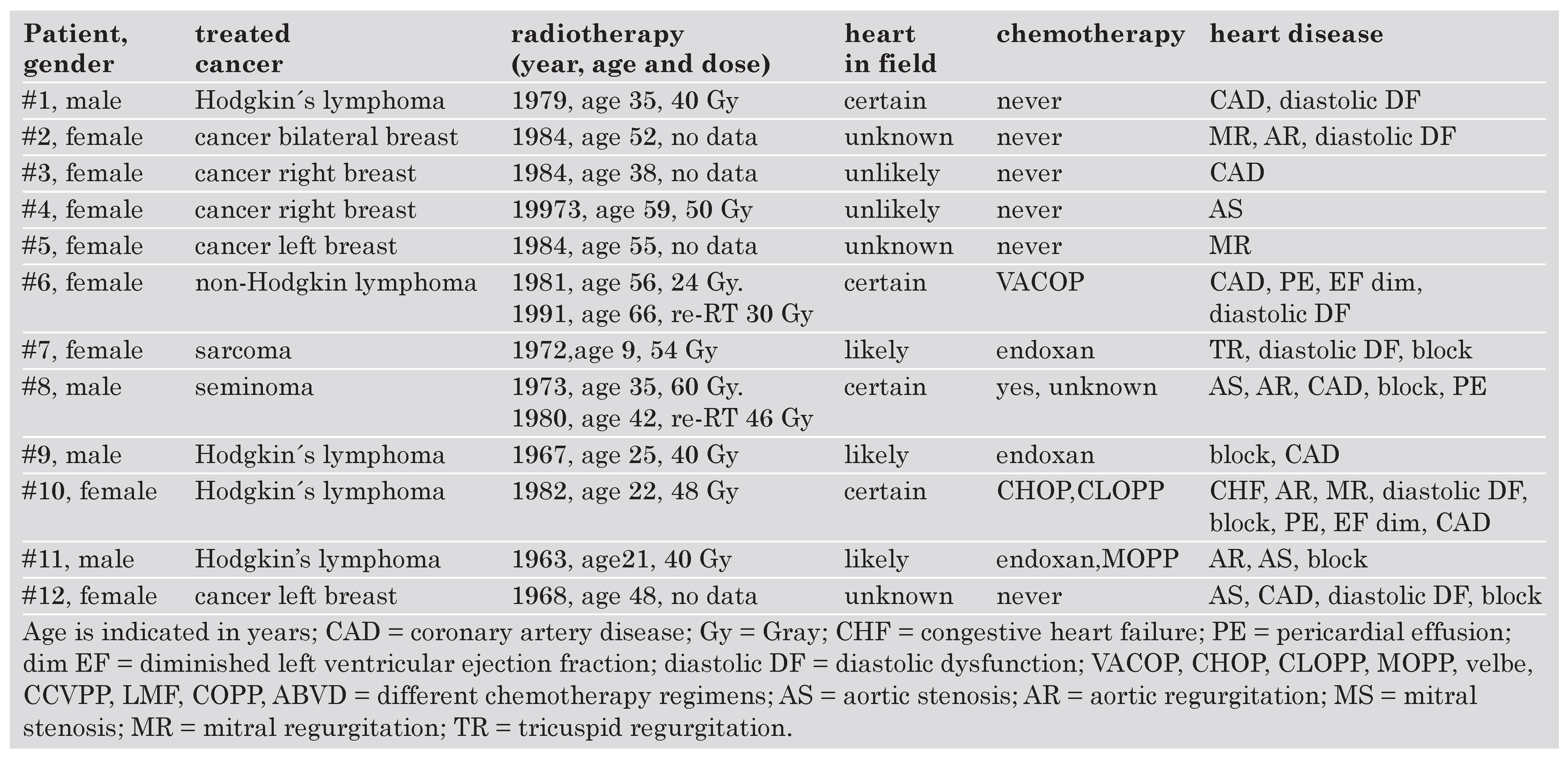
 |
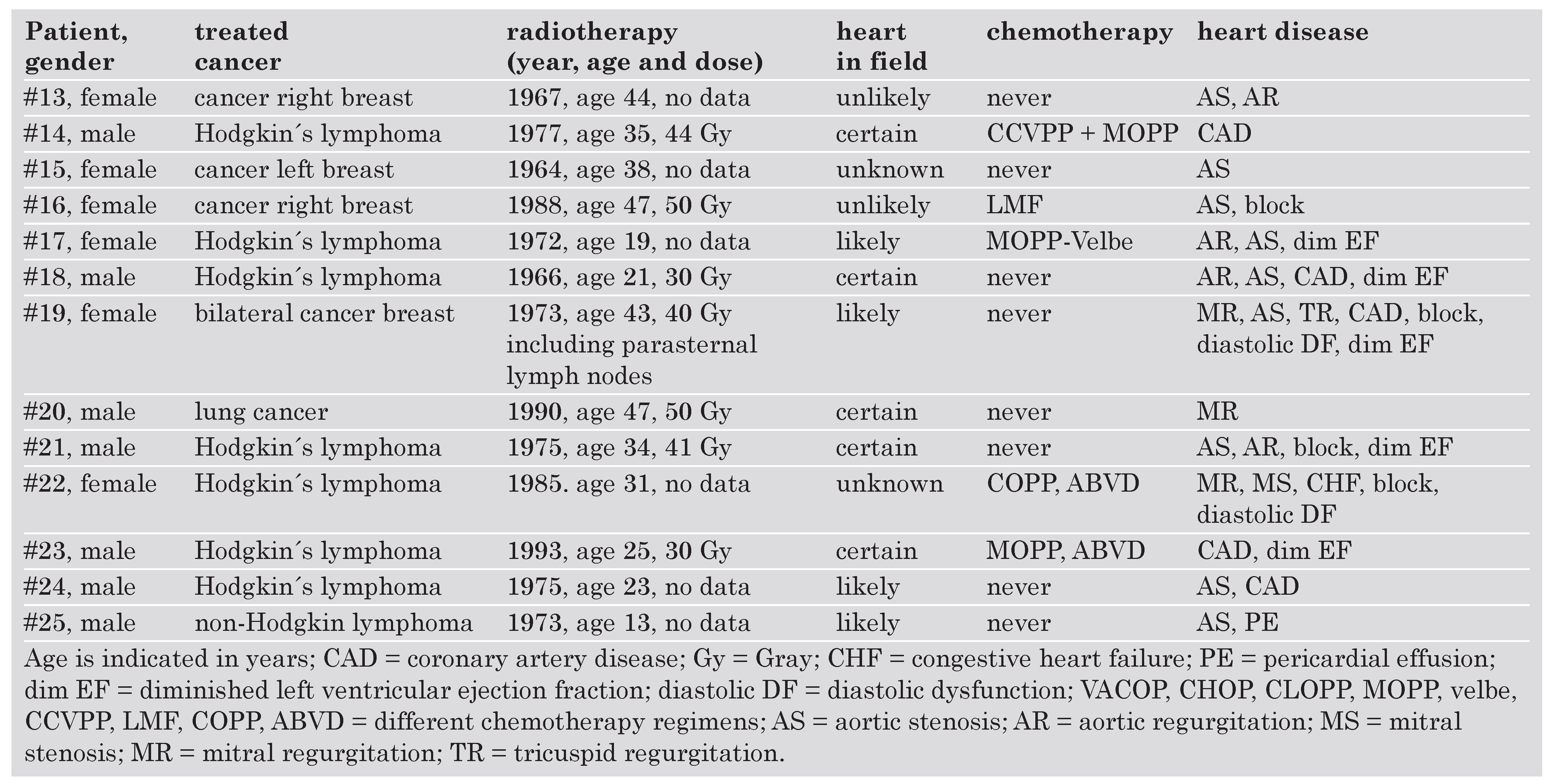
 |
 |
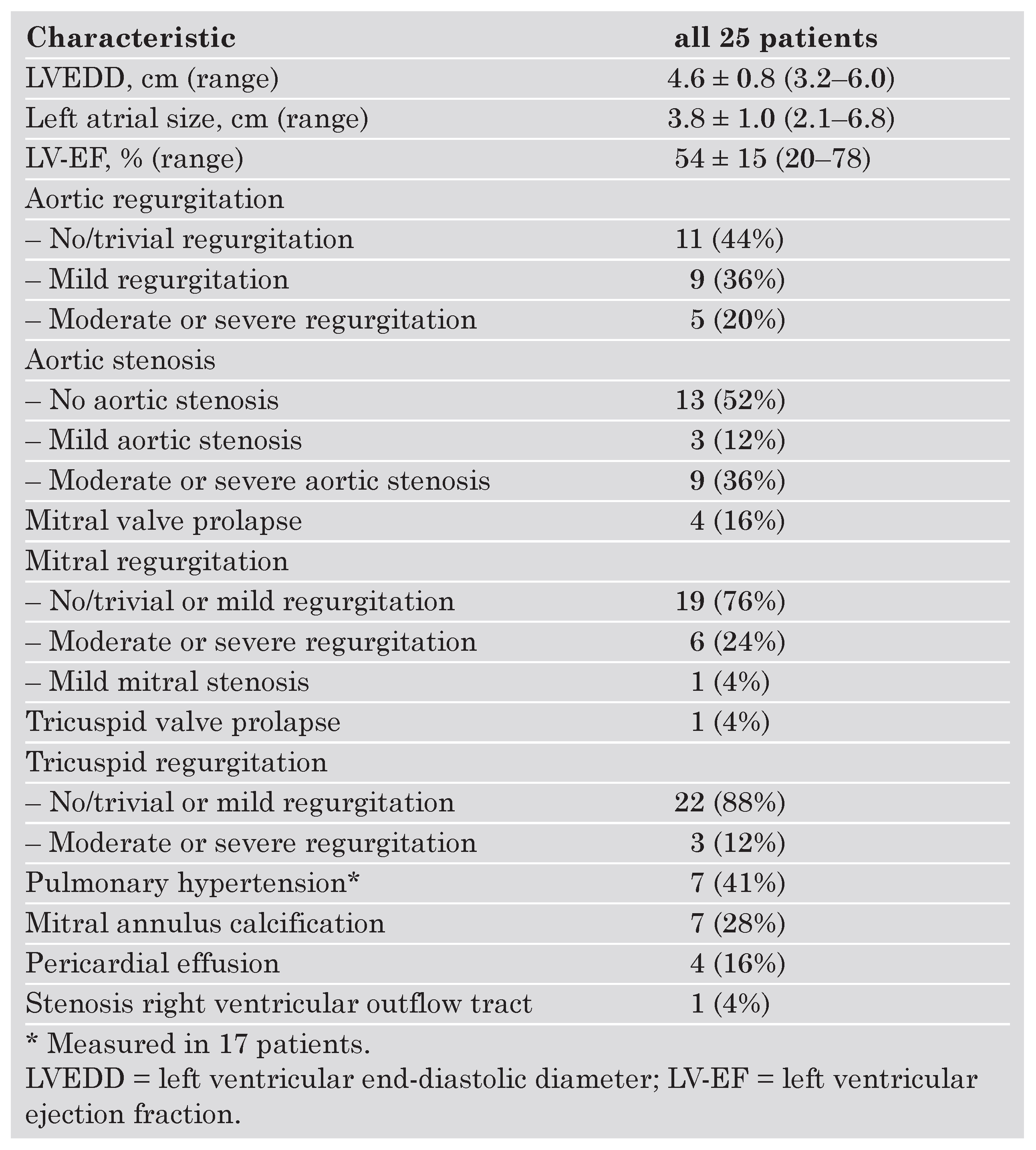
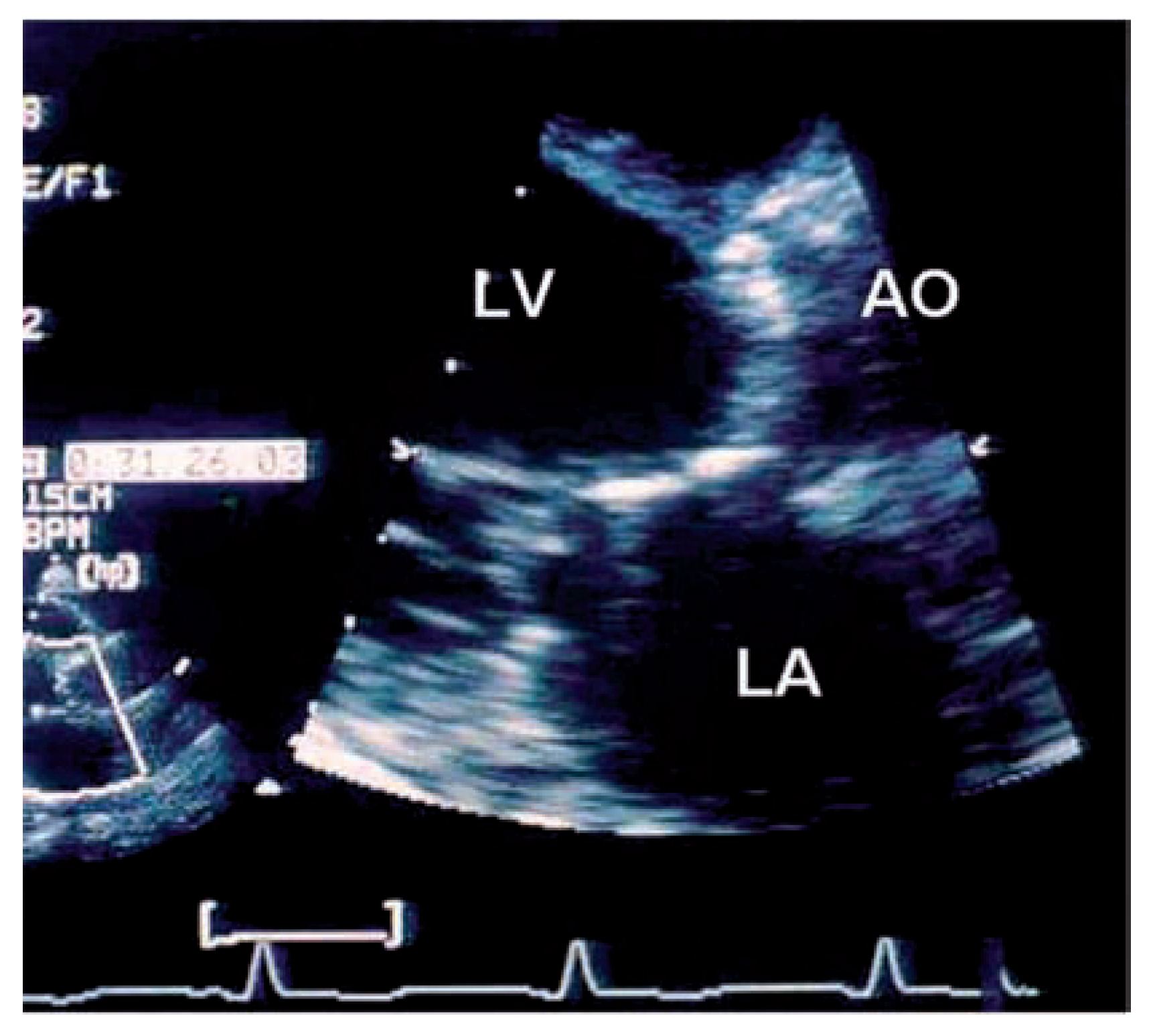
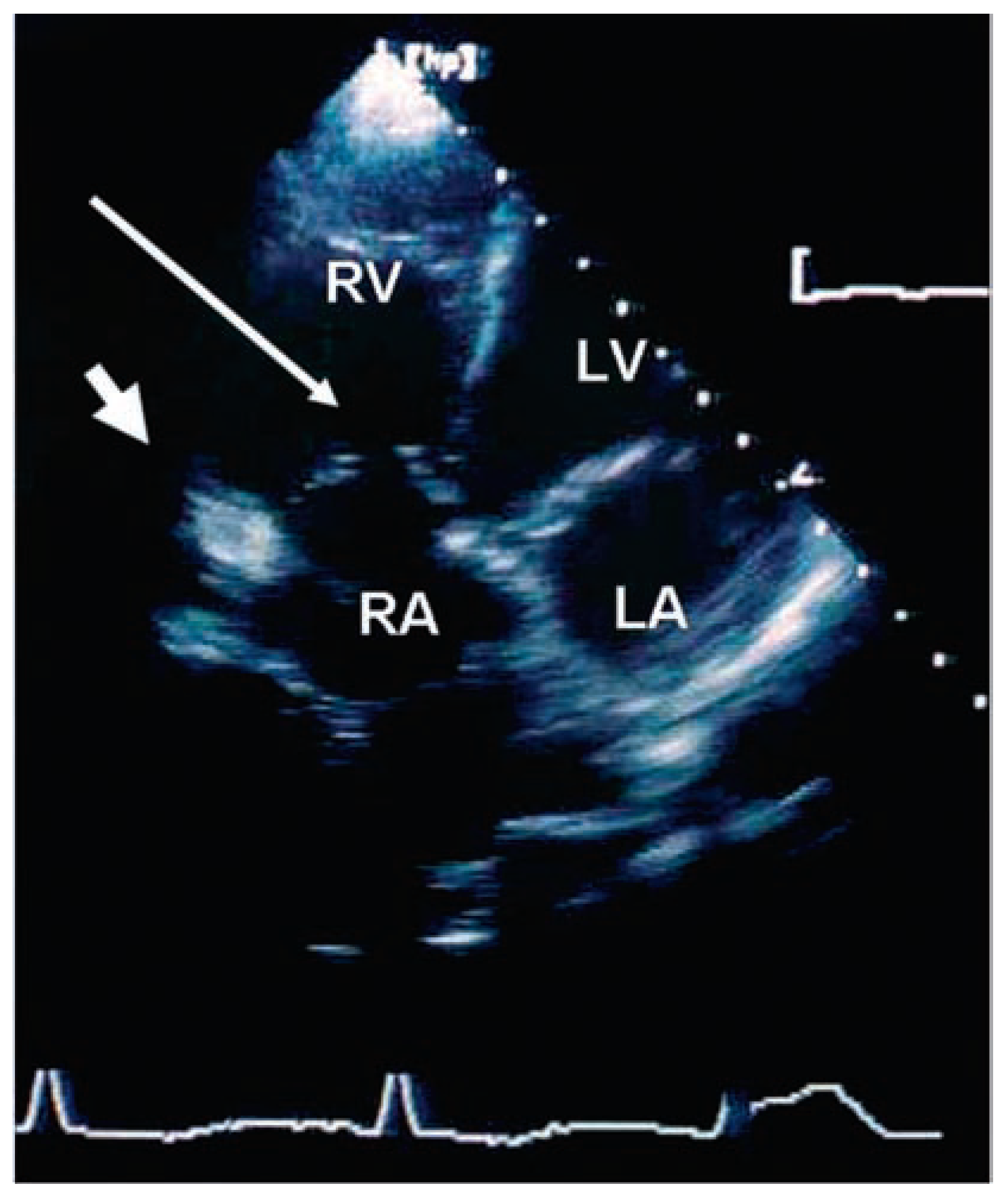
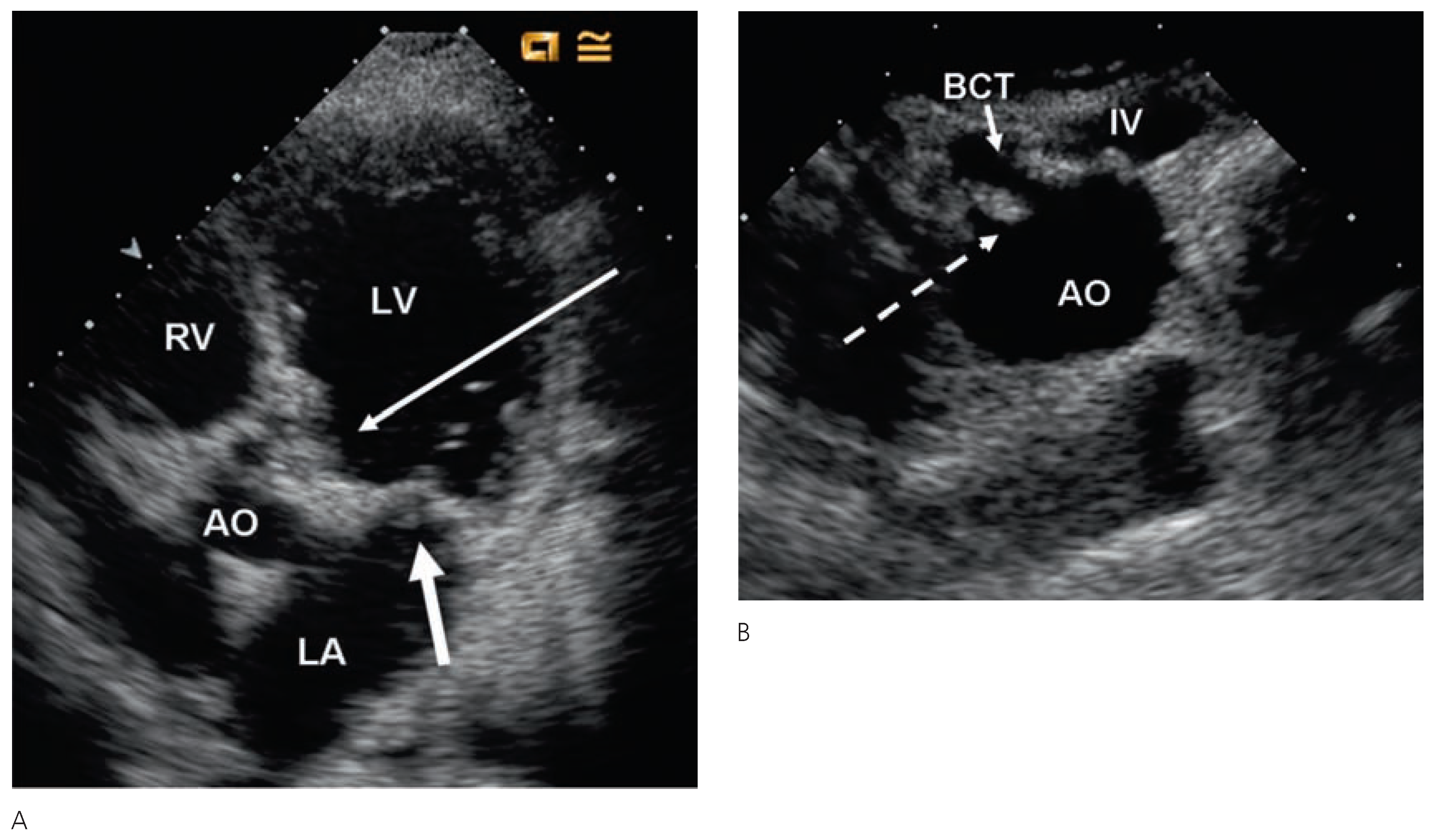
Echocardiographic findings
 |
Cardiac Interventions
 |
Follow-up and causes of death
Discussion
- Spectrum of radiation induced heart disease
Pericardial disease
Valvular heart disease
Coronary artery disease after radiation therapy
Conduction system disease
Surgery
Limitations
Conclusions
Conflicts of Interest
References
- Cohn, K.E.; Stewart, J.R.; Fajardo, L.F.; Hancock, E.W. Heart disease following radiation. Medicine (Baltimore). 1967, 46, 281–98. [Google Scholar] [CrossRef]
- Benoff, L.J.; Schweitzer, P. Radiation therapy-induced cardiac injury. Am Heart J. 1995, 129, 1193–6. [Google Scholar] [CrossRef]
- Hancock, S.L.; Donaldson, S.S.; Hoppe, R.T. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol. 1993, 11, 1208–15. [Google Scholar] [CrossRef] [PubMed]
- Applefeld, M.M. Radiation-induced cardiac disease. Am Heart J. 1996, 131, 1235–6, author reply 1237–8. [Google Scholar] [CrossRef]
- Brosius, F.C., 3rd; Waller, B.F.; Roberts, W.C. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med. 1981, 70, 519–30. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Hancock, S.L.; Lee, B.K.; Mariscal, C.S.; Schnittger, I. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003, 42, 743–9. [Google Scholar] [CrossRef]
- McEniery, P.T.; Dorosti, K.; Schiavone, W.A.; Pedrick, T.J.; Sheldon, W.C. Clinical and angiographic features of coronary artery disease after chest irradiation. Am J Cardiol. 1987, 60, 1020–4. [Google Scholar] [CrossRef] [PubMed]
- Dautzenberg, B.; Arriagada, R.; Chammard, A.B.; Jarema, A.; Mezzetti, M.; Mattson, K.; et al. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Groupe d’Etude et de Traitement des Cancers Bronchiques. Cancer. 1999, 86, 265–73. [Google Scholar] [CrossRef]
- Hooning, M.J.; Aleman, B.M.; van Rosmalen, A.J.; Kuenen, M.A.; Klijn, J.G.; van Leeuwen, F.E. Cause-specific mortality in long-term survivors of breast cancer: A 25-year follow-up study. Int J Radiat Oncol Biol Phys. 2006, 64, 1081–91. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Ribeiro, G.G. Mortality patterns over 34 years of breast cancer patients in a clinical trial of post-operative radiotherapy. Clin Radiol. 1989, 40, 204–8. [Google Scholar] [CrossRef]
- Prosnitz, R.G.; Chen, Y.H.; Marks, L.B. Cardiac toxicity following thoracic radiation. Semin Oncol. 2005, 32 (Suppl. 3), S71–80. [Google Scholar] [CrossRef]
- Glanzmann, C.; Huguenin, P.; Lutolf, U.M.; Maire, R.; Jenni, R.; Gumppenberg, V. Cardiac lesions after mediastinal irradiation for Hodgkin’s disease. Radiother Oncol. 1994, 30, 43–54. [Google Scholar] [CrossRef]
- Glanzmann, C.; Kaufmann, P.; Jenni, R.; Hess, O.M.; Huguenin, P. Cardiac risk after mediastinal irradiation for Hodgkin’s disease. Radiother Oncol. 1998, 46, 51–62. [Google Scholar] [CrossRef]
- Schiller, N.B.; Shah, P.M.; Crawford, M.; DeMaria, A.; Devereux, R.; Feigenbaum, H.; et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989, 2, 358–67. [Google Scholar] [CrossRef]
- Appleton, C.P.; Firstenberg, M.S.; Garcia, M.J.; Thomas, J.D. The echo-Doppler evaluation of left ventricular diastolic function. A current perspective. Cardiol Clin. 2000, 18, 513–46, ix. [Google Scholar] [CrossRef]
- Hatle, L.K.; Appleton, C.P.; Popp, R.L. Differentiation of constrictive pericarditis and restrictive cardiomyopathy by Doppler echocardiography. Circulation. 1989, 79, 357–70. [Google Scholar] [CrossRef]
- Bonow, R.O.; Carabello, B.A.; Chatterjee, K.; de Leon, A.C., Jr.; Faxon, D.P.; Freed, M.D.; et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006, 48, e1–148. [Google Scholar] [CrossRef] [PubMed]
- Connolly, H.M.; Crary, J.L.; McGoon, M.D.; Hensrud, D.D.; Edwards, B.S.; Edwards, W.D.; et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997, 337, 581–8. [Google Scholar] [CrossRef] [PubMed]
- Weissman, N.J.; Tighe, J.F., Jr.; Gottdiener, J.S.; Gwynne, J.T. An assessment of heart-valve abnormalities in obese patients taking dexfenfluramine, sustained-release dexfenfluramine, or placebo. Sustained-Release Dexfenfluramine Study Group. N Engl J Med. 1998, 339, 725–32. [Google Scholar] [CrossRef]
- Hancock, E.W. Subacute effusive-constrictive pericarditis. Circulation. 1971, 43, 183–92. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, R.D.; Rosenthal, A.; Cassady, R.; Jaffe, N.; Nadas, A.S. Constrictive pericarditis in childhood due to mediastinal irradiation. Circulation. 1974, 50, 1033–9. [Google Scholar] [CrossRef]
- Hancock, S.L.; Tucker, M.A.; Hoppe, R.T. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA. 1993, 270, 1949–55. [Google Scholar] [CrossRef]
- Pierga, J.Y.; Maunoury, C.; Valette, H.; Socie, G.; Girinski, T.; Tchernia, G.; et al. Follow-up thallium-201 scintigraphy after mantle field radiotherapy for Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 1993, 25, 871–6. [Google Scholar] [CrossRef]
- Amromin, G.D.; Gildenhorn, H.L.; Solomon, R.D.; Nadkarni, B.B. The Synergism Of X-Irradiation And Cholesterol-Fat Feeding On The Development Of Coronary Artery Lesions. J Atheroscler Res. 1964, 4, 325–34. [Google Scholar] [CrossRef]
- Hancock, S.L.; Cox, R.S.; McDougall, I.R. Thyroid diseases after treatment of Hodgkin’s disease. N Engl J Med. 1991, 325, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Gottdiener, J.S.; Katin, M.J.; Borer, J.S.; Bacharach, S.L.; Green, M.V. Late cardiac effects of therapeutic mediastinal irradiation. Assessment by echocardiography and radionuclide angiography. N Engl J Med. 1983, 308, 569–72. [Google Scholar] [CrossRef] [PubMed]
- Pohjola-Sintonen, S.; Totterman, K.J.; Salmo, M.; Siltanen, P. Late cardiac effects of mediastinal radiotherapy in patients with Hodgkin’s disease. Cancer. 1987, 60, 31–7. [Google Scholar] [CrossRef]
- Applefeld, M.M.; Cole, J.F.; Pollock, S.H.; Sutton, F.J.; Slawson, R.G.; Singleton, R.T.; et al. The late appearance of chronic pericardial disease in patients treated by radiotherapy for Hodgkin’s disease. Ann Intern Med. 1981, 94, 338–41. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; McGregor, C.G.; Danielson, G.K.; Daly, R.C.; Dearani, J.A.; Mullany, C.J.; et al. Valvular heart operation in patients with previous mediastinal radiation therapy. Ann Thorac Surg. 2001, 71, 1880–4. [Google Scholar] [CrossRef]
- Veeragandham, R.S.; Goldin, M.D. Surgical management of radiation-induced heart disease. Ann Thorac Surg. 1998, 65, 1014–9. [Google Scholar] [CrossRef]
- Crestanello, J.A.; McGregor, C.G.; Danielson, G.K.; Daly, R.C.; Dearani, J.A.; Orszulak, T.A.; et al. Mitral and tricuspid valve repair in patients with previous mediastinal radiation therapy. Ann Thorac Surg. 2004, 78, 826–31, discussion 826–31. [Google Scholar] [CrossRef] [PubMed]
© 2007 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Attenhofer Jost, C.H.; Huguenin, P.; Prêtre, R.; Turina, J.; Seifert, B.; Amann, F.W.; Glanzmann, C.; Vogt, P.R.; Jenni, R. Long-Term Follow-Up After Thoracic Radiotherapy: Symptomatic Heart Disease Is an Ominous Sign. Cardiovasc. Med. 2007, 10, 387. https://doi.org/10.4414/cvm.2007.01285
Attenhofer Jost CH, Huguenin P, Prêtre R, Turina J, Seifert B, Amann FW, Glanzmann C, Vogt PR, Jenni R. Long-Term Follow-Up After Thoracic Radiotherapy: Symptomatic Heart Disease Is an Ominous Sign. Cardiovascular Medicine. 2007; 10(12):387. https://doi.org/10.4414/cvm.2007.01285
Chicago/Turabian StyleAttenhofer Jost, Christine H., Pia Huguenin, René Prêtre, Juraj Turina, Burkhardt Seifert, F. Wolfgang Amann, Christoph Glanzmann, Paul R. Vogt, and Rolf Jenni. 2007. "Long-Term Follow-Up After Thoracic Radiotherapy: Symptomatic Heart Disease Is an Ominous Sign" Cardiovascular Medicine 10, no. 12: 387. https://doi.org/10.4414/cvm.2007.01285
APA StyleAttenhofer Jost, C. H., Huguenin, P., Prêtre, R., Turina, J., Seifert, B., Amann, F. W., Glanzmann, C., Vogt, P. R., & Jenni, R. (2007). Long-Term Follow-Up After Thoracic Radiotherapy: Symptomatic Heart Disease Is an Ominous Sign. Cardiovascular Medicine, 10(12), 387. https://doi.org/10.4414/cvm.2007.01285




