Abstract
Palmitoylethanolamide (PEA) is a naturally occurring fatty acid amide and an endocannabinoid-related lipid that has been extensively studied for its analgesic, immunomodulatory, antimicrobial, and anti-inflammatory properties. It has demonstrated efficacy in various applications and is currently utilized as a nutraceutical for its antinociceptive, neuroprotective, and immunomodulatory effects, particularly in supporting brain and joint health and in mitigating inflammatory processes. Background/Objectives: Despite its significant therapeutic potential, the clinical effectiveness of PEA is limited by its poor water solubility and, consequently, low oral bioavailability. Additionally, degradation in the acidic gastrointestinal environment further compromises its absorption. To address these challenges, several technological strategies have been explored to improve its pharmacokinetic profile, including conventional micronization and ultra-micronization techniques. The objective of this study was to characterize a novel liposomal formulation based on PEA and evaluate its intestinal permeation and absorption. Methods: Comparative permeation studies of PEA were conducted using ex vivo models to evaluate its absorption characteristics across gastrointestinal mucosae. The experiments were performed in a Franz diffusion cell system using a porcine colon mucosa in two physiologically relevant media: Simulated Gastric Fluid (SGF) and Fasted State Simulated Intestinal Fluid (FaSSIF). Results: Liposomal PEA showed a more efficient and continuous release over time, reaching higher concentrations of PEA permeated through the membrane. Conclusions: Our findings demonstrate a significant improvement in PEA’s permeability and absorption in an ex vivo simulated gastrointestinal environment. Liposomal PEA appears to be more affine to biological membranes. These results suggest that liposomal PEA may represent a promising therapeutic strategy for managing chronic pain and inflammatory conditions such as chronic pelvic pain.
1. Introduction
Palmitoylethanolamide (PEA), originally discovered in the 1950s [1], is an endogenous fatty acid amide that has recently gained significant attention for its therapeutic potential across a variety of health conditions [2]. PEA is known for its anti-inflammatory, analgesic, and neuroprotective properties. PEA’s main mechanism of action is through the modulation of the endocannabinoid system, and through interactions with peroxisome proliferator-activated receptor-alpha (PPAR-α) [3]. One of the most compelling benefits of PEA is its anti-inflammatory effect. Studies have demonstrated that PEA can effectively reduce chronic pain and inflammation by inhibiting the release of pro-inflammatory cytokines and mast cell degranulation [4]. In models of neuropathic pain, PEA administration led to a significant decrease in pain perception, underscoring its potential as an alternative to traditional pain medications [2]. PEA also exhibits neuroprotective properties, making it a promising candidate for treating neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease [5]. Research indicates that PEA can mitigate oxidative stress and neuroinflammation, thereby slowing the progression of these debilitating conditions [6]. Additionally, its ability to cross the blood–brain barrier allows for direct action on central nervous system pathologies [7]. The role of PEA in modulating the immune response further expands its therapeutic applications. By influencing macrophage activity and promoting the resolution of inflammation, PEA helps maintain immune homeostasis [8]. Clinical trials have shown that PEA supplementation can improve symptoms in patients with conditions such as multiple sclerosis and inflammatory bowel disease [9].
Chronic pain affects millions worldwide and is often resistant to conventional treatment. Through its action on cannabinoid receptors and its ability to reduce neuroinflammation, PEA offers a novel approach to pain management [10]. Studies have shown that PEA can alleviate pain in conditions like fibromyalgia and diabetic neuropathy, improving the quality of life for many patients [11].
The safety profile of PEA further enhances its appeal as a therapeutic agent. Unlike many pharmaceuticals, PEA is well-tolerated with minimal side effects, even at high doses [12]. This safety, combined with its efficacy, positions PEA as a favorable option for long-term use in managing chronic conditions [10]. In summary, PEA stands out as a multifaceted molecule with significant therapeutic potential. Its anti-inflammatory, analgesic, and neuroprotective properties, coupled with a favorable safety profile, make it a promising candidate for treating a wide range of conditions, from chronic pain to neurodegenerative diseases [7]. As research continues to unravel the mechanisms and benefits of PEA, it holds the promise of improving the lives of many patients suffering from chronic and inflammatory diseases.
PEA exerts its therapeutic effects not only through interactions with the endocannabinoid system but also by modulating transient receptor potential (TRP) channels. Specifically, PEA influences cannabinoid receptors indirectly, enhancing the effects of anandamide by inhibiting its degradation, thus potentiating the overall endocannabinoid tone [7]. This interaction primarily involves the CB2 receptors, which are implicated in anti-inflammatory and analgesic processes [10]. In addition to cannabinoid receptors, PEA also targets TRP channels, particularly TRPV1 (vanilloid receptor 1), which plays a crucial role in pain and inflammation pathways [13]. TRPV1 activation can lead to the desensitization of pain pathways, offering relief from chronic pain conditions. PEA modulates these channels, providing a dual mechanism of action in pain management by both enhancing endocannabinoid activity and directly interacting with TRP channels [14]. This multifaceted approach underscores PEA’s potential as a versatile therapeutic agent in treating pain and inflammation through multiple molecular targets.
The bioavailability of PEA is a critical factor influencing its therapeutic efficacy. PEA, being a lipid molecule, has relatively low solubility in water, which can limit its absorption and bioavailability when administered orally [14]. Studies have shown that standard formulations of PEA may result in suboptimal plasma concentrations, which necessitate the development of improved delivery systems to enhance its absorption and therapeutic potential [7]. For instance, micronization and ultramicronization techniques have been employed to reduce the particle size of PEA, thereby increasing its surface area and improving its dissolution rate and bioavailability [10]. To further enhance the bioavailability of PEA, several innovative formulations have been developed. In this study, we evaluated and characterized a novel liposomal-based formulation of PEA and compared the solubility, absorption, and permeation with non-liposomal formulations of PEA.
2. Materials and Methods
2.1. Reagents
The standard PEA used for the calibration curve was obtained from Sigma-Aldrich, (Milan, Italy). Native-PEA (herein referred to as PEA, 80 mesh, particle size 175 µm) and liposomal PEA were generously provided by Lipo Science Laboratory (Oosterhout, The Netherlands). Liposomal PEA formulation is based on PEA and soy lecithin phospholipids as the lipid component. The liposomes were prepared using a methodology that ensures effective incorporation of PEA at a concentration >60% into the liposomal bilayer. Micronized PEA (fine PEA) (particle size 90% ~10 µm) was kindly supplied by Gonmisol (Madrid, Spain). These three PEA raw materials (PEA, liposomal PEA, and fine PEA) were compared for their absorption and permeation characteristics using an ex vivo gastrointestinal permeation model. All compounds were solubilized directly in phosphate-buffered saline (PBS) prior to testing.
2.2. Dissolution Test
The in vitro release test of PEA, simulating gastric conditions, was conducted using a dissolution test at 37 °C under sink conditions, in accordance with the guidelines of the United States Pharmacopeia [15].
2.3. Calibration Curve
A stock solution of 1 mg/mL of standard PEA in PBS/EtOH (1:1 v/v) was prepared and stirred for two hours. The solution was then filtered through a 0.2 μm cellulose filter. Samples with concentrations between 0.01 and 0.1 mg/mL were prepared by serial dilutions and analyzed by UV spectrophotometry (Jasco V-760, 201 nm, Jasco UK Limited, West Yorkshire, UK).
2.4. Preparation of Mediums
Physiologically relevant mediums: Simulated Gastric Fluid (SGF), representing the acidic environment of the stomach, and Fasted State Simulated Intestinal Fluid (FaSSIF), mimicking the conditions of the small intestine under fasting conditions, were prepared according to Anissa [16], with slight modifications, detailed specifically as follows.
2.4.1. Simulated Gastric Fluid (SGF)
Simulated Gastric Fluid (SGF) medium was prepared by dissolving 1.6 g of pepsin and 1.0 g of NaCl in 1 L of deionized water, stirring for 10 min. Next, 3.5 mL of HCl 6 M was added to the mixture, stirring for 30 min. Subsequently, the pH of the mixture was measured and adjusted using 2 M NaOH to attain a pH of 2.0. The mixture was finally sonicated for 10 min to obtain a homogeneous solution.
2.4.2. Fasted State Simulated Intestinal Fluid (FaSSIF)
Fasted State Simulated Intestinal Fluid (FaSSIF) medium was prepared by dissolving 4.43 g of NaH2PO4 and 2.265 g of NaCl in 1 L of deionized water, stirring for 10 min. Subsequently, 0.24 g of soy lecithin was added to the mixture, and the solution was stirred for 30 min. Following this, 0.8 g of Sodium Taurocholate was introduced to the mixture, which was then stirred for an additional 20 min to achieve homogeneity. The pH of the solution was measured and adjusted using 2 M NaOH to reach a pH of 6.8. The solution was then sonicated for 10 min until a homogeneous solution was obtained.
2.5. Experimental Protocol
The experimental protocol was structured into 2 phases, aimed at evaluating the effects of the samples in terms of permeation and absorption of PEA derived from the different raw materials. Permeation studies were performed with a Franz cell and porcine colon mucosae within an orbiting incubator at 37 °C (±1 °C). Five mL of DPBS/EtOH (50:50) were placed in the acceptor compartment, and 1 mL of 100 mg/mL analyte in SGF was placed in the donor compartment.
In the first phase of the experiment, the selected concentrations of the three types of PEA raw materials (PEA, fine PEA, and liposomal PEA) were used to test the effects on the Franz cell and porcine colon mucosae. At the same time, the permeability test was performed in a time-course study.
Two-hundred microliter samples from the acceptor compartment were withdrawn and replaced with the same amount of solvent. The sample was analyzed by UV spectrophotometry, UV Jasco V-760 (cuvette 1 mL, optical path 10 mm, 210 nm). After two hours, the donor compartment medium was replaced with FaSSIF (pH 6.8), and the experiment was carried out for 22 h. Samples were withdrawn and read every hour for 10 h and after 24 h.
In the second phase, the mucosae were placed in 5 mL of octanol, stirring for 72 h. Then, the solvent was filtered through a 0.2 μm filter, and the sample was read by UV spectroscopy.
2.6. Scanning Electron Microscopy
Samples were observed by SEM using a Phenom ProXSEM (Alfatest, Cernusco sul Naviglio, Italy). The SEM analysis was performed with an electron-accelerating voltage of 10 kV. Samples were prepared by depositing the samples on a double-sided adhesive tape previously applied on a stainless-steel stub and dried under vacuum (0.1 Torr) before analysis. All the SEM analyses were performed at 25.0 °C ± 0.1 °C.
2.7. Statistical Analysis
The data were analyzed using one-way ANOVA to determine whether statistically significant differences existed among the three PEA raw materials (PEA, liposomal PEA, and fine PEA). To assess whether the differences in AUC between the three groups are statistically significant, a one-way ANOVA was performed using simulated replicates based on the AUC values. Due to the lack of replicate AUC measurements, a formal post hoc test such as Tukey’s HSD could not be performed. Instead, independent t-tests were performed between each pair of formulations (liposomal PEA vs. PEA, liposomal PEA vs. fine PEA, and PEA vs. fine PEA) to explore pairwise differences. For PEA permeation studies, two-way ANOVA and post hoc comparisons were evaluated.
3. Results
3.1. Evaluation of the Dissolution of PEA
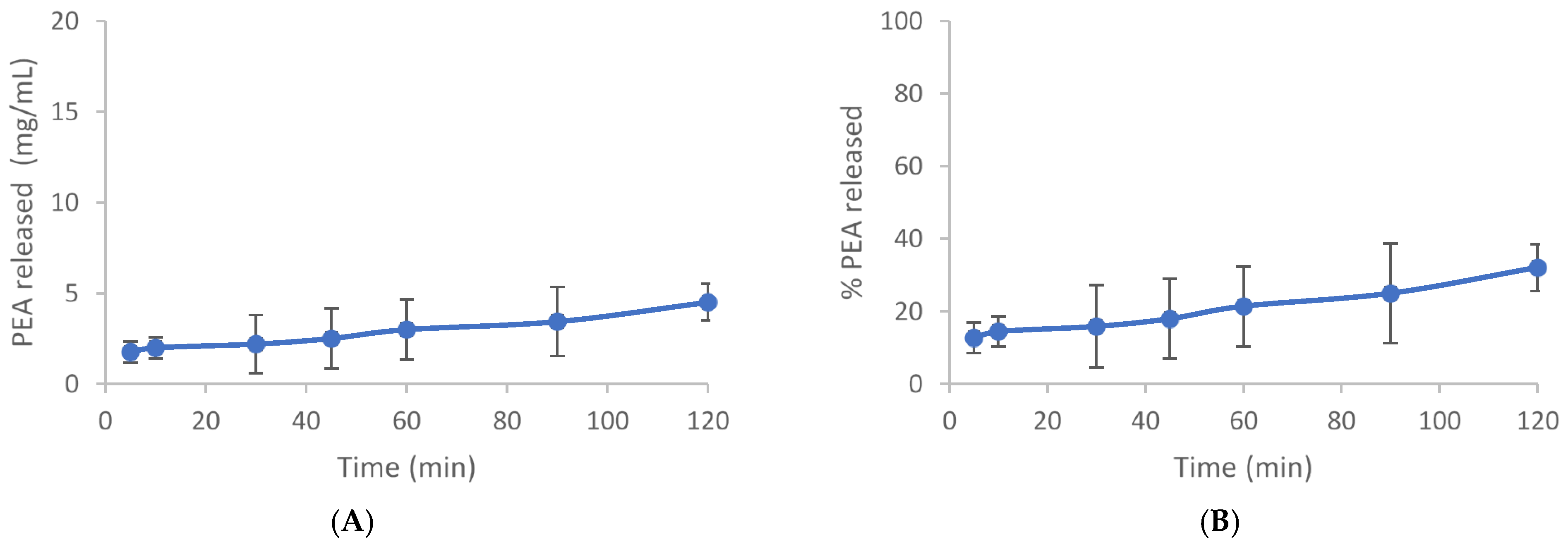
The dissolution test was carried out to provide valuable insights into the behavior of PEA in a controlled medium. The use of a closed system with a blade at 60 rpm ensures consistency in agitation, while maintaining sink conditions prevents saturation effects that could alter dissolution behavior. The test was carried out under sink conditions, using an extract quantity equal to 33% of the solubility of the active in the dissolution medium and in the selected conditions (46.2 mg/L). Fourteen milligrams of PEA were dispersed in 1.0 L of the mixture of gastric fluid/ethanol in the 60/40 ratio at 37 °C 0.1. At set times (5, 15, 30, 45, 60, 90, 120 min), 5 mL aliquots were taken and refilled with an equal volume of thermostatic fluid. Samples were filtered with 0.22-micron porosity filters to remove any suspended particles, diluted with acetonitrile, and analyzed by HPLC. The test was conducted in duplicate. The resulting release profiles expressed both in mg/L and in percentage as a function of time are shown in Figure 1A,B and Table A1 and Table A2. The release profile indicates that after 120 min, only 32% of Cmax is achieved, suggesting limited dissolution within this timeframe under the specified conditions (Figure 1).
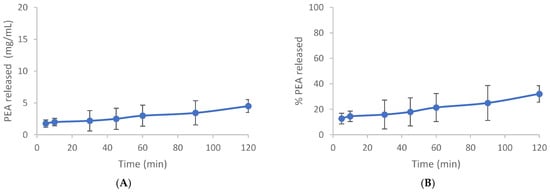
Figure 1.
Time course dissolution test. (A) The release profile of PEA is expressed in mg/mL. (B) Release % of PEA. Mean values expressed in mg/mL or % ± SD.
3.2. Evaluation of Permeability in an Ex Vivo Gastrointestinal Model
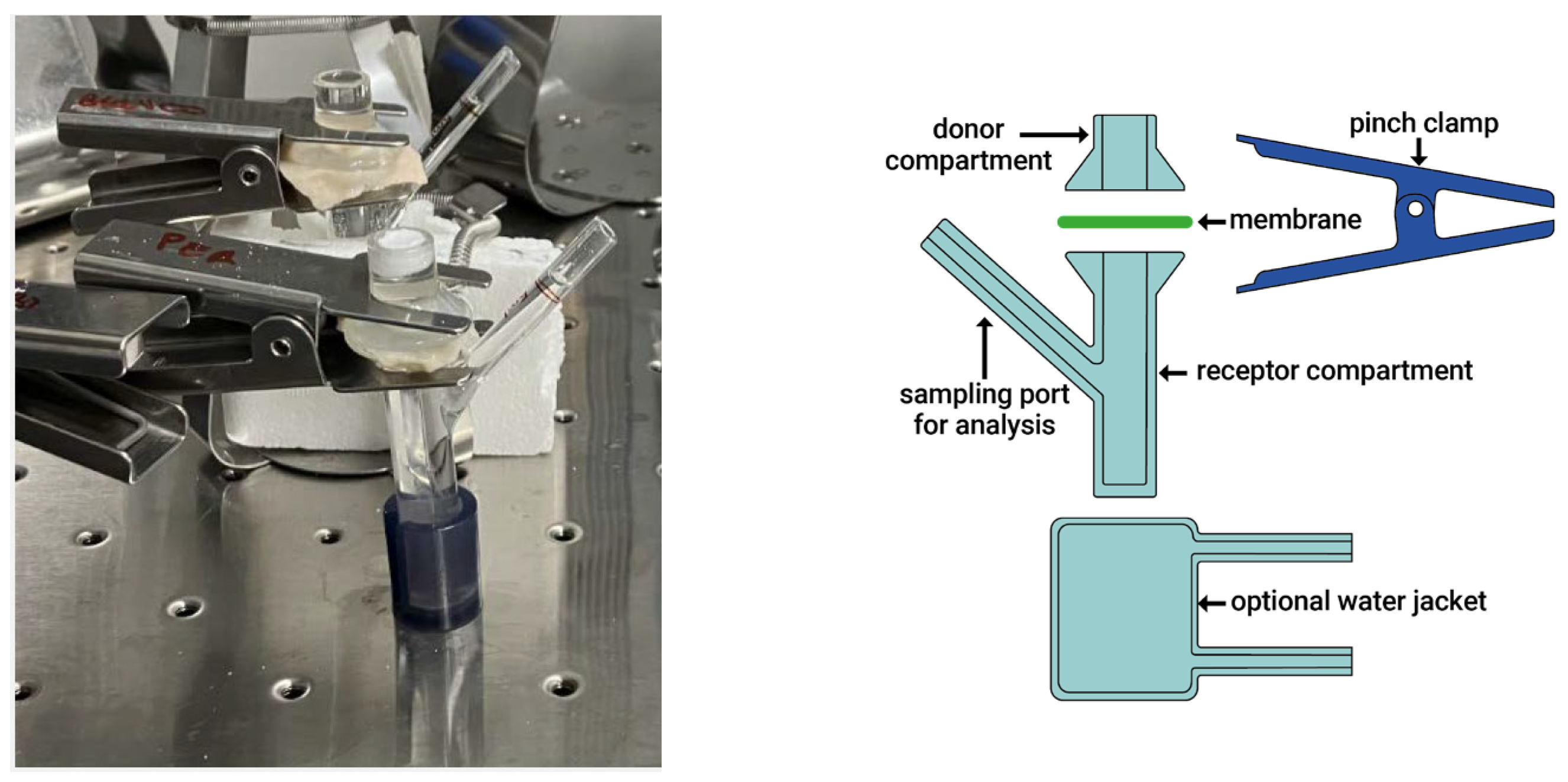
Investigations were carried out using an ex vivo gastrointestinal model designed to replicate the functional complexity of the intestinal environment. A 24 h gastro-enteric permeation study was conducted using Simulated Gastric Fluid (SGF) and simulated intestinal fluid (FaSSIF) within a Franz diffusion cell system, allowing for the evaluation of molecular permeability across porcine biological membranes. The Franz cell (Figure 2) system is widely used in in vitro diffusion studies to assess membrane permeation and drug absorption potential.
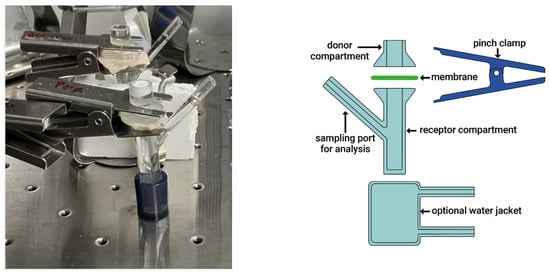
Figure 2.
Photograph and image of the Franz cell system.
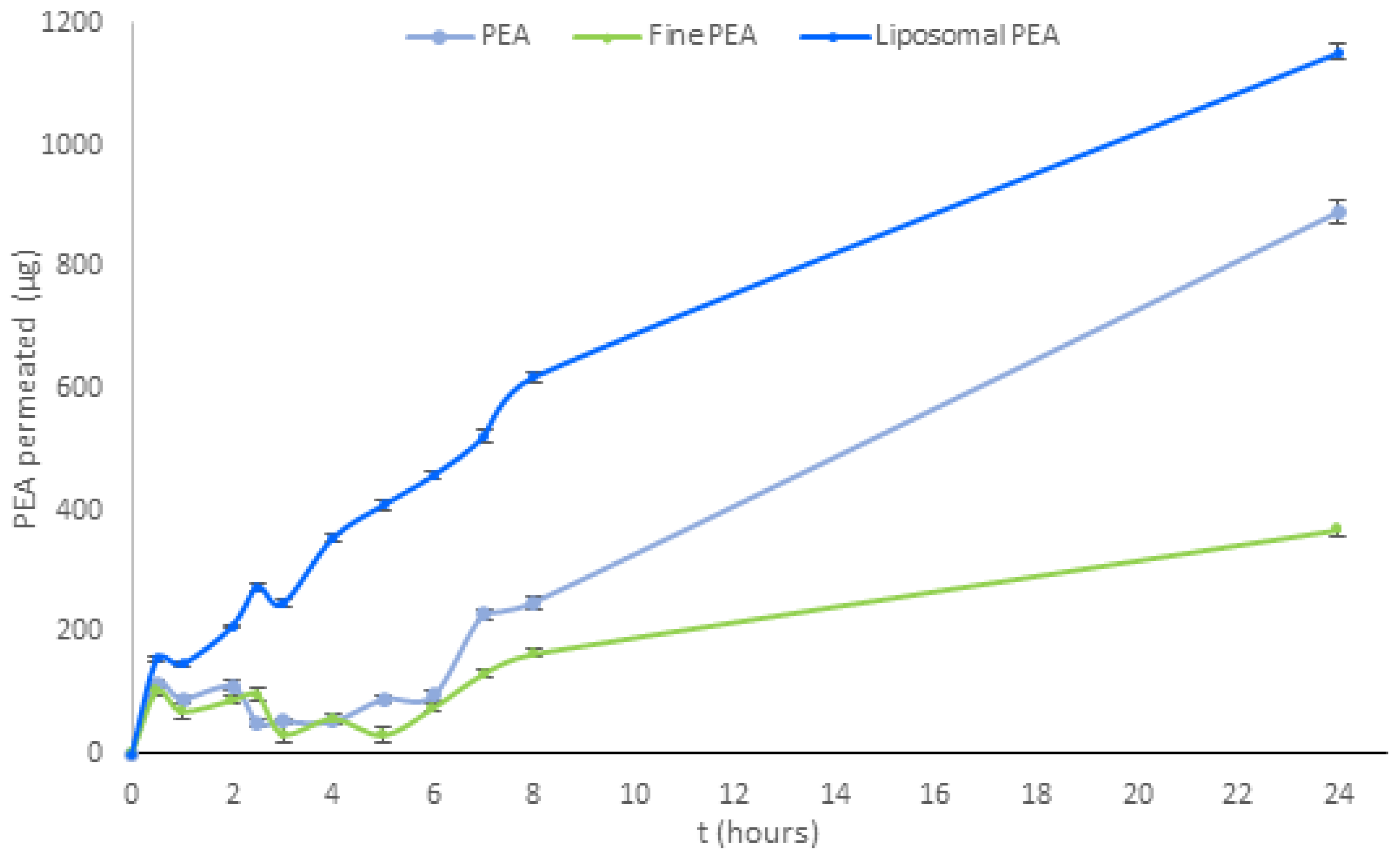
Subsequent investigations were conducted on three PEA raw materials (PEA, liposomal PEA, and fine PEA) and compared for their permeation at 0′, 30 min, 1-2-2,5-3-4-5-6-7-8, and -24 h. As demonstrated in Figure 3, liposomal PEA exhibited a greater time-dependent absorption from 30 min to 24 h compared to the other samples. Specifically, it is shown that liposomal PEA demonstrated good absorption, with sustained absorption throughout the experiment compared to the two types of PEAs that were evaluated (Figure 3 and Table A3).
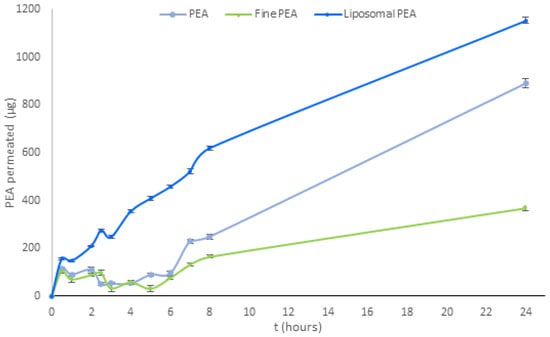
Figure 3.
Analysis of permeation of PEA over time for three formulations (PEA, liposomal PEA, and fine PEA). Data are expressed as permeated concentrations (µg) means ± SD. Statistical analysis was performed using two-way ANOVA, followed by Tukey’s HSD post hoc comparisons. Significant differences (p < 0.001) were observed between PEA and both liposomal PEA and fine PEA, indicating superior permeation performance of the liposomal formulation. p < 0.05, liposomal PEA vs. PEA (Table A4).
Liposomal PEA has demonstrated a total release of 1152.56 μg after 24 h, PEA reaches 891.76 μg, and fine PEA only 370.43 μg. Liposomal technology demonstrated more efficacious and continuous release of PEA over time in the intestinal environment. Throughout the analysis period, the liposomal PEA obtained a better absorption profile compared to the non-liposomal formulations, suggesting that the combination of PEA with liposomes is responsible for the increased permeability. PEA and fine PEA recorded permeability data for less than 23% and 67%, respectively, compared to liposomal PEA. Of note, fine PEA showed a reduced permeability compared to the other two.
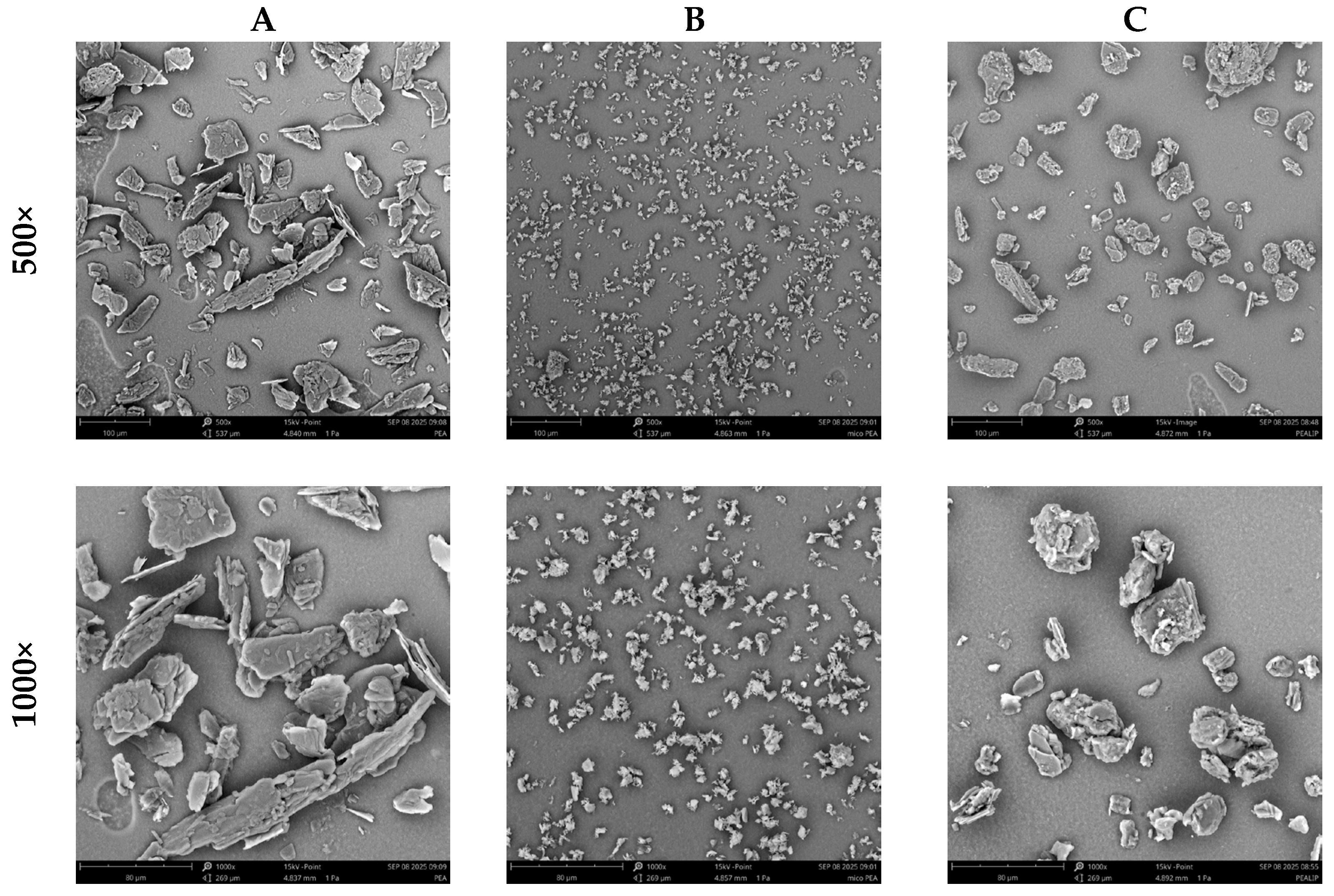
To obtain a deeper understanding of permeability differences, Scanning Electron Microscopy (SEM) morphological analysis was performed on three PEA formulations: PEA, fine PEA, and liposomal PEA. SEM imaging revealed distinct structural characteristics (Figure 4). These morphological traits could be directly relevant to the interaction with biological membranes and may significantly influence absorption and bioavailability.
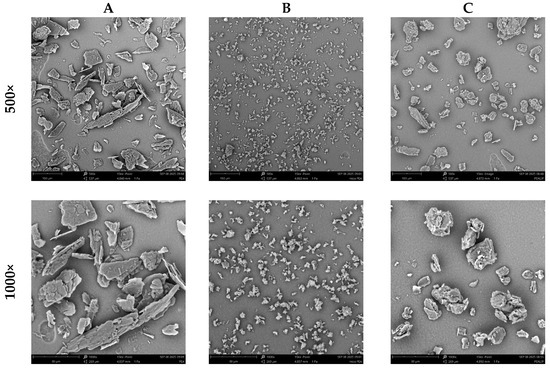
Figure 4.
SEM analysis of (A) PEA, (B) Fine PEA, and (C) = liposomal PEA at 500× and 1000×.
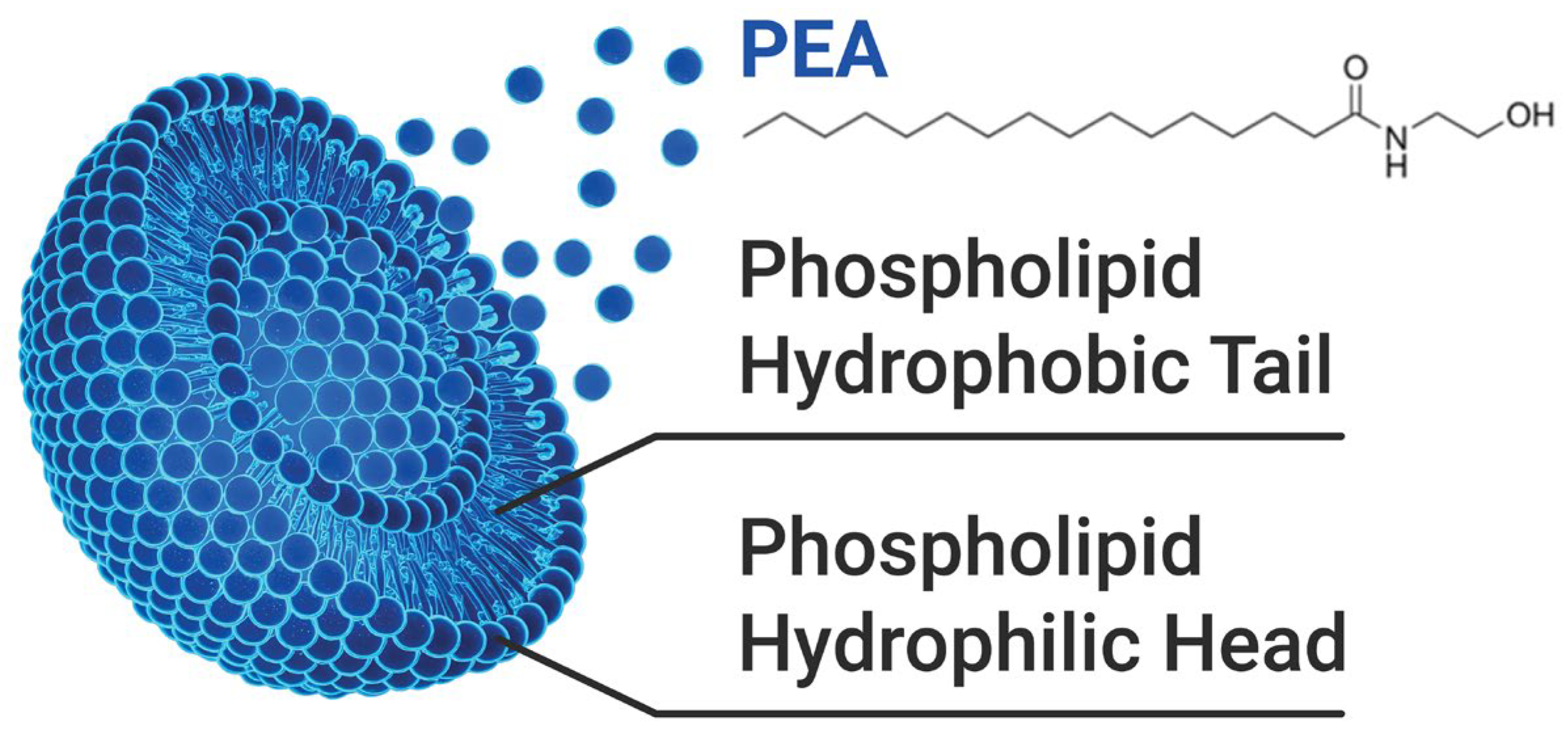
In this context, the SEM findings provide a complementary perspective, linking physical structure to functional performance. Figure 5 illustrates the chemical structure of liposomal PEA, whose encapsulation properties may enhance its permeation and therapeutic efficacy [17].
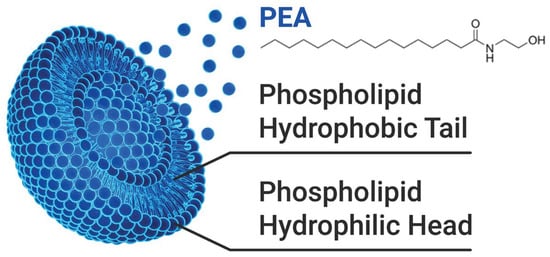
Figure 5.
Illustration of the chemical structure of PEA and its association with the liposome composed of a phospholipid bilayer.
3.3. Evaluation of Absorption in an Ex Vivo Gastrointestinal Model
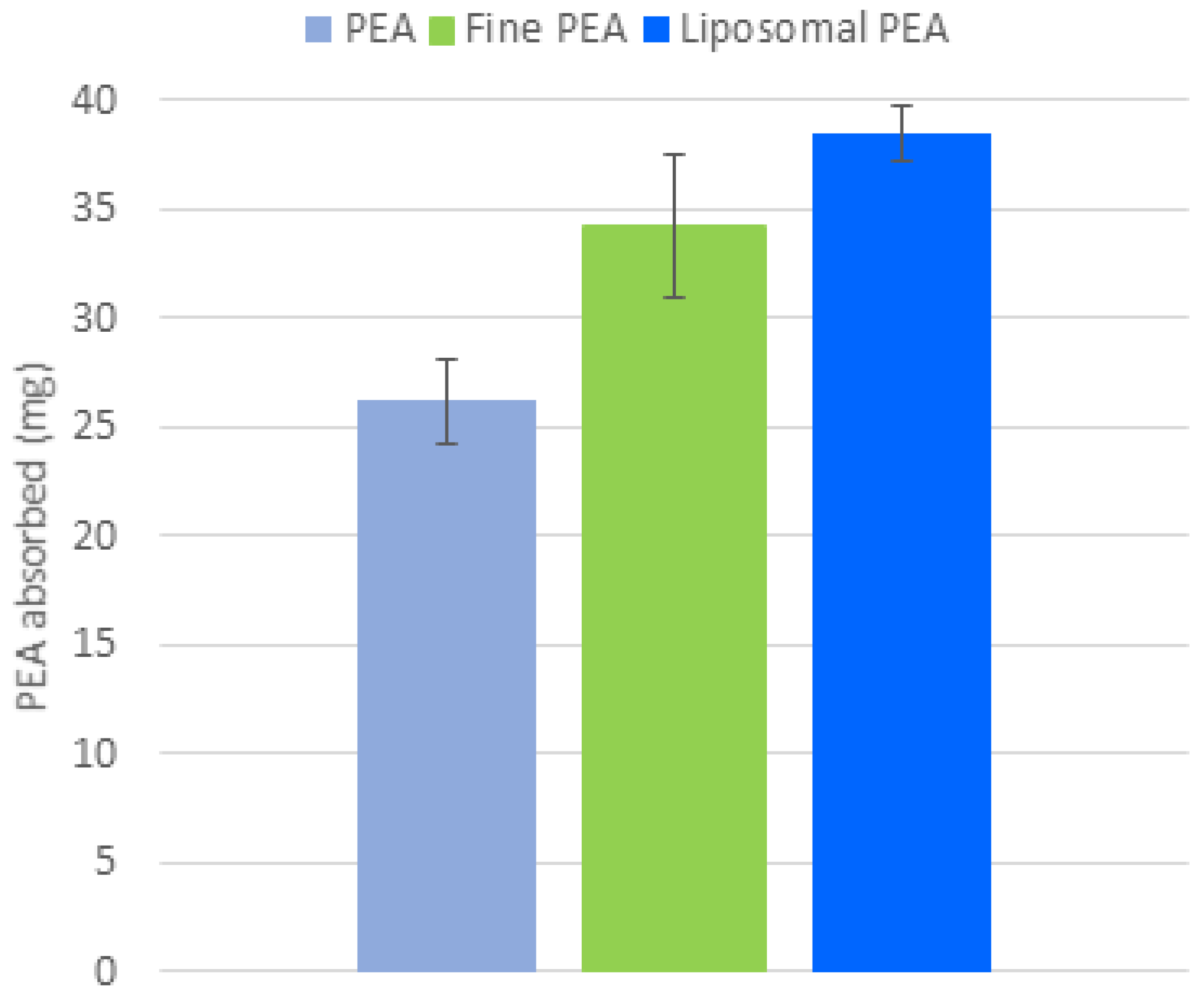
Following the analysis conducted on the ex vivo gastrointestinal model, the membrane was removed, and the biological material absorbed in the membrane was quantified. The quantitative analysis of the amount of PEA absorbed into the membranes used to predict the membrane interaction in the gut (Figure 6) reveals that liposomal PEA shows an absorption of 37 mg, compared to 24.23 mg PEA and 30.4 mg for fine PEA (Figure 6). This finding is consistent with the permeation data described above, within the context of this study. This test can provide valuable insights into bioavailability, permeability, and potential efficacy of the compound.
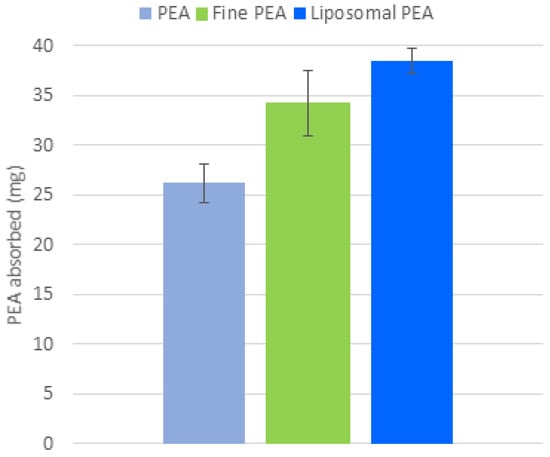
Figure 6.
Comparison of PEA absorption (mg) across three formulations: PEA, fine PEA, and liposomal PEA. Data are expressed as mean values of PEA absorbed ± SD, p < 0.001, liposomal PEA vs. PEA.
3.4. Evaluation of Bioavailability
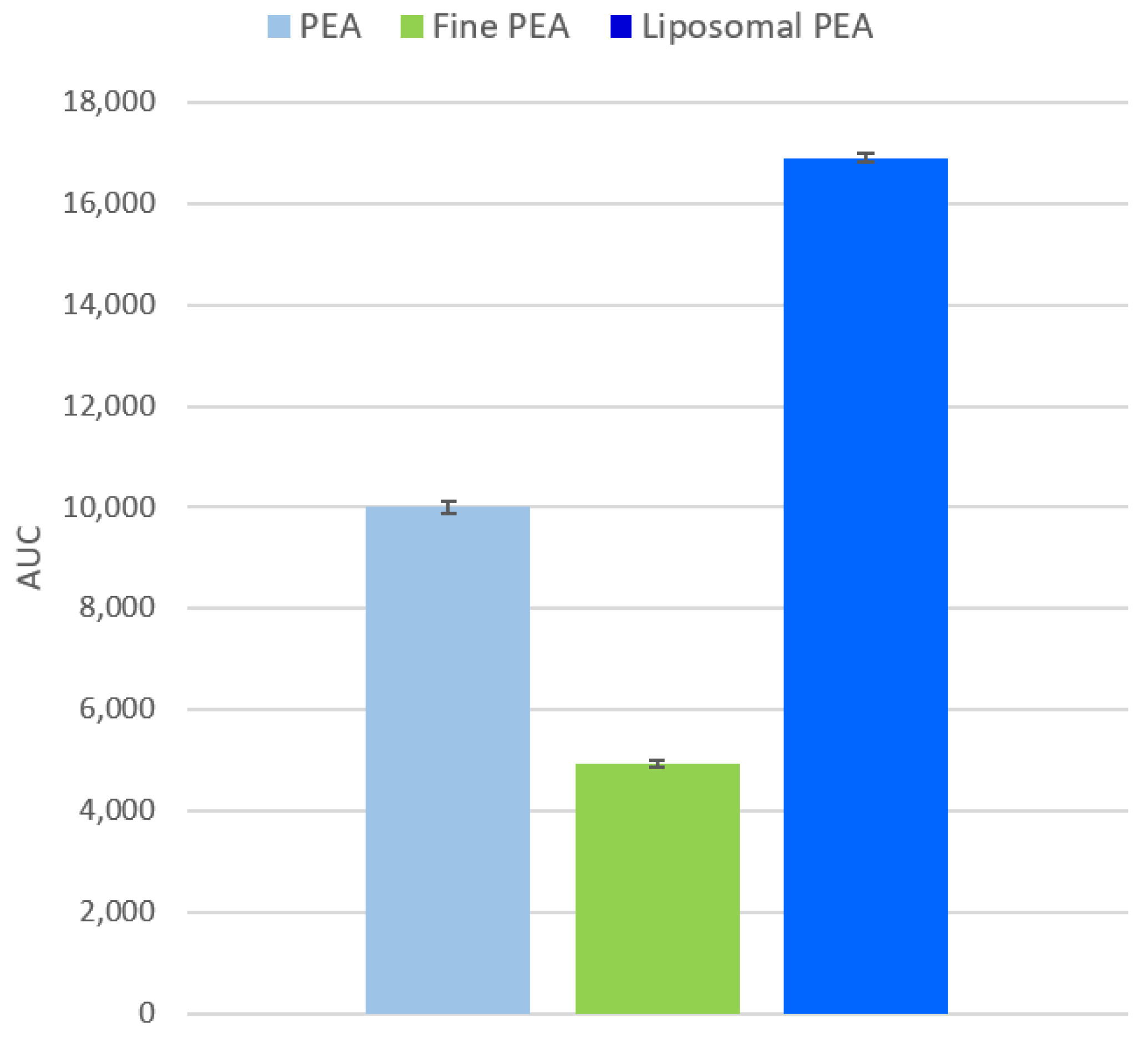
Bioavailability was assessed by calculating the Area Under the Curve (AUC) for each formulation (Figure 7). The analysis shows that liposomal PEA has the highest bioavailability, followed by PEA, with fine PEA showing the lowest. The AUC values were as follows: PEA: 10,007 ± 124; fine PEA: 4919 ± 71; and liposomal PEA: 16,906 ± 86.
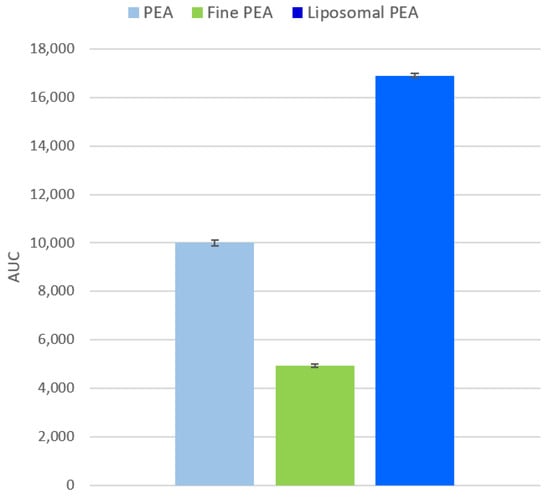
Figure 7.
Analysis of the Area Under the Curve (AUC) for each formulation, considering sample permeability data. Data are expressed as mean values ± SD derived from absorption data. A one-way ANOVA was performed using simulated replicates based on the AUC values: PEA: 10,007.47, Fine PEA: 4919.07, and liposomal PEA: 16,906.10. (p < 0.0001). The t-tests confirmed that liposomal PEA had significantly higher AUC values compared to both PEA and Fine PEA (p < 0.05).
Liposomal formulation likely enhances absorption due to improved metabolic stability and membrane permeability.
The improved bioavailability and permeation of liposomal PEA observed in this study may be attributed to several key mechanisms associated with liposomal delivery systems. First, liposomes can facilitate direct interaction with biological membranes through fusion or endocytosis. Second, the liposomal structure enhances the absorption of hydrophobic compounds, such as PEA, in aqueous environments, promoting better permeation and absorption. Additionally, liposomes may offer protection against enzymatic degradation, preserving the integrity of PEA during transit through the gastrointestinal tract. Their ability to adhere to mucosal surfaces can prolong residence time at the absorption site, further enhancing uptake. The observed gradual release profile suggests a sustained delivery mechanism, which may contribute to maintaining therapeutic levels over time. Finally, liposomes may transiently modulate tight junctions between epithelial cells, facilitating paracellular transport [18].
Together, these mechanisms support the potential of liposomal formulations to improve the pharmacokinetic profile and therapeutic efficacy of PEA.
4. Discussion
Enhancing the absorption of PEA may offer significant advantages in the management of pain, particularly in cases of chronic and acute pain, including pelvic pain. Improved bioavailability ensures that a greater proportion of the administered dose reaches systemic circulation and is available to exert therapeutic effects. Liposomal PEA is a form of PEA that is encapsulated in liposomes. Liposomes can improve the delivery and absorption of PEA in the body. This increased absorption and permeation can lead to more effective modulation of pain pathways, reducing inflammation, and providing substantial pain relief [7]. When PEA is more readily absorbed and utilized by the body, it can better influence the endocannabinoid system and its associated receptors, which are crucial for pain regulation [19].
Moreover, constant concentration enhances the molecule’s efficacy by allowing for a prolonged onset of its effects and mechanism of action. This rapid onset is particularly beneficial for managing chronic pain episodes and conditions requiring relief, such as chronic pelvic pain. Chronic pelvic pain, often associated with conditions like endometriosis or pelvic inflammatory disease, can significantly impact quality of life and may require prompt intervention to alleviate discomfort. Formulations of PEA with better gastrointestinal absorption enable more immediate pain relief, which can lead to quicker improvements in patient symptoms and overall well-being [10]. In addition, the protection of PEA by the lipid involucre can determine greater stability through the gastrointestinal tract and enhanced concentration reaching the target organs, and this can enhance its clinical efficacy. Studies have shown that effective management of chronic pain conditions, including those characterized by persistent inflammation and neuropathic pain, is significantly improved with formulations that allow for higher and more sustained plasma concentrations of the drug [20]. This means that patients can experience prolonged relief from pain and inflammation, potentially reducing the need for additional medications and improving their overall therapeutic experience. Furthermore, the benefits of such optimized formulations extend to enhancing patient adherence and satisfaction. When pain relief is achieved more rapidly and effectively, patients are more likely to adhere to their treatment regimen and report higher levels of satisfaction with their pain management [6].
Recent formulations of PEA have focused on micronized or ultra-micronized forms, in which the particle size is typically reduced to below 10 micrometers. This reduction is widely believed to enhance the compound’s dissolution rate and facilitate more efficient absorption in the gastrointestinal tract, thereby improving bioavailability [21,22,23]. However, data obtained from our ex vivo gastrointestinal model suggest a contrasting outcome. Specifically, the fine PEA exhibited significantly reduced absorption compared to the liposomal form, leading us to believe that small particle size might lead to degradation during the permeation process, which may compromise its stability and limit its effective absorption. These findings challenge the prevailing assumption that micronization universally enhances gastrointestinal uptake and underscore the importance of evaluating formulation performance under physiologically relevant conditions. In real biological systems, factors such as enzymatic degradation, pH variability, mucosal barriers, and intestinal transit time can influence the stability and absorption of micronized compounds [24]. For instance, smaller particles may be more susceptible to chemical or enzymatic breakdown, or may interact differently with the mucosal lining, potentially reducing their effective uptake [24]. In this regard, it is widely accepted that liposomes improve the solubility and absorption of hydrophobic molecules.
Consequently, the characterization of this novel liposomal PEA with enhanced absorption and intestinal permeability may represent a crucial advancement in the formulation of PEA products with better gastric absorption.
Possible Limitations: While the study demonstrates that liposomal PEA has superior bioavailability and permeation through porcine colon mucosae, the use of a single ex vivo model (porcine tissue) may not fully represent the complexity of human gastrointestinal absorption. Differences in species-specific membrane composition, enzymatic activity, and immune responses could affect the generalizability of the results to human physiology. Further studies involving human subjects or advanced in vitro models, such as organoids, may further confirm and predict clinical outcomes.
5. Conclusions
In conclusion, liposomal PEA demonstrated superior bioavailability compared to other non-liposomal formulations. Liposomal PEA showed a more efficient and continuous release over time, reaching higher concentrations of PEA permeated through the membrane. This increased absorption and permeation may be the result of greater stability of PEA and a better affinity to the biological membrane. Taken together, the gradual release and the high quantity of PEA permeated suggest liposomes as novel and effective ingredients to supplement PEA. These findings support the potential of liposomal delivery systems to enhance the therapeutic efficacy of PEA.
6. Patents
The Patent named Peasomial, Patent Num. 102025000015505 by S&R Farmaceutici S.p.A., Bastia Umbria, Italy, has resulted from the work reported in this manuscript.
Author Contributions
Conceptualization, G.C. and C.P.; methodology, G.C. and F.M.; formal analysis, F.M. and M.L.; investigation, F.M. and M.L.; resources, R.G.I.; writing—original draft preparation, C.P. and R.G.I.; writing—review and editing, C.P., G.C., V.M. and R.G.I.; project administration, R.G.I.; funding acquisition, R.G.I. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the S&R Farmaceutici and Liposcience for donating the substances. The authors declare that this study received funding from the Italian Ministry of Enterprises and Made in Italy, Project number: F/350076/01/X60.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The corresponding author can provide this study’s data upon request.
Acknowledgments
The authors have reviewed and edited the output and take full responsibility for the content of this publication. Materials used for experiments were kindly provided by S&R Farmaceutici (Bastia Umbria, Italy), Liposcience Laboratory (Oosterhout, The Netherlands), and Technology Scientifics (Palermo, Italy). We thank A. Dottorini for the illustrations.
Conflicts of Interest
G.C., C.P., V.M. and R.G.I. are employees of S&R Farmaceutici and do not play a role in analyzing the data. Francesco Montalbano is the CEO of Technology Scientific S.r.l. All other authors declare no conflicts of interest. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Abbreviations
The following abbreviations are used in this manuscript:
| PEA | Palmitolyethanolammide |
| SGF | Simulated Gastric Fluid |
| FaSSIF | Fasted State Simulated Intestinal Fluid |
Appendix A
The tables below show the data observed in the graphs reported in the figures in the main text.

Table A1.
PEA release concentration (mg/mL).
Table A1.
PEA release concentration (mg/mL).
| Time (min.) | PEA (mg/mL) | SD |
|---|---|---|
| 5 | 1.76 | 0.58 |
| 10 | 2.00 | 0.58 |
| 30 | 2.20 | 1.60 |
| 45 | 2.51 | 1.66 |
| 60 | 3.00 | 1.66 |
| 90 | 3.44 | 1.90 |
| 120 | 4.51 | 1.00 |

Table A2.
PEA release %.
Table A2.
PEA release %.
| Time (min.) | PEA (%) | SD |
|---|---|---|
| 5 | 12.70 | 4.20 |
| 10 | 14.50 | 4.10 |
| 30 | 15.90 | 11.40 |
| 45 | 18.00 | 11.00 |
| 60 | 21.44 | 11.00 |
| 90 | 25.00 | 13.70 |
| 120 | 32.10 | 6.50 |

Table A3.
PEA permeation expressed in mg/mL.
Table A3.
PEA permeation expressed in mg/mL.
| Time (min.) | PEA | SD | Fine PEA | SD | Liposomal PEA | SD |
|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 00 | 0 | 00 |
| 0.50 | 116.51 | 4.11 | 103.72 | 6.24 | 156.29 | 3.51 |
| 1 | 91.33 | 6.13 | 70.93 | 14.31 | 148.33 | 3.51 |
| 2 | 111.85 | 8.50 | 89.23 | 6.94 | 210.85 | 3.51 |
| 2.5 | 52.06 | 5.35 | 98.21 | 11.90 | 275.10 | 6.00 |
| 3 | 53.65 | 5.44 | 33.53 | 16.44 | 247.22 | 6.66 |
| 4 | 53.82 | 5.66 | 58.48 | 8.26 | 355.20 | 6.66 |
| 5 | 90.88 | 6.94 | 31.83 | 11.90 | 409.79 | 10.21 |
| 6 | 96.78 | 7.41 | 76.50 | 7.41 | 458.02 | 6.08 |
| 7 | 230.00 | 7.48 | 132.87 | 6.60 | 521.70 | 10.02 |
| 8 | 250.00 | 10.61 | 165.84 | 6.18 | 620.04 | 8.19 |
| 24 | 891.76 | 19.26 | 370.44 | 10.87 | 1152.57 | 12.90 |

Table A4.
Tukey’s HSD post hoc test (formulation comparison on permeation data).
Table A4.
Tukey’s HSD post hoc test (formulation comparison on permeation data).
| Comparison | Mean Difference | p-Value |
|---|---|---|
| Fine PEA vs. PEA | 67.2 µg | 0.298 |
| Fine PEA vs. LIPOSOMAL PEA | 276.9 µg | <0.001 |
| PEA vs. Liposomal PEA | 209.7 µg | <0.001 |
References
- Kuehl, F.A., Jr.; Jacob, T.A.; Ganley, O.H.; Ormond, R.E.; Meisinger, M.A.P. The identification of N-(2-hydroxyethyl)-Palmitamide as a naturally occurring anti-inflammatory agent. J. Am. Chem. Soc. 1957, 79, 5577–5578. [Google Scholar] [CrossRef]
- Lang-Illievich, K.; Klivinyi, C.; Lasser, C.; Brenna, C.T.A.; Szilagyi, I.S.; Bornemann-Cimenti, H. Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients 2023, 15, 1350. [Google Scholar] [CrossRef] [PubMed]
- Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef]
- Costa, B.; Conti, S.; Giagnoni, G.; Colleoni, M. Therapeutic Effect of the Endogenous Fatty Acid Amide, Palmitoylethanolamide, in Rat Acute Inflammation: Inhibition of Nitric Oxide and Cyclo-Oxygenase Systems. Br. J. Pharmacol. 2002, 137, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Landolfo, E.; Cutuli, D.; Petrosini, L.; Caltagirone, C. Effects of Palmitoylethanolamide on Neurodegenerative Diseases: A Review from Rodents to Humans. Biomolecules 2022, 12, 667. [Google Scholar] [CrossRef]
- Scuderi, C.; Stecca, C.; Valenza, M.; Ratano, P.; Bronzuoli, M.R.; Bartoli, S.; Steardo, L.; Pompili, E.; Fumagalli, L.; Campolongo, P.; et al. Palmitoylethanolamide Controls Reactive Gliosis and Exerts Neuroprotective Functions in a Rat Model of Alzheimer’s Disease. Cell Death Dis. 2014, 5, e1419. [Google Scholar] [CrossRef]
- Petrosino, S.; Di Marzo, V. The Pharmacology of Palmitoylethanolamide and First Data on the Therapeutic Efficacy of Some of Its New Formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Calignano, A.; La Rana, G.; Giuffrida, A.; Piomelli, D. Control of Pain Initiation by Endogenous Cannabinoids. Nature 1998, 394, 277–281. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Fusco, M.; Della Valle, M.F.; Zusso, M.; Costa, B.; Giusti, P. Palmitoylethanolamide, a Naturally Occurring Disease-Modifying Agent in Neuropathic Pain. Inflammopharmacology 2014, 22, 79–94. [Google Scholar] [CrossRef]
- Gabrielsson, L.; Mattsson, S.; Fowler, C.J. Palmitoylethanolamide for the Treatment of Pain: Pharmacokinetics, Safety and Efficacy. Br. J. Clin. Pharmacol. 2016, 82, 932–942. [Google Scholar] [CrossRef]
- Paladini, A.; Fusco, M.; Cenacchi, T.; Schievano, C.; Piroli, A.; Varrassi, G. Palmitoylethanolamide, a Special Food for Medical Purposes, in the Treatment of Chronic Pain: A Pooled Data Meta-Analysis. Pain Physician 2016, 19, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Keppel Hesselink, J. Chronic Idiopathic Axonal Neuropathy and Pain, Treated with the Endogenous Lipid Mediator Palmitoylethanolamide: A Case Collection. Int. Med. Case Rep. J. 2013, 6, 49–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ambrosino, P.; Soldovieri, M.V.; Russo, C.; Taglialatela, M. Activation and Desensitization of TRPV1 Channels in Sensory Neurons by the PPARα Agonist Palmitoylethanolamide. Br. J. Pharmacol. 2013, 168, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular Targets for Cannabidiol and Its Synthetic Analogues: Effect on Vanilloid VR1 Receptors and on the Cellular Uptake and Enzymatic Hydrolysis of Anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- General Chapter: USP. In <711> Dissolution; USP-NF: Rockville, MD, USA, 2011.
- Annisa, V.; Sulaiman, T.N.S.; Nugroho, A.K.; Nugroho, A.E. Utilization of Alginate with Gum Acacia/Pectin/Carrageenan as Precipitation Inhibitor to Improve Bioavailability in Drug Supersaturation: A Case Study of Ketoconazole. Carbohydr. Polym. Technol. Appl. 2023, 6, 100389. [Google Scholar] [CrossRef]
- Liu, W.; Hou, Y.; Jin, Y.; Wang, Y.; Xu, X.; Han, J. Research Progress on Liposomes: Application in Food, Digestion Behavior and Absorption Mechanism. Trends Food Sci. Technol. 2020, 104, 177–189. [Google Scholar] [CrossRef]
- Daeihamed, M.; Dadashzadeh, S.; Haeri, A.; Faghih Akhlaghi, M. Potential of Liposomes for Enhancement of Oral Drug Absorption. Curr. Drug Deliv. 2017, 14, 289–303. [Google Scholar] [CrossRef]
- Lambert, D.M.; Vandevoorde, S.; Jonsson, K.-O.; Fowler, C.J. The Palmitoylethanolamide Family: A New Class of Anti-Inflammatory Agents? Curr. Med. Chem. 2002, 9, 663–674. [Google Scholar] [CrossRef]
- Artukoglu, B.B.; Beyer, C.; Zuloff-Shani, A.; Brener, E.; Bloch, M.H. Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain Physician 2017, 20, 353–362. [Google Scholar]
- Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Micronized/Ultramicronized Palmitoylethanolamide Displays Superior Oral Efficacy Compared to Nonmicronized Palmitoylethanolamide in a Rat Model of Inflammatory Pain. J. Neuroinflammation 2014, 11, 136. [Google Scholar] [CrossRef]
- Fusco, R.; Gugliandolo, E.; Campolo, M.; Evangelista, M.; Paola, R.D.; Cuzzocrea, S. Correction: Effect of a New Formulation of Micronized and Ultramicronized N-Palmitoylethanolamine in a Tibia Fracture Mouse Model of Complex Regional Pain Syndrome. PLoS ONE 2018, 13, e0201501. [Google Scholar] [CrossRef]
- Nobili, S.; Micheli, L.; Lucarini, E.; Toti, A.; Ghelardini, C.; Di Cesare Mannelli, L. Ultramicronized N-Palmitoylethanolamine Associated with Analgesics: Effects against Persistent Pain. Pharmacol. Ther. 2024, 258, 108649. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Lu, Y.; Quan, H.; Wang, Y.; Song, S.; Guo, H. Advanced Oral Drug Delivery Systems for Gastrointestinal Targeted Delivery: The Design Principles and Foundations. J. Nanobiotechnol. 2025, 23, 400. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).