Mechanism of Action and Antimicrobial Potential of Weissellicin LM85 from Weissella confusa
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.3. Determination of Kill Kinetics and Estimation of Live/Dead Cells
2.4. Determination of Efflux of Potassium (K+) Ions
2.5. Determination of Dissipation of Membrane Potential (∆ψ)
2.6. Determination of Dissipation of Transmembrane pH Gradient (∆pH)
2.7. Effects of Weissellicin LM85 on Genomic DNA
2.8. Morphological Analysis
2.9. In Vitro Applications
2.10. Statistical Analysis
3. Results
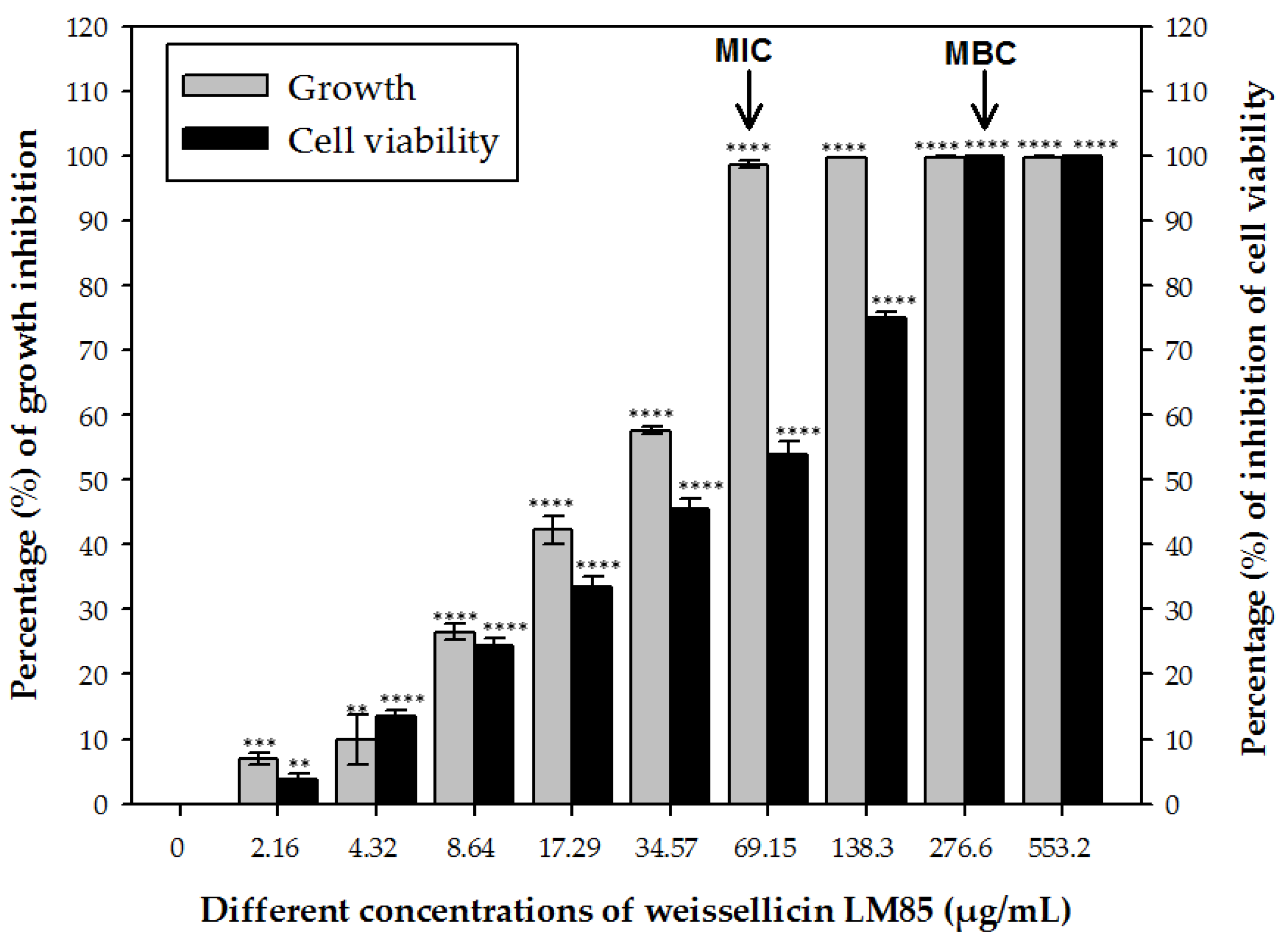
3.1. MIC and MBC
3.2. Kill Kinetics and Estimation of Live/Dead Cells
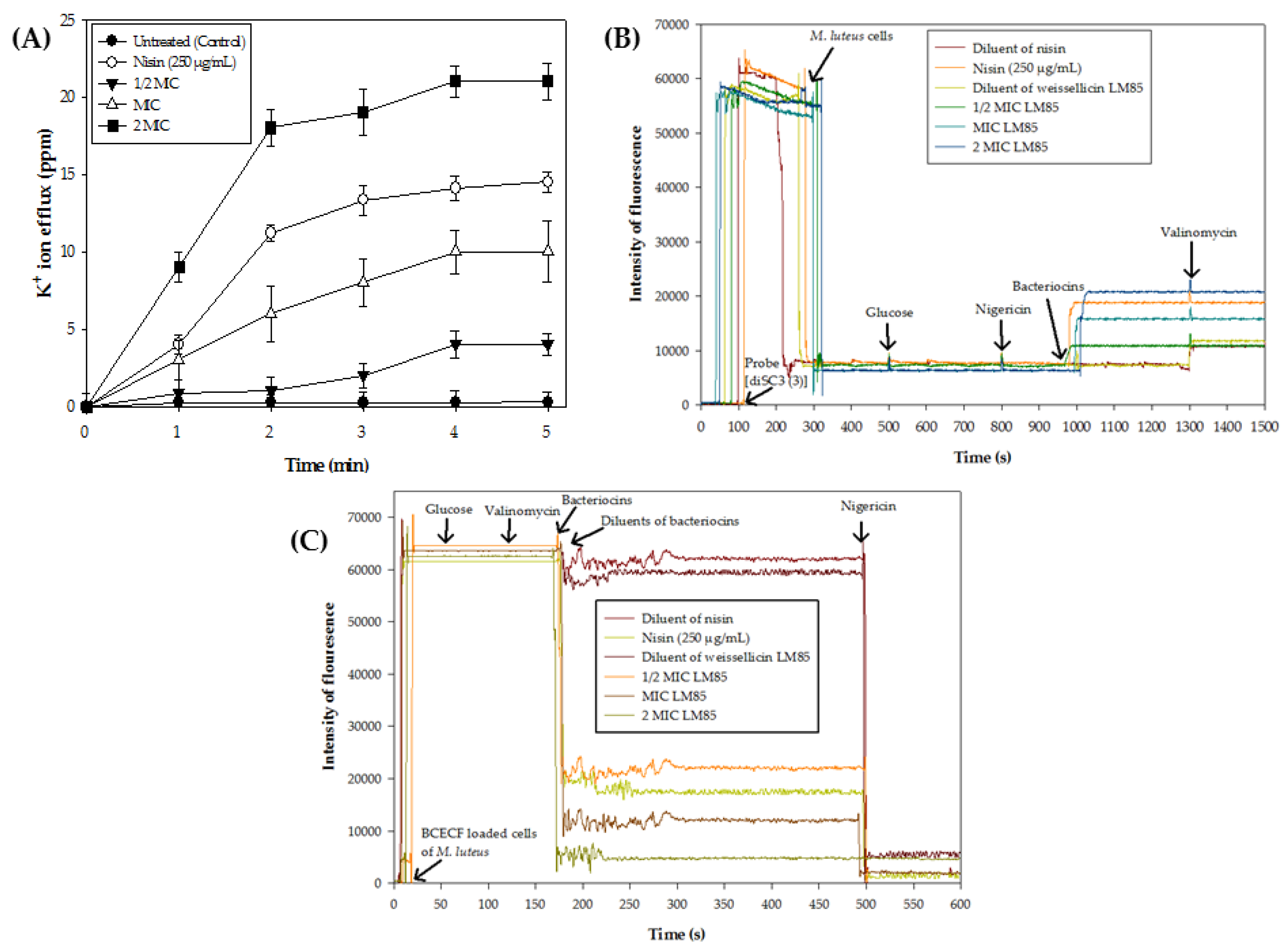
3.3. Efflux of Potassium (K+) Ions
3.4. Dissipation of Membrane Potential (∆ψ)
3.5. Transmembrane pH Gradient (∆pH)
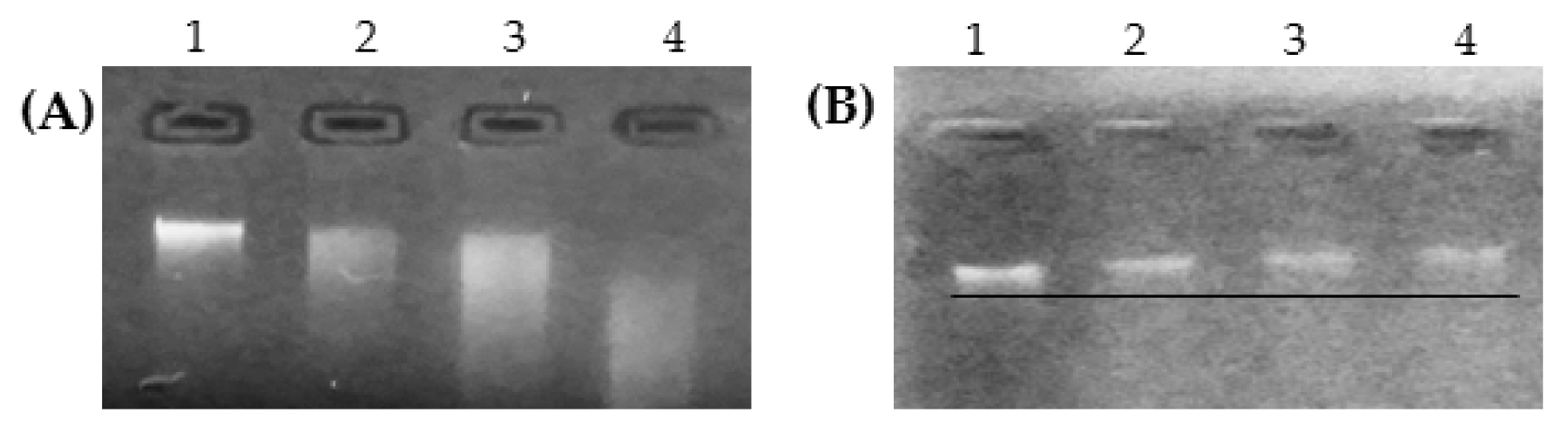
3.6. Effects on Genomic DNA
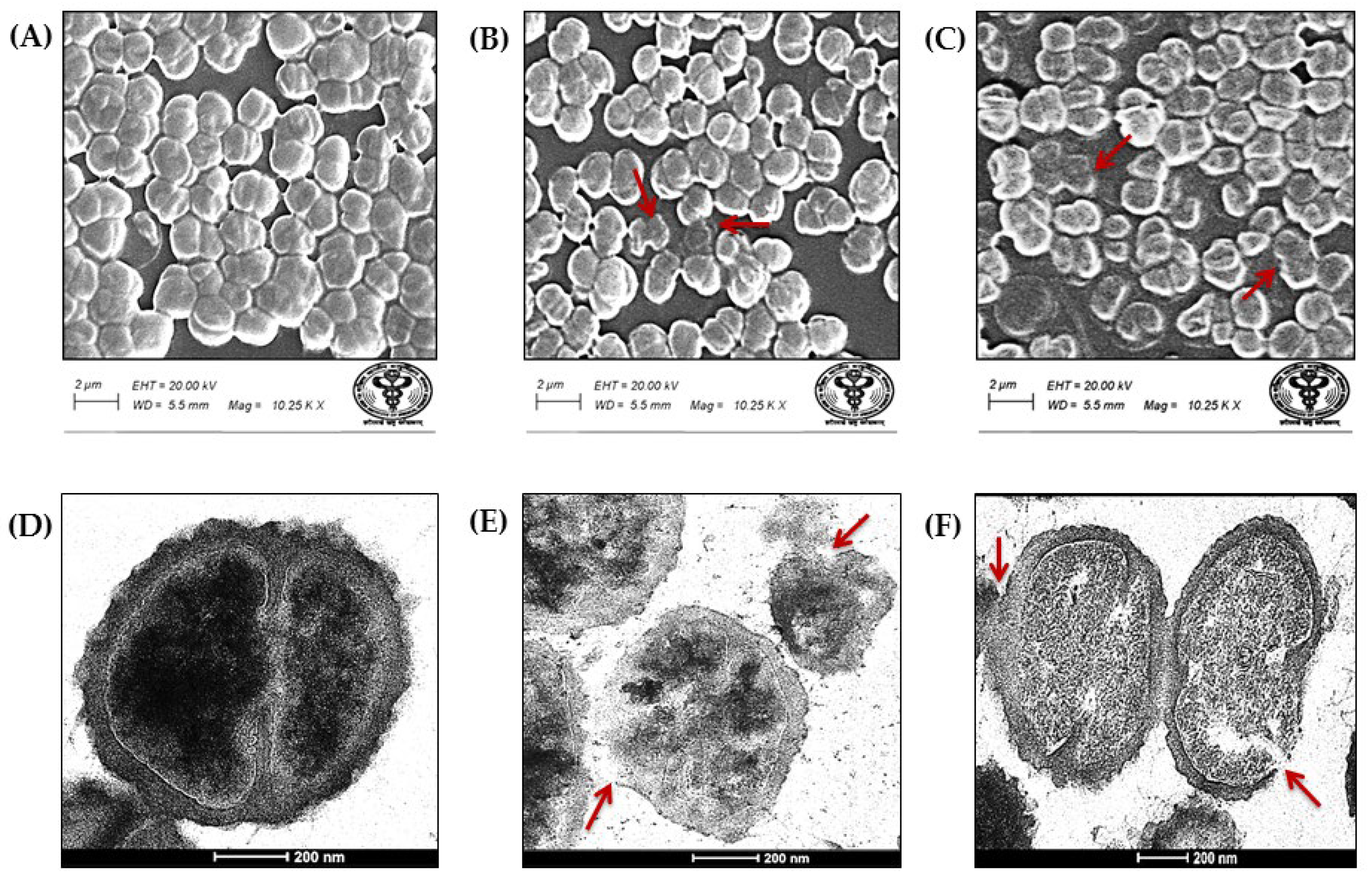
3.7. Morphological Analysis
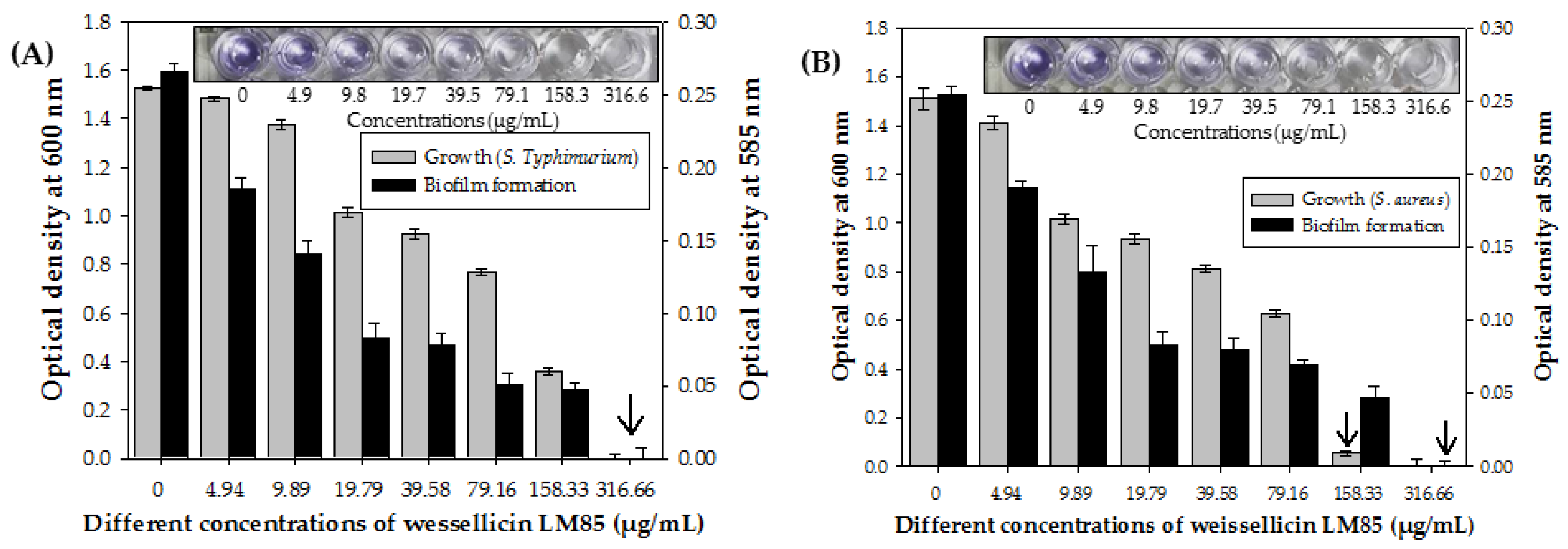
3.8. Inhibition of Growth and Biofilm Formation of S. Typhimurium ATCC13311 and S. aureus ATCC 25923
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPs | Antimicrobial peptides |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| K+ | Potassium ion |
| ∆ψ | Membrane potential |
| ∆pH | Transmembrane pH gradient |
| MRS | De Man, Rogosa, and Sharpe |
| LAB | Lactic acid bacteria |
| PMF | Proton motive force |
| NB | Nutrient broth |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| PI | Propidium iodide |
| RT | Room temperature |
| NaCl | Sodium chloride |
| KCl | Potassium chloride |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| di-S-C3-(3) | 3,3′-dipropylthicarbocyanine iodide |
| BCECF, AM | 2′,7′-Bis-(2-Carboxyethyl)-5-(and-6)-Carboxyfluorescein, Acetoxymethyl Ester |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
| ddH2O | Double distilled water |
| HMDS | Hexamethyldisilazane |
| CV | Crystal violet |
| PBS | Phosphate buffer saline |
| CFU/mL | Colony-forming units per milliliter |
References
- Goh, H.F.; Philip, K. Purification and characterization of bacteriocin produced by Weissella confusa A3 of dairy origin. PLoS ONE 2015, 10, e0140434. [Google Scholar] [CrossRef]
- Singh, J.K.; Devi, P.B.; Reddy, G.B.; Jaiswal, A.K.; Kavitake, D.; Shetty, P.H. Biosynthesis, classification, properties, and applications of Weissella bacteriocins. Front. Microbiol. 2024, 15, 1406904. [Google Scholar] [CrossRef]
- Allen, H.K.; Trachsel, J.; Looft, T.; Casey, T.A. Finding alternatives to antibiotics. Ann. N. Y. Acad. Sci. 2014, 1323, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Popov, I.; Weeks, R.; Chikindas, M.L. Use of bacteriocins and bacteriocinogenic beneficial organisms in food products: Benefits, challenges, concerns. Foods 2022, 11, 3145. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zeng, Y.; Shen, J.; Li, K.; Zhou, Y.; Zeng, X.; Pan, D. Enhanced bacteriocin production by lactic acid bacteria by co-culture of Lactiplantibacillus plantarum and Limosilactobacillus fermentum. Food Biosci. 2025, 68, 106358. [Google Scholar] [CrossRef]
- Yadav, M.K.; Tiwari, S.K. Weissellicin LM85 purified from Weissella confusa LM85 effluxes potassium ions and depletes proton motive force in Escherichia coli ATCC 25922. Int. J. Pept. Res. Ther. 2024, 30, 44. [Google Scholar] [CrossRef]
- Cui, Y.; Luo, L.; Wang, X.; Lu, Y.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Mining, heterologous expression, purification, antibactericidal mechanism, and application of bacteriocins: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 863–899. [Google Scholar] [CrossRef]
- Manberger, A.; Verbrugghe, P.; Guðmundsdóttir, E.E.; Santesson, S.; Nilsson, A.; Hreggviðsson, G.Ó.; Linares-Pastén, J.A.; Nordberg Karlsson, E. Taxogenomic assessment and genomic characterisation of Weissella cibaria strain 92 able to metabolise oligosaccharides derived from dietary fibres. Sci. Rep. 2020, 10, 5853. [Google Scholar] [CrossRef]
- Kavitake, D.; Devi, P.B.; Shetty, P.H. Overview of exopolysaccharides produced by Weissella genus—A review. Int. J. Biol. Macromol. 2020, 164, 2964–2973. [Google Scholar] [CrossRef]
- Fhoula, I.; Boumaiza, M.; Tayh, G.; Rehaiem, A.; Klibi, N.; Ouzari, I.H. Antimicrobial activity and safety features assessment of Weissella spp. from environmental sources. Food Sci. Nutr. 2022, 10, 2896–2910. [Google Scholar] [CrossRef]
- Srionnual, S.; Yanagida, F.; Lin, L.H.; Hsiao, K.N.; Chen, Y.S. Weissellicin 110, a newly discovered bacteriocin from Weissella cibaria 110, isolated from plaa-som, a fermented fish product from Thailand. Appl. Environ. Microbiol. 2007, 73, 2247–2250. [Google Scholar] [CrossRef]
- Leong, K.H.; Chen, Y.S.; Lin, Y.H.; Pan, S.F.; Yu, B.; Wu, H.C.; Yanagida, F. Weissellicin L, a novel bacteriocin from sian-sianzih-isolated Weissella hellenica 4-7. J. Appl. Microbiol. 2013, 115, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, X.; Jiang, M.; Rui, X.; Li, W.; Dong, M. A newly discovered bacteriocin from Weissella hellenica D1501 associated with Chinese Dong fermented meat (Nanx Wudl). Food Control 2014, 42, 116–124. [Google Scholar] [CrossRef]
- Papagianni, M.; Papamichael, E.M. Purification, amino acid sequence and characterization of the class IIa bacteriocin weissellin A, produced by Weissella paramesenteroides DX. Bioresour. Technol. 2011, 102, 6730–6734. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Zendo, T.; Sawa, N.; Perez, R.H.; Nakayama, J.; Sonomoto, K. Characterization and identification of weissellicin Y and weissellicin M, novel bacteriocins produced by Weissella hellenica QU 13. J. Appl. Microbiol. 2012, 112, 99–108. [Google Scholar] [CrossRef]
- Woraprayote, W.; Pumpuang, L.; Tosukhowong, A.; Roytrakul, S.; Perez, R.H.; Zendo, T.; Sonomoto, K.; Benjakul, S.; Visessanguan, W. Two putatively novel bacteriocins active against Gram-negative food borne pathogens produced by Weissella hellenica BCC 7293. Food Control 2015, 55, 176–184. [Google Scholar] [CrossRef]
- Tenea, G.N.; Lara, M.I. Antimicrobial compounds produced by Weissella confusa Cys2-2 strain inhibit Gram-negative bacteria growth. CyTA-J. Food 2019, 17, 105–111. [Google Scholar] [CrossRef]
- Sreelakshmi, K.; Kadirvelu, K.; Ramasubramanian, V. Probiotic efficacy, antioxidant, antimicrobial and anticancer activities of cell free supernatant derived from Weissella confusa isolated from Oreochromis niloticus—An in vitro study. The Microbe 2025, 6, 100291. [Google Scholar] [CrossRef]
- Kaur, R.; Tiwari, S.K. Isolation, identification and characterization of Pediococcus pentosaceus LB44 and Weissella confusa LM85 for the presence of bacteriocin-like inhibitory substances (BLIS). Microbiology 2016, 85, 540–547. [Google Scholar] [CrossRef]
- Kaur, R.; Tiwari, S.K. Optimization of culture conditions for bacteriocin production by soil isolates Pediococcus pentosaceus LB44 and Weissella confusa LM85. Int. J. Infect. 2017, 4, e15842. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D. Antibacterial mode of action of garviecin LG34 against Gram-negative bacterium Salmonella typhimurium. FEMS Microbiol. Lett. 2024, 371, fnae066. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, P.; Yadav, M.K.; Kumari, I.; Tiwari, S.K. Enterocin LD3 from Enterococcus hirae LD3 Inhibits the Growth of Salmonella enterica subsp. enterica serovar Typhimurium ATCC13311 in Fruit Juice. Probiotics Antimicrob. Proteins 2024, 16, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Liu, G.; Ke, C.; Fan, W.; Li, C.; Chen, Y.; Dixon, W.; Song, M.; Cao, Y.; Xiao, H. Inhibitory effects of a novel antimicrobial peptide from kefir against Escherichia coli. Food Control 2016, 65, 63–72. [Google Scholar] [CrossRef]
- Leslie, V.A.; Alarjani, K.M.; Malaisamy, A.; Balasubramanian, B. Bacteriocin producing microbes with bactericidal activity against multidrug resistant pathogens. J. Infect. Public Health 2021, 14, 1802–1809. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Y.; Li, Y.; Wang, X. Mode of action of leucocin K7 produced by Leuconostoc mesenteroides K7 against Listeria monocytogenes and its potential in milk preservation. Biotechnol. Lett. 2016, 38, 1551–1557. [Google Scholar] [CrossRef]
- Du, H.; Zhou, L.; Lu, Z.; Bie, X.; Zhao, H.; Niu, Y.D.; Lu, F. Transcriptomic and proteomic profiling response of methicillin-resistant Staphylococcus aureus (MRSA) to a novel bacteriocin, plantaricin GZ1-27 and its inhibition of biofilm formation. Appl. Microbiol. Biotechnol. 2020, 104, 7957–7970. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, S.; Zhang, T.; Wu, Y.; Ma, J.; Zhao, L.; Li, X.; Zhang, J. Characterization and antibacterial modes of action of bacteriocins from Bacillus coagulans CGMCC 9951 against Listeria monocytogenes. LWT 2022, 160, 113272. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Q.; Lu, F.; Bie, X.; Zhao, H.; Lu, Z.; Lu, Y. A novel plantaricin 827 effectively inhibits Staphylococcus aureus and extends shelf life of skim milk. LWT 2022, 154, 112849. [Google Scholar] [CrossRef]
- Shastry, R.P.; Arunrenganathan, R.R.; Rai, V.R. Characterization of probiotic Enterococcus lactis RS5 and purification of antibiofilm enterocin. Biocatal. Agric. Biotechnol. 2021, 31, 101897. [Google Scholar] [CrossRef]
- Yap, P.G.; Lai, Z.W.; Tan, J.S. Bacteriocins from lactic acid bacteria: Purification strategies and applications in food and medical industries: A review. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 51. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef]
- Deng, Z.; Hou, K.; Zhao, J.; Wang, H. The probiotic properties of lactic acid bacteria and their applications in animal husbandry. Curr. Microbiol. 2022, 79, 22. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, H.; Zhang, C.; Lu, F.; Bie, X.; Lu, Z. Expression of a novel bacteriocin the plantaricin Pln1-in Escherichia coli and its functional analysis. Protein Expr. Purif. 2016, 119, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Tenea, G.N.; Hurtado, P.; Ortega, C. A novel Weissella cibaria strain UTNGt21O isolated from wild Solanum quitoense fruit: Genome sequence and characterization of a peptide with highly inhibitory potential toward Gram-negative bacteria. Foods 2020, 9, 1242. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Xin, G.; Niu, H.; Huang, W. Chlorinated emodin as a natural antibacterial agent against drug-resistant bacteria through dual influence on bacterial cell membranes and DNA. Sci. Rep. 2017, 7, 12721. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Criss, A.K. Fluorescence microscopy methods for determining the viability of bacteria in association with mammalian cells. J. Vis. Exp. 2013, 79, e50729. [Google Scholar] [CrossRef]
- Jiang, Y.; Mei, C.; Huang, X.; Gu, Q.; Song, D. Antibacterial activity and mechanism of a bacteriocin derived from the valine-cecropin a (1-8)-plantaricin zj5 (1-18) hybrid peptide against Escherichia coli O104. Food Biophys. 2020, 15, 442–451. [Google Scholar] [CrossRef]
- Yadav, M.; Tiwari, S.K. Mechanism of cell-killing activity of plantaricin LD1 against Escherichia coli ATCC 25922. Appl. Biochem. Biotech. 2024, 196, 7570–7587. [Google Scholar] [CrossRef]
- Perez-Ramos, A.; Madi-Moussa, D.; Coucheney, F.; Drider, D. Current knowledge of the mode of action and immunity mechanisms of LAB-bacteriocins. Microorganisms 2021, 9, 2107. [Google Scholar] [CrossRef]
- Elsilk, S.E.; Azab, E.A.; Tahwash, A.M. Bacteriocins-like substances produced by Enterococcus sanguinicola isolated from traditional Egyptain food Sires (Chicorium pumilum). JSM Microbiol. 2015, 3, 1018. [Google Scholar]
- Tenea, G.N.; Pozo, T.D. Antimicrobial peptides from Lactobacillus plantarum UTNGt2 prevent harmful bacteria growth on fresh tomatoes. CyTA-J. Food 2019, 17, 105–111. [Google Scholar] [CrossRef]
- Sheoran, P.; Tiwari, S.K. Enterocin LD3 from Enterococcus hirae LD3 causing efflux of intracellular ions and UV-absorbing materials in Gram-negative bacteria. J. Appl. Microbiol. 2019, 126, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tang, X.; Zhou, Q.; Zou, J.; Li, P.; Breukink, E.; Gu, Q. Plantaricin NC8 from Lactobacillus plantarum causes cell membrane disruption to Micrococcus luteus without targeting lipid II. Appl. Microbiol. Biotechnol. 2018, 102, 7465–7473. [Google Scholar] [CrossRef]
- Buttress, J.A.; Halte, M.; Te Winkel, J.D.; Erhardt, M.; Popp, P.F.; Strahl, H. A guide for membrane potential measurements in Gram-negative bacteria using voltage-sensitive dyes. Microbiology 2022, 168, 001227. [Google Scholar] [CrossRef]
- Gong, H.S.; Meng, X.C.; Wang, H. Mode of action of plantaricin MG, a bacteriocin active against Salmonella typhimurium. J. Basic Microbiol. 2010, 50, S37–S45. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, L.; Wei, Y.; Shang, N.; Zhang, X.; Li, P. Two-peptide bacteriocin PlnEF causes cell membrane damage to Lactobacillus plantarum. Biochim. Biophys. Acta Biomembr. 2016, 1858, 274–280. [Google Scholar] [CrossRef]
- Yadav, M.K.; Baldia, A.; Tiwari, S.K. Plantaricin LD1 inhibits the growth and biofilm formation of Staphylococcus aureus in milk. J. Explor. Res. Pharmacol. 2024, 9, 1–7. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Chaiyasut, C.; Kantachote, D.; Sirilun, S. Cell-free supernatants from cultures of lactic acid bacteria isolated from fermented grape as biocontrol against Salmonella typhi and Salmonella typhimurium virulence via autoinducer-2 and biofilm interference. PeerJ 2019, 7, e7555. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, M.K.; Tiwari, S.K. Mechanism of Action and Antimicrobial Potential of Weissellicin LM85 from Weissella confusa. Nutraceuticals 2025, 5, 33. https://doi.org/10.3390/nutraceuticals5040033
Yadav MK, Tiwari SK. Mechanism of Action and Antimicrobial Potential of Weissellicin LM85 from Weissella confusa. Nutraceuticals. 2025; 5(4):33. https://doi.org/10.3390/nutraceuticals5040033
Chicago/Turabian StyleYadav, Manoj Kumar, and Santosh Kumar Tiwari. 2025. "Mechanism of Action and Antimicrobial Potential of Weissellicin LM85 from Weissella confusa" Nutraceuticals 5, no. 4: 33. https://doi.org/10.3390/nutraceuticals5040033
APA StyleYadav, M. K., & Tiwari, S. K. (2025). Mechanism of Action and Antimicrobial Potential of Weissellicin LM85 from Weissella confusa. Nutraceuticals, 5(4), 33. https://doi.org/10.3390/nutraceuticals5040033





