Abstract
Pistacia terebinthus L. which has been traditionally used in diet and medicine, remains underexplored in Greece, particularly regarding its chemical composition and antioxidant activity. The current study aims to comparatively evaluate the chemical profile of cold-pressed terebinth fruit oils, obtained from wild trees growing in the Greek Island of Chios (North East Aegean Sea), harvested during three years (2019, 2020 and 2021). The oils’ lipid profile was dominated by oleic acid (C18:1 cis-9) (42–45%) followed by palmitic acid (C16:0) (24–30%) and linoleic acid (C18:2 cis-9,12) (19–22%). Their phenolic acid content, expressed as anacardic acids—known for their bioactive properties—was quantified via q-1H-NMR and found to be markedly high (1.91–2.98 mmol/kg oil). Total phenolic content (TPC) of the fruit extract showed interesting high value (185.92 ± 2.61 mg GAE/g) accompanied by strong antioxidant activity (DPPH, exhibiting > 80% inhibition at a concentration of 100 µg/mL) which was positively correlated with TPC. Additionally, the fruits demonstrated a rich nutritional profile, particularly in crude fibers (38.9%) and essential minerals (K, Mg, and Zn), along with low sodium content, suggesting notable dietary benefits. The cold-pressed oil exhibited high lipid content and low specific extinction coefficients (K232, K270), indicating minimal oxidation and confirming the oil’s freshness. These findings highlight the potential of P. terebinthus fruit oil as a high-value functional raw material with nutritional and antioxidant properties. Comparable to olive oil in lipid quality, Greek turpentine fruit and oil could play a promising role towards further applications in the food, cosmetic and pharmaceutical sectors.
1. Introduction
The rising global interest in natural, health-promoting bioactive plant materials has renewed attention toward lesser-known Mediterranean plant species. Among these is Pistacia terebinthus L. (Anacardiaceae), commonly known as the terebinth or turpentine tree, which is a deciduous species native to the eastern Mediterranean basin, including Greece. It is a broad, bushy tree which grows slowly, reaching a height and spread of 25–30 ft, and is characterized by its shiny, resin-scented leaves. The tree produces reddish-purple flowers arranged in compact compound clusters, which bloom between March and April. Its fruits are small, globular nutlets that turn brown upon ripening [1,2].
Traditionally valued for its aromatic resin and medicinal properties, P. terebinthus has been used in folk medicine for a wide range of medicinal purposes across the Mediterranean and the Middle East [2,3]. In Greece, its resin is employed as an antidote, aphrodisiac, expectorant, and for treating leprosy [4]. In Iran, the resin is burned to purify air and act as an antiseptic, while the leaves and bark are used as astringents to treat diarrhea [4]. In Jordan, both the resin and leaves are valued: the resin is used as a diuretic, laxative, stimulant, and aphrodisiac, while the leaves help manage hypertension, act as diuretics, and treat jaundice [5]. In Spain, the aerial parts of the plant are traditionally used for their hypotensive and anti-cephalalgic effects, and the branches for their antiseptic properties. Additionally, its flowers and leaves are used to relieve tooth pain and assist in treating dislocated joints, while the fruit is employed in the management of prostatitis [6]. In Turkey, both fruit and leaves are widely used in ethnomedicine. The fruit is traditionally consumed to alleviate symptoms of cold, flu, stomachache, rheumatism, and urinary issues. It is also valued as a stimulant, antitussive, and appetite enhancer, and has been used in soap production and as a coffee substitute. The leaves are used to treat stomachaches, fungal infections, and diabetes. The resin, meanwhile, is applied for its antiseptic, antipyretic, anti-inflammatory, and respiratory benefits [7,8].
Furthermore, in Greece—particularly on the island of Chios (famous also for the biggest cultivation of Pistacia lentiscus trees worldwide, and production of the unique resin of mastic)—the species holds a unique place in both culinary and cultural traditions. The fruits of P. terebinthus, locally known as “tsikouda”, are collected from wild plants and consumed either fresh or dried, often served together with traditional alcoholic beverages like souma or raki. When cold-pressed, the fruits produce a rare and high-value aromatic oil, locally referred to as tsikoudolado or agiolado. Fruits and oil are traditionally used in the preparation of a local pie, called tremithopita, sweets, as an ingredient in smoked and salted cured pork sausages, and for frying fish. Additionally, the fresh shoots of P. terebinthus are pickled and consumed as food. In addition to their culinary use, fruits are employed as natural dyes for textiles [9].
In addition to its culinary and ethnobotanical importance, P. terebinthus has attracted scientific interest due to its rich phytochemical composition. The fruits are rich in dietary fiber, carotenoids, tocopherols, and phenolic compounds, while the cold-pressed oil contains significant levels of unsaturated fatty acids, tocopherols, carotenoids, and phenolics [10,11,12]. Among the phenolic constituents associated with the family are anacardic acids-characteristic phenolic compounds of Anacardiaceae family. Anacardic acids are biologically significant due to their broad spectrum of bioactivities. Their amphiphilic structure enables them to scavenge free radicals, chelate transition metals, and integrate into lipid bilayers, thereby modulating oxidative processes and cellular signaling. These properties explain their reported antioxidant, antimicrobial, and gastroprotective properties [13,14], which highlight their potential as functional bioactive compounds. Anacardic acids, the main phenolic lipids in terebinth fruit oil, were quantified using q-1H-NMR, a method selected for its ability to provide direct, reproducible quantification without chromatographic separation and with minimal sample preparation. This approach also circumvents the limitations of HPLC, where co-elution and standard availability can hinder accurate quantification.
Although several previous studies, have examined the essential oils and resins of various Pistacia species [4,15,16], no comprehensive research to date has focused on the fruit and cold-pressed oil of wild P. terebinthus trees native to Greece—despite the region’s long history of traditional use.
To our knowledge, the present study is the first systematic characterization of cold-pressed fruit oil from wild growing P. terebinthus populations on Chios Island, Greece. The study specifically evaluates its chemical composition (fatty acids, phenolic compounds, and anacardic acids)-antioxidant capacity as well as nutritional profile, with the hypothesis that P. terebinthus fruit oil represents a valuable functional raw material comparable to established Mediterranean oils.
2. Materials and Methods
2.1. Chemicals and Reagents
All the chemicals and reagents were purchased from the following suppliers: Merck (Darmstadt, Germany) ethanol absolute, Folin–Ciocalteu reagent, gallic acid, sodium carbonate (Na2CO3), cyclohexane, acetonitrile, deuterated chloroform (CDCl3), Sigma-Aldrich (St. Louis, MO, USA) sulfuric acid 95–98% (H2SO4), 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), Carlo Erba Reagents (Val-de-Reuil, France) (hexane, petroleum ether, dimethyl sulfoxide (DMSO), Sigma-Aldrich (Steinheim, Germany) syringaldehyde (98% purity) used as internal standard (IS), Lab-Scan (Dublin, Ireland) chloroform, Panreac (Barcelona, Spain) sodium sulfate (Na2SO4) anhydrous, Fisher Scientific (Loughborough, UK) methanol high-performance liquid chromatography (HPLC) grade, hydrochloric acid 37% (HCl), Glentham Life Sciences (Corsham, UK) 2,2-diphenyl-1-picrylhydrazyl (DPPH•), and Lachner (Neratovice, Czechia) sodium hydroxide micropearls (NaOH).
2.2. Plant Material
Fruit Oil Extraction: Cold-pressed P. terebinthus fruits were harvested in September from wild trees on the island of Chios (North-East Aegean, Greece). Harvesting was carried out at full fruit maturity (dark red to brown color) under typical late-summer Mediterranean conditions (warm, dry weather). Oils were obtained by cold pressing, and the 2021 harvest (TSO1) had a yellow colour and was stored in amber glass bottles at 4 °C until further analysis. Terebinth oils from previous harvest years were also included in the study (TSO2 = harvest year 2020 and TSO3 = harvest year 2019). The oil samples were provided by a certified local producer (Chios Island, Greece) who performed the cold-press extraction.
Methanolic extract of Oil and Fruits: A volume of 10 mL of terebinth fruit oil (TSO1) was extracted twice with 20 mL of methanol (MeOH) each time. The combined methanolic extracts were evaporated to dryness in a rotary evaporator at room temperature. The resulting precipitates were weighed, redissolved in MeOH, and kept in the dark at 4 °C for further analysis (TSOME1). Methanolic extracts were similarly prepared from oil samples of previous harvest years (TSOME2, TSOME3) for comparative analysis.
In addition, fruit samples (TSF) were processed as previously described by Ozcan et al. [17] to evaluate the methanolic extract (TSFME). Powdered samples (5 g) were mixed with 25 mL of methanol and subjected to sonicator (Elma, Jena, Germany) for 30 min in an ultrasonic bath. The methanolic extracts were evaporated to dryness in a rotary evaporator at room temperature.
2.3. Fatty Acid Determination
The fatty acid profile was analyzed by gas chromatography following transesterification, as previously described [18]. The FAMEs were prepared by mixing a solution in n-hexane (0.1 g in 5 mL) with 1 mL of 1 M methanolic sodium hydroxide. The mixture was incubated at 50 °C for 20 min. After phase separation, the upper phase containing FAMEs was collected and transferred to vials for GC analysis. The analysis was conducted on an Agilent Technologies Gas Chromatograph 7820A (Shanghai, China) linked to Agilent Technologies 5977B mass spectrometer system (Santa Clara, CA, USA) equipped with a 30 m length, 0.25 mm i.d. and 0.5 µm film thickness HP5-MS capillary column. The column temperature was first set at 60 °C; subsequently increased to 140 °C at a rate of 15 °C/min; then increased to 240 °C at a rate of 3 °C/min and finally increased to 280 °C at a rate of 15 °C/min. The identification of components was conducted using mass spectral databases (Wiley275, ADAMS07) and in comparison with existing literature data. The matching quality was consistently high (98–99% for all identified compounds).
2.4. Total Phenolic Content (TPC) Determination
The total phenolic content of terebinth fruits methanolic extract (TSFME), cold-pressed oils (TSO1) and methanolic oil extracts (TSOME), were quantified using the Folin–Ciocalteu method [19]. Results were expressed as gallic acid equivalents (GAE). All experiments were carried out in triplicate and data are presented as mean values ± standard deviation (n = 3).
2.5. Quantification of Anacardic Acids-qNMR Method Validation
Instrumentation: The quantitative determination of phenols in terebinth oil was performed using NMR spectroscopy on a DRX 400 MHz (Bruker). CDCls was used as a solvent due to its advantage not to react with the studied compounds. The spectra were processed using either the MNova (Mestrelab Research) or the TOPSPIN program as described previously [20].
Oil Extraction for Analysis: Terebinth oil (5.0 g) was mixed with cyclohexane (20 mL) and acetonitrile (25 mL). The mixture was homogenized using a vortex mixer (VXMTAL multi-tube vortex mixer, OHAUS Corporation, Parsippany, NJ, USA) for 30 s and centrifuged at 4000 rpm for 5 min. A part of the acetonitrile phase (25 mL) was collected, mixed with 1.0 mL of a syringaldehyde solution (0.5 mg/mL) in acetonitrile, and evaporated under vacuum using a rotary evaporator (Buchi, Flawil, Switzerland).
The residue was dissolved in CDCl3 and was transferred to a 5 mm NMR tube. Each sample was analyzed in triplicate using a standard 90 degree excitation pulse, with a relaxation delay of 10 s.
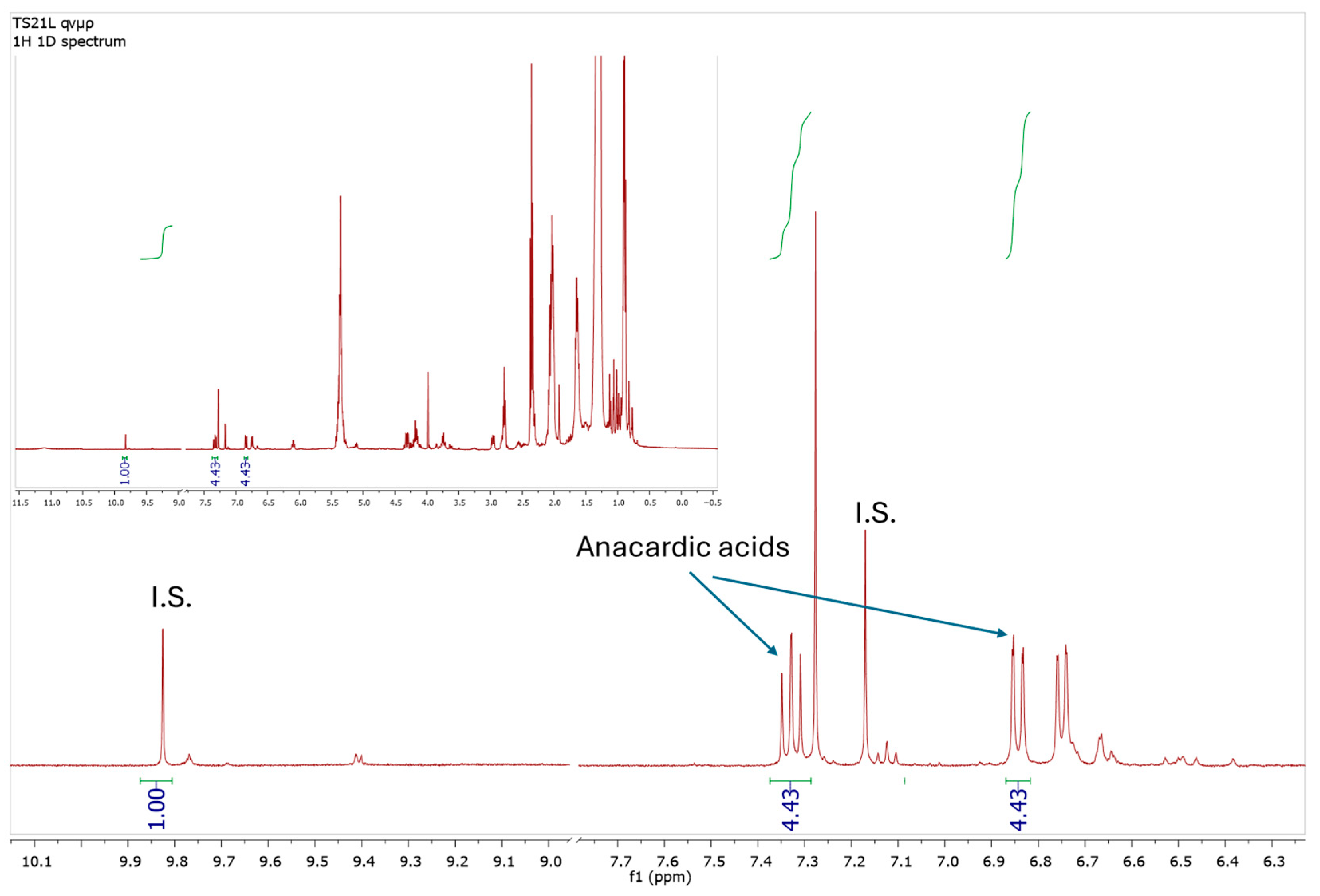
The concentration of total Anacardic acids (AAs) was measured by comparing the area of the selected signal at 6.85 ppm with that of the internal standard (IS) at 9.81 ppm, which was set as 1. The calculation of the concentration in mmol/kg was performed using the following formula: C = [IAAs × mmolI.S.]/Msample, where Msample = 0.0050 kg and mmolI.S. = 0.5 mg/MWsyringaldehyde = 0.00270.
The recovery was calculated by the comparison of successive extractions of samples regarding the Anacardic acids studied. The recovery achieved after one extraction was >98%. The intraday and interday precision was determined by analyzing three replicates of a random sample prepared the same day and from three samples prepared in different days, and the %RSD was found to be <10%. LOQ = 0.02 mmol/kg (S/N > 10) determined after serial dilutions.
2.6. Determination of Antioxidant Activity of Terebinth Fruits—Radical Scavenging Activity
The extraction procedure was carried out following a previously described method [17] with slight modifications. The powdered and sonicated TSF sample (5 g/25 mL methanol) was centrifuged for 10 min. The resulting supernatant was filtered through a 0.45 μm membrane and transferred into a separatory funnel with 10 mL of n-hexane. The mixture was shaken to achieve phase separation between methanol and hexane. This step was repeated three times, after which the methanol fractions were combined and evaporated by rotary evaporator at 50 °C to dryness.
The antioxidant potential of Terebinth fruit extracts was assessed using the DPPH• radical scavenging assay, following a previously reported protocol [19] with minor modifications. A stock solution of DPPH• (314 µM) was prepared in absolute ethanol and stored in the dark at room temperature until use. Gallic acid served as the positive control (IC50: 4.5 μg/mL). Test samples were dissolved in DMSO to the desired concentrations.
For the assay, 190 µL of DPPH• solution and 10 µL of gallic acid or extract were added into wells of a 96-well microplate and incubated for 30 min at room temperature in the dark. Absorbance was recorded at 517 nm using a TECAN microplate reader (Tecan Group Ltd., Männedorf, Switzerland). Negative controls contained 10 µL of DMSO and 190 µL of DPPH•, while blanks consisted of 10 µL of sample and 190 µL of ethanol.
The percentage of DPPH• scavenging was estimated by the following equation: AA% = {[(A − B) − (C − D)]/(A − B)} × 100, A: Control (without sample), B: Blank (without sample, without DPPH•), C: Sample, D: Blank sample (without DPPH•). The measurements were carried out in triplicates.
2.7. Determination of Nutritional Value of Terebinth Fruit and Oil
The fruits (TSF) and oil (TSO1) of P. terebinthus were examined for their nutritional characteristics. Physical and chemical properties of terebinth fruits were determined (energy, protein, fatty acids, ash, dietary fibers, mineral content as well as K232 and K270 indexes). Ash content was determined using the conventional dry ashing method (AOAC 940.26), while crude fiber content was measured according to the acid–alkali digestion procedure (AOAC 962.09). Protein content was estimated using the modified Kjeldhal method, while ash and crude fiber content was determined through conventional procedures. All experiments were performed in triplicate. Details of nutritional estimation parameters have been previously described [21].
2.8. Statistical Analysis
Mean values for each compound were calculated from three replicates of oils harvested in three different years. Differences in fatty acid concentrations among the three oils were analyzed by two-way ANOVA followed by Tukey’s multiple comparison test. Differences in total phenolic content (TPC) among the three methanolic extracts of the oils and concentrations in total anacardic acids in the three oils were assessed using one-way ANOVA with Tukey’s post hoc test. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism version 8.0.1 for Windows (GraphPad Software, La Jolla, CA, USA).
3. Results
P. terebinthus fruit oil (TSO1) has been analyzed by GC-MS and the dominant compounds were monounsaturated, polyunsaturated, and saturated fatty acids. Results were compared with those of previous harvest years (TSO2 and TSO3) (Table 1).

Table 1.
Fatty acid profile of P. terebinthus oils.
The terebinth fruit oil methanolic extract and fruits methanolic extracts were analysed for their TPC and DPPH inhibitory activity (Table 2 and Table 3). The concentration of anacardic acids in turpentine oils has been evaluated by q-NMR spectra as shown in Table 4. Furthermore, the nutritional profile and microelement contents of P. terebinthus fruit and oil were thoroughly analyzed (Table 5).

Table 2.
TPC of P. terebinthus oils.

Table 3.
Determination of TPC and antioxidant activity by DPPH• assay of P. terebinthus fruits’ methanolic extract (TSFME).

Table 4.
Concentration of anacardic acids from 3 harvest years by q-NMR spectra.

Table 5.
Nutritional Characteristics and Microelement Contents of P. terebinthus Fruits and Oil (2021 Harvest).
3.1. Fatty Acid Profiles of P. terebinthus Oils (Harvest 2021 vs. Previous Years)
The fatty acid composition of cold-pressed turpentine’s tree fruit oil from the 2021 harvest (TSO1) was analyzed, revealing a lipid profile rich in oleic acid (C18:1 cis-9), an omega-9 monounsaturated fatty acid; linoleic acid (C18:2 n-6), a polyunsaturated fatty acid; and palmitic acid (C16:0), a saturated fatty acid. These three fatty acids constitute the primary lipid components of P. terebinthus oils, consistent with previous reports [22]. Comparative analysis included oils from the 2019 and 2020 harvests (TSO3 and TSO2, respectively), all exhibiting qualitatively similar fatty acid profiles.
Oleic acid was the predominant fatty acid across all samples, with its percentage observed in the 2020 oil (TSO2: 45.45 ± 0.52%) being significantly higher (p < 0.05) compared to the percentages observed in 2019 and 2021, suggesting influences of fruit maturity depending on climatic factors on monounsaturated fatty acid (MUFA) content. Linoleic acid, a nutritionally valuable polyunsaturated fatty acid (PUFA), showed a statistically significant gradual annual increase, peaking in the 2021 sample (TSO1: 22.30 ± 0.14%), followed by a decrease in 2020 (TSO2: 20.95 ± 0.47%) and 2019 oils (TSO3: 19.71 ± 0.65%). This trend clearly reflects a shift from polyunsaturated to monounsaturated forms during fruit ripening or in response to environmental variables as it is already proven that the oil composition of fruit varies in different growth periods [22]. Palmitic acid, the main saturated fatty acid (SFA), was measured at 24.99 ± 0.15% in TSO1, slightly lower than in 2020 oil (TSO2: 25.34 ± 0.77%) and significantly less than in 2019 oil (TSO3: 30.28 ± 1.35%) compared to both TSO1 and TSO2 (p < 0.05). These findings indicate that palmitic and oleic acid concentrations increase with fruit maturation, while linoleic acid tends to decrease, demonstrating that oil composition varies across different growth stages. This is in line with previous developmental studies [22], which showed that in Pistacia terebinthus L. naturally growing in the south-eastern Anatolian region of Turkey, ripening was associated with an increase in oleic and palmitic acid contents and a decrease in linoleic acid.
Minor fatty acids such as palmitoleic (3.04–4.49%) and stearic acids (1.9–2.4%) were also detected, consistent with earlier reports [2]. Additional minor fatty acids previously identified—arachidic acid (<0.2%) [17], cis-vaccenic acid [10], cis-13-octadecenoic acid (0.2–2.6%), linolenic acid (0.4–1.4%) [22], myristic and lauric acids (~0.1%), and eicosenoic acid (0.2%) [2]—were likewise found in comparable proportions in the present samples.
These results confirm that terebinth fruit oil is a rich source of dietary fats, particularly ω-9 and ω-6 fatty acids, which are linked to numerous health benefits. Diets high in oleic acid, for example, have been associated with reduced LDL cholesterol levels [23,24].
Across all harvest years, saturated fatty acids (ΣSFA) ranged from 27.44% to 32.29%, monounsaturated fatty acids (ΣMUFA) dominated with 47.95% to 55.36%, and polyunsaturated fatty acids (ΣPUFA) ranging from 19.71% to 22.30%. The 2021 oil (TSO1) values fall well within these ranges, corroborating earlier literature data [2,25,26] which report typical ranges for P. terebinthus oils: oleic acid (34.8–55.2%), linoleic acid (16.2–29.6%), and palmitic acid (13.7–25.7%).
Fatty acid profiling was conducted following standard lipid analysis procedures involving saponification and methylation (transesterification) to produce fatty acid methyl esters (FAMEs).
3.2. Total Phenolic Content (TPC) of Oils
The total phenolic content (TPC) of 2021 P. terebinthus oil (TSOME1) is shown in Table 2 and compared with values of the previous harvest years (TSOME2 and TSOME3). The extracts of 2021 harvest (TSOME1) exhibited the highest content, measured at 11.10 ± 0.50 g GAE/kg oil, followed by the 2019 oil (TSOME3: 9.17 ± 0.01 g GAE/kg oil) and the lowest in the 2020 sample (TSOME2: 7.73 ± 0.09 g GAE/kg oil).
3.3. Total Phenolic Content and Antioxidant Activity of P. terebinthus Fruits
To evaluate the antioxidant potential of P. terebinthus fruits, their total phenolic content was quantified, and their free radical scavenging activity was determined using the DPPH• assay.
The methanolic extract of P. terebinthus fruits demonstrated a remarkably high total phenolic content (185.92 ± 2.61 mg GAE/g extract). In line with this, the DPPH• radical scavenging test revealed strong inhibitiory activity across all the tested concentrations, reaching nearly 50% inhibition even at the lowest dose. The calculated IC50 value (58.37 μg/mL), confirmed the notable antioxidant efficiency of the extract.
3.4. Quantification of Fruits’ Anacardic Acid Content
The bioactive phenolic acids’ content, expressed as anacardic acids (AAs) has been measured using a reliable and simple analytical method based on 1D qNMR [20].
Anacardic acids were quantitated as a total due to the possible differences in the side chain (length or number of double bonds), using the characteristic peaks at 7.33 ppm and 6.85 ppm that did not show any overlapping. The amount of each compound in the oil sample was calculated in comparison to the amount of syringaldehyde that was used as internal standard (singlet peak at 9.81 ppm) (Figure 1).
Figure 1.
1H-NMR spectrum of the aromatic region of the P. terebinthus oil extract showing the peaks that were used for the quantification of anacardic acids.
The Greek terebinth fruit oil samples exhibited high amounts of anacardic acids 1.91–2.98 mmol/kg oil as shown in Table 4. TSO3 (2019 harvest) exhibited the highest AAs levels, while subsequent years showed notable concentrations. However, the results reported herein add an important dimension to P. terebinthus nutritional profile. Anacardic acids are phenolic lipids that are increasingly recognized for their bioactive properties, including anti-inflammatory, antioxidant, antibacterial, gastroprotective, antitumoral and lipoxygenase inhibitory activities [13,14,27], highlighting their pharmacological interest.
3.5. Nutritional Evaluation of TSF and TSO1
The fruits (TSF) and oil (TSO1) of P. terebinthus (2021 harvest), obtained through the cold-pressing method—a green and safe method for consumers—were examined for their nutritional values (Table 5). Macronutrient analysis was performed by the determination of several parameters (energy, protein, ash, crude fibers).
The fruits were particularly rich in crude fibers (38.9%), well-known nutritional compounds as they play a key role in modulating blood glucose and cholesterol levels. TSF also contained significant amounts of proteins (7.3%), total sugars (1.9%) and digestible carbohydrates (10.2%), while its fat content was 34.0%, resulting in an energy value of 454 Kcal/100 g.
The mineral contents of terebinth fruits were also determined revealing an excellent source that offers nutritional advantages. Greek turpentine fruits showed even greater mineral concentrations compared to previous reports [2]. Furthermore, their nutritional profile can be comparable with well-known commercial fruits, such as olive, pistachio, banana and figs in terms of certain minerals [21]. Fruits showed high levels of K, Mg and Zn along with other crucial minerals for human health, such as Fe and Zn, all of which are essential for cardiovascular health, bone health and oxygen transport. Moreover, the very low sodium content (<0.3%) is beneficial for cardiovascular health, particularly in individuals managing salt-sensitive hypertension.
Variations in physical properties across previously reported studies [2,17,25,28] can be attributed to the differences in geographic and environmental conditions, the fruit size and the analytical methods used. In addition, moisture, crude protein, ash, crude fiber and crude oil contents of fruits are strongly influenced by variety and agronomic conditions.
In addition, the oil exhibited a higher fat content (99.7%) and an elevated calorific value (879 Kcal/100 g), as expected for a lipid-rich product. Additionally, the specific extinction coefficients at 232 nm (K232) and 270 nm (K270), commonly used to evaluate oxidative stability and quality in edible oils, particularly in extra virgin olive oil. Our results showed that both K-values were relatively low, suggesting minimal oxidation and confirming the freshness and integrity of terebinth oil. These results reinforce the high-quality profile of P. terebinthus oil and its potential as a functional, nutritionally rich lipid source.
4. Discussion
GC-MS analysis revealed that P. terebinthus fruit oil is dominated by oleic, palmitic, and linoleic acids, similar to previous studies from Anatolia [4,22]. However, the oleic acid levels reported in the current study (42.7–45.5 g/100 g) fall within the upper range of reported values and rank among the highest documented in the literature [2,10,25,26,28].
This is nutritionally relevant since oleic acid (a MUFA) is strongly associated with cardiovascular benefits [23,24], and improved oxidative stability of oils. In contrast, the relatively high palmitic acid levels exceed values reported for Turkish terebinth oils [22] and may reduce some of the health advantages, given the less favorable metabolic profile of saturated fats. This deviation could be linked to geographic variation, fruit maturity stage, or environmental factors. One-way ANOVA confirmed that the differences in oleic, palmitic, and linoleic acid contents across samples were statistically significant (p < 0.05), thereby supporting the validity of these compositional variations. Thus, while the oil composition resembles high-quality olive oils in MUFA dominance, the elevated palmitic fraction may represent a unique characteristic of Greek P. terebinthus.
Compared to olive oil, P. terebinthus oil demonstrates a similarly high MUFA content (47.9–55.4 g/100 g), but lower PUFA (15–20.9 g/100 g). This lower PUFA fraction reduces susceptibility to oxidation, supporting a longer shelf-life and potential for industrial use. At the same time, linoleic acid levels (19–22 g/100 g) are nutritionally valuable, as ω-6 fatty acids are essential for human health. Taken together, the balance of MUFA and PUFA suggests that terebinth oil could be positioned as a premium edible oil, nutritionally competitive with olive oil while offering distinctive compositional traits.
The detection of anacardic acids (1.91–2.98 mmol/kg oil) adds further value, since these phenolic lipids are associated with antioxidant, antimicrobial, and anticancer prop-erties [14]. Although literature on terebinth-derived anacardic acids is scarce, parallels with cashew and other anacardic-rich sources suggest that these compounds may significantly contribute to the bioactivity of the oil. In this respect, our findings extend prior reports by demonstrating the dual nutritional and functional potential of P. terebinthus oil.
The methanolic extract exhibited a very high TPC (185.92 ± 2.61 mg GAE/g) and strong radical scavenging activity (>80% inhibition in DPPH assay). These values are higher than those reported for related Pistacia species, suggesting that Greek P. terebinthus fruits are particularly rich in antioxidant phenolics. The synergistic interaction of phenolic acids and unsaturated fatty acids may further enhance this capacity, a point that warrants future mechanistic studies. Nutritional analysis revealed that terebinth fruits are also high in dietary fiber (38.9%), comparable to fiber-rich foods such as olives, pistachios, and figs. This supports their traditional dietary use and highlights their potential role in lipid metabolism regulation and gut health. Combined with favorable oil quality indices (low K232 and K270 values indicating stability), the fruits and oils present promising opportunities for both nutritional and functional applications.
Finally, one-way ANOVA analysis confirmed that the overall differences highlighted and discussed herein were statistically significant (p < 0.05), thereby supporting the interpretation of the observed trends.
5. Conclusions
In the current study, the fatty acid composition of P. terebinthus (terebinth) fruit oil, foraged from the island of Chios (Greek North East Aegean Sea) was analyzed for the first time using GC-MS following conventional procedures, including saponification, methylation, and direct saponification. Additionally, the total phenolic content (TPC) was determined using the Folin–Ciocalteu method. The results demonstrated that terebinth fruits and their oil are rich sources of mono- and polyunsaturated fatty acids, particularly omega-9 (oleic acid) and omega-6 (linoleic acid) types. Oleic acid, the predominant fatty acid, is known for its health-promoting properties, including its association with reduced levels of low-density lipoprotein (LDL) cholesterol. The fatty acid profile of the oil was comparable to that of olive oil, often regarded as the “prince” of vegetable oils. The 2021 sample (TSO1) exhibited a balanced and high-quality lipid composition, consistent with previous harvests. Minor year-to-year variations in composition highlight the dynamic nature of oil biosynthesis, which is influenced by both biological maturity and environmental factors. Based on its dominant fatty acid composition, terebinth oil can be classified as a high oleic acid-rich oil, aligning with the classification system of vegetable oils [29].
A notable feature of the oil is its high content of anacardic acids (AAs), a class of phenolic lipids primarily found in the Anacardiaceae family, known for their antioxidant, antimicrobial, and anti-inflammatory properties. However, AAs are also recognized as potential allergens, capable of acting as skin sensitizers and inducing allergic contact dermatitis in sensitive individuals [30]. While their presence in P. terebinthus oil enhances its functional potential, particularly in nutraceutical and cosmetic applications, their allergenic properties necessitate careful evaluation. Given these safety concerns, precautionary measures are recommended: in cosmetic applications, controlled concentrations and clear labeling are essential, while in dietary uses, exposure levels should be further assessed through toxicological studies. Thus, although the presence of AAs was confirmed through a validated and reliable analytical method, their inclusion in consumer products should be guided by appropriate safety evaluations and regulatory considerations.
Nutritionally, both the fruits and the oil demonstrated valuable characteristics. The high fiber content of the fruits is particularly relevant for promoting lipid homeostasis and may contribute to antihyperlipidemic effects. Additionally, the notable levels of protein and oil, combined with the pleasant aroma and flavor, support the potential use of terebinth in the food industry.
Growing global interest in natural, traditional, and health-promoting products has increased demand for terebinth fruits, which are still consumed in various regions as roasted snacks and ingredients in baked goods. This study offers meaningful insights into the nutritional value of this underutilized Mediterranean species, particularly in the context of wild edible fruits and nuts. Notably, it presents the first report on Greek terebinth oil (Chios turpentine), highlighting its unique chemical composition and bioactive properties. The data supports its potential as a sustainable, value-added resource derived from wild flora and foraged traditionally. Given its promising health benefits, P. terebinthus oil shows strong potential for use in the food, cosmetic, and pharmaceutical industries, aligning with eco-conscious and health-driven consumer trends. Overall, this work contributes to a more comprehensive understanding of the nutritional and functional value of P. terebinthus, emphasizing its relevance as a promising natural source.
Author Contributions
Conceptualization, I.C. and K.G.; methodology, E.-F.V., P.M. and O.G.; validation, E.-F.V., P.M. and O.G.; formal analysis, K.G., E.-F.V., P.M. and O.G.; investigation, E.-F.V., P.M. and O.G.; resources, I.C.; data curation, K.G., E.-F.V. and I.C.; writing—original draft preparation, K.G., E.-F.V. and I.C.; writing—review and editing, K.G., E.-F.V. and I.C.; supervision, I.C.; funding acquisition, I.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
Abbreviations
| DPPH 2 | 2-diphenyl-1-picrylhydrazyl |
| FAMEs | Fatty acid methyl esters |
| GAE | Gallic acid equivalents |
| MUFA | Monounsaturated fatty acids |
| PUFA | Polyunsaturated fatty acids |
| qNMR | Quantitative NMR |
| SFA | Saturated fatty acids |
| TPC | Total phenolic content |
| TSF | Terebinth dried fruit sample |
| TSFME | Terebinth fruit methanolic extract |
| TSO1 | Terebinth oil harvest year 2021 |
| TSO2 | Terebinth oil harvest year 2020 |
| TSO3 | Terebinth oil harvest year 2019 |
| TSOME1 | Methanolic extract of Oil TSO1 |
| TSOME2 | Methanolic extract of Oil TSO2 |
| TSOME3 | Methanolic extract of Oil TSO3 |
References
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1968; Volume 2, p. 237. [Google Scholar]
- Özcan, M. Characteristics of fruit and oil of terebinth (Pistacia terebinthus L.) growing wild in Turkey. J. Sci. Food Agric. 2004, 84, 517–520. [Google Scholar] [CrossRef]
- Topçu, G.; Ay, M.; Bilici, A.; Sarıkürkçü, C.; Öztürk, M.; Ulubelen, A. A new flavone from antioxidant extracts of Pistacia terebinthus. Food Chem. 2007, 103, 816–822. [Google Scholar] [CrossRef]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Salehi-Surmaghi, M.H.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. lentiscus and P. khinjuk): A review of their traditional uses, phytochemistry, and pharmacology. Sci. World J. 2013, 2013, 219815. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Bader, A.; Cioni, P.L.; Katbeh-Bader, A.; Morelli, I. Composition of the essential oil of leaves, galls, and ripe and unripe fruits of Jordanian Pistacia palaestina Boiss. J. Agric. Food Chem. 2004, 52, 572–576. [Google Scholar] [CrossRef]
- Grant Wyllie, S.; Brophy, J.J.; Sarafis, V.; Hobbs, M. Volatile components of the fruit Pistacia lentiscus. J. Food Sci. 1990, 55, 1325–1326. [Google Scholar] [CrossRef]
- Badem, A. Some Traditional Terebinth Dishes in Turkey and Their Health Effects. J. Gastron. Hosp. Travel 2021, 4, 306–320. [Google Scholar] [CrossRef]
- Fidan, M.S.; Baltacı, C.; Öz, M.; Akar, Z. Chemical Composition of Pistacia terebinthus L. and Its Phytochemical and Biological Properties. BioResources 2023, 18, 6862–6881. [Google Scholar] [CrossRef]
- Savvides, A.M.; Stavridou, C.; Ioannidou, S.; Zoumides, C.; Stylianou, A. An ethnobotanical investigation into the traditional uses of mediterranean medicinal and aromatic plants: The case of Troodos mountains in Cyprus. Plants 2023, 12, 1119. [Google Scholar] [CrossRef]
- Matthäus, B.; Özcan, M.M. Quantitation of Fatty Acids, Sterols, and Tocopherols in Turpentine (Pistacia terebinthus Chia) Growing Wild in Turkey. J. Agric. Food Chem. 2006, 54, 7667–7671. [Google Scholar] [CrossRef]
- Özcan, M.M.; Tzakou, O.; Couladis, M. Essential oil composition of the turpentine tree (Pistacia terebinthus L.) fruits growing wild in turkey. Food Chem. 2009, 114, 282–285. [Google Scholar] [CrossRef]
- Kıvçak, B.; Akay, S. Quantitative determination of α-tocopherol in Pistacia lentiscus, Pistacia lentiscus var. Chia, and Pistacia terebinthus by TLC-densitometry and colorimetry. Fitoterapia 2005, 76, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Gomes Junior, A.L.; Islam, M.T.; Nicolau, L.A.D.; de Souza, L.K.M.; Araújo, T.D.S.L.; Lopes de Oliveira, G.A.; Melo-Cavalcante, A.A.D.C. Anti-inflammatory, antinociceptive, and antioxidant properties of anacardic acid in experimental models. ACS Omega 2020, 5, 19506–19515. [Google Scholar] [CrossRef]
- Salehi, B.; Gültekin-Özgüven, M.; Kirkin, C.; Özçelik, B.; Morais-Braga, M.F.; Carneiro, J.N.; Bezerra, C.F.; Silva, T.G.; Coutinho, H.D.; Amina, B.; et al. Antioxidant, antimicrobial, and anticancer effects of anacardium plants: An ethnopharmacological perspective. Front. Endocrinol. 2020, 11, 295. [Google Scholar] [CrossRef]
- Najibullah, S.N.M.; Ahamad, J.; Sultana, S.; Zafar, A. Chemical Characterization and Evaluation of Anticancer Activity of Pistacia terebinthus Linn. Fruits Essential Oil. J. Essent. Oil Bear. Plants 2022, 25, 180–187. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Papageorgiou, V.P. GC-MS analysis of penta- and tetra-cyclic triterpenes from resins of Pistacia species. Part II. Pistacia terebinthus var. Chia. Biomed. Chromatogr. 2005, 19, 586–605. [Google Scholar] [CrossRef]
- Özcan, M.M.; Al Juhaimi, F.; Uslu, N.; Ahmed, I.A.M.; Babiker, E.E.; Osman, M.A.; Ghafoor, K. Effect of sonication process of terebinth (Pistacia terebinthus L.) fruits on antioxidant activity, phenolic compounds, fatty acids and tocopherol contents. J. Food Sci. Technol. 2020, 57, 2017–2025. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhang, P.; Cui, P.L.; Cheng, W.G.; Zhang, S.J. Glycerol carbonate synthesis from glycerol and dimethyl carbonate using guanidine ionic liquid. Chin. J. Chem. Eng. 2017, 25, 1182–1186. [Google Scholar] [CrossRef]
- Varvouni, E.F.; Graikou, K.; Gortzi, O.; Cheilari, A.; Aligiannis, N.; Chinou, I. Chemical and biological evaluation of the oil and seedcake from seeds of a Greek cardoon cultivar as potential functional vegetable oil. Comparison with sesame, flaxseed and extra virgin olive oils. Foods 2021, 10, 2665. [Google Scholar] [CrossRef] [PubMed]
- Diamantakos, P.; Ioannidis, K.; Papanikolaou, C.; Tsolakou, A.; Rigakou, A.; Melliou, E.; Magiatis, P. A new definition of the term “high-phenolic olive oil” based on large scale statistical data of Greek olive oils analyzed by qNMR. Molecules 2021, 26, 1115. [Google Scholar] [CrossRef] [PubMed]
- Graikou, K.; Kourti, P.M.; Zengin, G.; Gortzi, O.; Danalatos, N.; Chinou, I. Chemical characterisation-biological evaluation of Greek cultivar cardoon seeds (Cynara cardunculus). A by-product with potential high added value. Planta Med. 2021, 87, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Inan, M. Seasonal variation of fatty and essential oil in terebinth (Pistacia terebinthus L.) fruit. Not. Bot. Horti Agrobot. 2021, 49, 12171. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef]
- Rauf, A.; Patel, S.; Uddin, G.; Siddiqui, B.S.; Ahmad, B.; Muhammad, N.; Hadda, T.B. Phytochemical, ethnomedicinal uses and pharmacological profile of genus Pistacia. Biomed. Pharmacother. 2017, 86, 393–404. [Google Scholar] [CrossRef]
- Kizil, S.; Turk, M. Microelement contents and fatty acid compositions of Rhus coriaria L. and Pistacia terebinthus L. fruits spread commonly in the southeastern Anatolia region of Turkey. Nat. Prod. Res. 2010, 24, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, G.; Gökmen, V. Changes in oxidative stability, antioxidant capacity and phytochemical composition of Pistacia terebinthus oil with roasting. Food Chem. 2011, 128, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D. Advancing Pistacia terebinthus L. (Anacardiaceae) Research: Food Preservation, Functional Foods, and Nutraceutical Potential. Foods 2025, 14, 1245. [Google Scholar] [CrossRef] [PubMed]
- Kaya, F.; Özer, A. Characterization of extracted oil from seeds of terebinth (Pistacia terebinthus L.) growing wild in Turkey. Turk. J. Sci. Technol. 2015, 10, 49–57. [Google Scholar]
- Gunstone, F. Edible Oil and Fat Products: Chemistry, Properties, and Health Effects. In Bailey’s Industrial Oil and Fat Products, 6th ed.; Shahidi, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 1. [Google Scholar]
- Mattison, C.P.; Malveira Cavalcante, J.; Izabel Gallão, M.; Sousa de Brito, E. Effects of industrial cashew nut processing on anacardic acid content and allergen recognition by IgE. Food Chem. 2018, 240, 370–376. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).