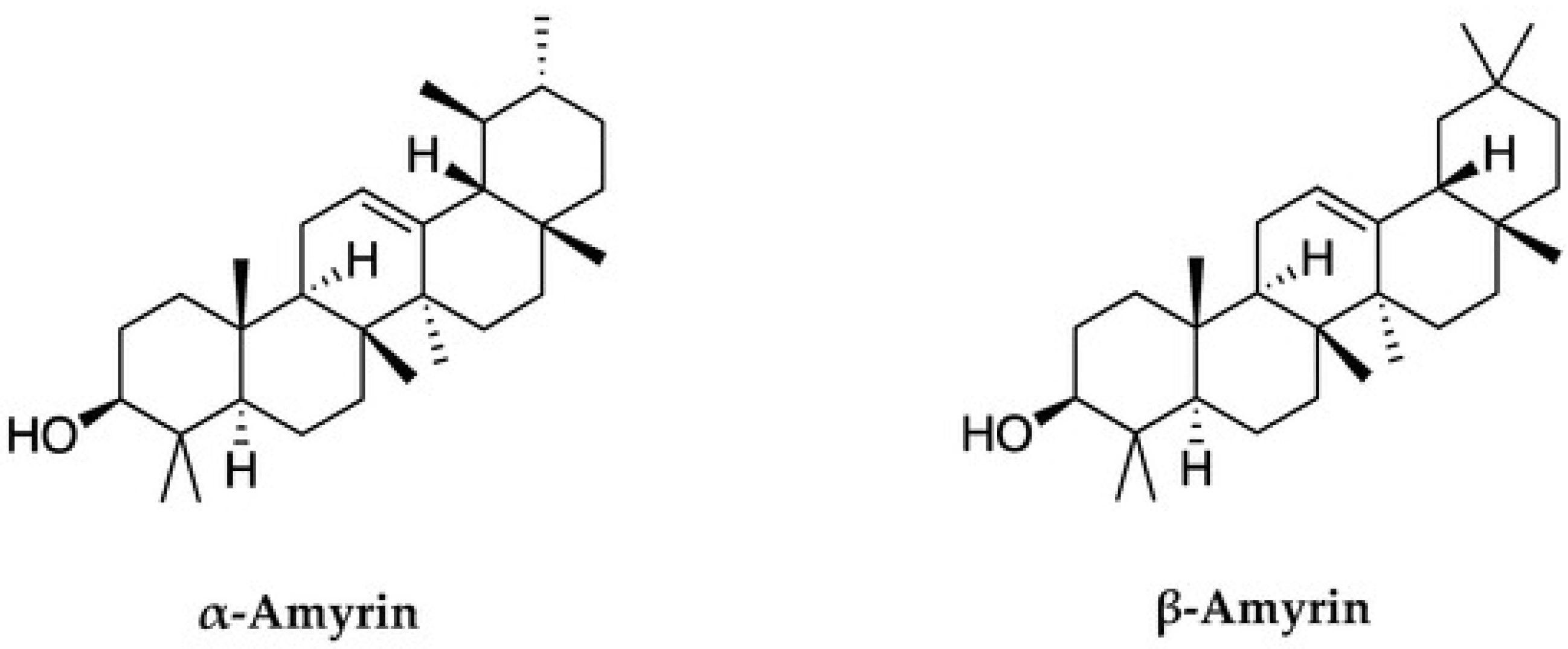
The Pharmaceutical Potential of α- and β-Amyrins
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Anti-Inflammatory Potential of α- and β-Amyrins
3.2. Antidiabetes Potential of α- and β-Amyrins
3.3. Antiatherosclerosis Effect of α- and β-Amyrins
3.4. Antinociceptive Effect of α- and β-Amyrins
3.5. Antigout Effect of α- and β-Amyrins
3.6. Positive Effects of α- and β-Amyrins on Nerves
3.7. Anti-Parkinsonian Effects of α- and β-Amyrins
3.8. Anticancer Potential of α- and β-Amyrins
3.9. Antibacterial Potential of α- and β-Amyrins
3.10. Anti-HIV Potential of α- and β-Amyrins
3.11. The Isolation α- and β-Amyrins
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.C.; Schellack, N.; Stokes, J.; Lancaster, R.; Zeeman, H.; Defty, D.; Steel, G. Ongoing initiatives to improve the quality and efficiency of medicine use within the public healthcare system in South Africa; a preliminary study. Front. Pharmacol. 2017, 8, 751. [Google Scholar] [CrossRef] [PubMed]
- Ding, B. Pharma Industry 4.0: Literature review and research opportunities in sustainable pharmaceutical supply chains. Process Saf. Environ. Prot. 2018, 119, 115–130. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in translation: The valley of death across preclinical and clinical divide–identification of problems and overcoming obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef]
- Algorri, M.; Abernathy, M.J.; Cauchon, N.S.; Christian, T.R.; Lamm, C.F.; Moore, C.M. Re-envisioning pharmaceutical manufacturing: Increasing agility for global patient access. J. Pharm. Sci. 2022, 111, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Anh, L.T.P.; Thuy, D.N. Role of Regulatory Policies and Benefits for Herbal Medicine Development in Vietnam. World News Nat. Sci. 2025, 59, 193–203. [Google Scholar]
- Gonzalez Pena, O.I.; López Zavala, M.Á.; Cabral Ruelas, H. Pharmaceuticals market, consumption trends and disease incidence are not driving the pharmaceutical research on water and wastewater. Int. J. Environ. Res. Public Health 2021, 18, 2532. [Google Scholar] [CrossRef]
- Wouters, O.J.; McKee, M.; Luyten, J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. JAMA 2020, 323, 844–853. [Google Scholar] [CrossRef]
- Perehudoff, K. Universal access to essential medicines as part of the right to health: A cross-national comparison of national laws, medicines policies, and health system indicators. Glob. Health Action 2020, 13, 1699342. [Google Scholar] [CrossRef]
- Abunna, F.; Mamo, G.; Megersa, B. One Health–A holistic solution for sustainable management of globalization-driven public health challenges. Ethiop. Vet. J. 2022, 26, 107–131. [Google Scholar] [CrossRef]
- Dalal, P.K.; Roy, D.; Choudhary, P.; Kar, S.K.; Tripathi, A. Emerging mental health issues during the COVID-19 pandemic: An Indian perspective. Indian J. Psychiatry 2020, 62, 354. [Google Scholar] [CrossRef] [PubMed]
- Gossling, S.; Scott, D.; Hall, C.M. Pandemics, tourism, and global change: A rapid assessment of COVID-19. J. Sustain. Tour. 2020, 29, 1–20. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Saraf, S. Limonoids: Overview of significant bioactive triterpenes distributed in plants kingdom. Biol. Pharm. Bull. 2006, 29, 191–201. [Google Scholar] [CrossRef]
- Garg, A.; Sharma, R.; Dey, P.; Kundu, A.; Kim, H.S.; Bhakta, T.; Kumar, A. Analysis of triterpenes and triterpenoids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 393–426. [Google Scholar]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis, and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Ha, T.K.Q.; Yang, J.L.; Pham, H.T.T.; Oh, W.K. Triterpenoids from the genus Gynostemma: Chemistry and pharmacological activities. J. Ethnopharmacol. 2021, 268, 113574. [Google Scholar] [CrossRef]
- Mahato, S.B.; Sen, S. Advances in triterpenoid research, 1990–1994. Phytochemistry 1997, 44, 1185–1236. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Li, L.; Song, W.; Li, M.; Hua, X.; Xue, Z. Natural products of pentacyclic triterpenoids: From discovery to heterologous biosynthesis. Nat. Prod. Rep. 2023, 40, 1303–1353. [Google Scholar] [CrossRef]
- Luchnikova, N.A.; Grishko, V.V.; Ivshina, I.B. Biotransformation of oleanane and ursane triterpenic acids. Molecules 2020, 25, 5526. [Google Scholar] [CrossRef]
- Morita, M.; Shibuya, M.; Kushiro, T.; Masuda, K.; Ebizuka, Y. Molecular cloning and functional expression of triterpene synthases from pea (Pisum sativum) New α-amyrin-producing enzyme is a multifunctional triterpene synthase. Eur. J. Biochem. 2000, 267, 3453–3460. [Google Scholar] [CrossRef]
- Viet, T.D.; Xuan, T.D.; Anh, L.H. α-Amyrin and β-amyrin isolated from Celastrus hindsii leaves and their antioxidant, anti-xanthine oxidase, and anti-tyrosinase potentials. Molecules 2021, 26, 7248. [Google Scholar] [CrossRef]
- Holanda Pinto, S.A.; Pinto, L.M.S.; Cunha, G.M.A.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Anti-inflammatory effect of α, β-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology 2008, 16, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, K.A.S.; Paszcuk, A.F.; Passos, G.F.; Silva, E.S.; Bento, A.F.; Meotti, F.C.; Calixto, J.B. Activation of cannabinoid receptors by the pentacyclic triterpene α, β-amyrin inhibits inflammatory and neuropathic persistent pain in mice. J. Pain 2011, 152, 1872–1887. [Google Scholar] [CrossRef] [PubMed]
- Vitor, C.E.; Figueiredo, C.P.; Hara, D.B.; Bento, A.F.; Mazzuco, T.L.; Calixto, J.B. Therapeutic action and underlying mechanisms of a combination of two pentacyclic triterpenes, α-and β-amyrin, in a mouse model of colitis. Br. J. Pharmacol. 2009, 157, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Matos, I.; Bento, A.F.; Marcon, R.; Claudino, R.F.; Calixto, J.B. Preventive and therapeutic oral administration of the pentacyclic triterpene α, β-amyrin ameliorates dextran sulfate sodium-induced colitis in mice: The relevance of cannabinoid system. Mol. Immunol. 2013, 54, 482–492. [Google Scholar] [CrossRef]
- Melo, C.M.; Carvalho, K.M.M.B.; de Sousa Neves, J.C.; Morais, T.C.; Rao, V.S.; Santos, F.A.; Chaves, M.H. α, β-amyrin, a natural triterpenoid ameliorates L-arginine-induced acute pancreatitis in rats. World J. Gastroenterol. 2010, 16, 4272. [Google Scholar] [CrossRef]
- Santos, F.A.; Frota, J.T.; Arruda, B.R.; de Melo, T.S.; da Silva, A.A.D.C.A.; Brito, G.A.D.C.; Rao, V.S. Antihyperglycemic and hypolipidemic effects of α, β-amyrin, a triterpenoid mixture from Protium heptaphyllum in mice. Lipids Health Dis. 2012, 11, 98. [Google Scholar] [CrossRef]
- Nair, S.A.; Sabulal, B.; Radhika, J.; Arunkumar, R.; Subramoniam, A. Promising anti-diabetes mellitus activity in rats of β-amyrin palmitate isolated from Hemidesmus indicus roots. Eur. J. Pharmacol. 2014, 734, 77–82. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Munvera, A.M.; Botezatu, A.V.D.; Talla, E.; Ceylan, O.; Fotsing, M.T.; Dinica, R.M. Synthesis of benzoyl esters of β-amyrin and lupeol and evaluation of their antibiofilm and antidiabetic activities. Results Chem. 2022, 4, 100322. [Google Scholar] [CrossRef]
- Giacoman-Martínez, A.; Alarcón-Aguilar, F.J.; Zamilpa, A.; Huang, F.; Romero-Nava, R.; Román-Ramos, R.; Almanza-Pérez, J.C. α-Amyrin induces GLUT4 translocation mediated by AMPK and PPARδ/γ in C2C12 myoblasts. Can. J. Physiol. Pharmacol. 2021, 99, 935–942. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, H.; Zhang, Q.; Xu, J. β-Amyrin ameliorates diabetic nephropathy in mice and regulates the miR-181b-5p/HMGB2 axis in high glucose-stimulated HK-2 cells. Environ. Toxicol. 2021, 37, 637–649. [Google Scholar] [CrossRef]
- Rathinavel, T.; Ammashi, S.; Gnanendra Shanmugam, S.T. Identification of anti-diabetic phytocompounds from Ficus racemosa and its validation through in silico molecular modeling. Int. J. Adv. Sci. Eng. 2019, 5, 1085–1098. [Google Scholar]
- Zhu, Q.J.; Lang, L.J.; Wang, Y.; Zhang, D.Q.; Jiang, B.; Xiao, C.J. Triterpenoids from the fruits of wild species of Crataegus scabrifolia and their lipid-lowering activities. Russ. J. Bioorg. Chem. 2022, 48, 1291–1298. [Google Scholar]
- Ding, Y.; Nguyen, H.T.; Kim, S.I.; Kim, H.W.; Kim, Y.H. The regulation of inflammatory cytokine secretion in macrophage cell line by the chemical constituents of Rhus sylvestris. Bioorg. Med. Chem. Lett. 2009, 19, 3607–3610. [Google Scholar] [CrossRef] [PubMed]
- De Lima, R.P.; Nunes, P.I.G.; Viana, A.F.S.C.; de Oliveira, F.T.B.; Silva, R.A.C.; Alves, A.P.N.N.; Santos, F.A. α, ß-Amyrin prevents steatosis and insulin resistance in a high-fat diet-induced mouse model of NAFLD via the AMPK-mTORC1-SREBP1 signaling mechanism. Braz. J. Med. Biol. Res. 2021, 54, e11391. [Google Scholar]
- Shih, M.F.; Cherng, J.Y. Reduction of adhesion molecule production and alteration of eNOS and endothelin-1 mRNA expression in endothelium by Euphorbia hirta L. through its beneficial β-amyrin molecule. Molecules 2014, 19, 10534–10545. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Nakahara, T.; Ikeuchi, S.; Nishimura, M. β-Amyrin induces angiogenesis in vascular endothelial cells through the Akt/endothelial nitric oxide synthase signaling pathway. Biochem. Biophys. Res. Commun. 2015, 467, 676–682. [Google Scholar] [CrossRef]
- Santos, F.A.; Carvalho, K.M.M.B.; Batista-Lima, F.J.; Nunes, P.I.G.; Viana, A.F.S.C.; da Silva, A.A.d.C.A.; de Brito, T.S. The triterpenoid alpha, beta-amyrin prevents the impaired aortic vascular reactivity in high-fat diet-induced obese mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 1029–1039. [Google Scholar] [CrossRef]
- Pinto, S.H.; Pinto, L.M.S.; Guedes, M.A.; Cunha, G.M.A.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Antinoceptive effect of triterpenoid α, β-amyrin in rats on orofacial pain induced by formalin and capsaicin. Phytomedicine 2008, 15, 630–634. [Google Scholar] [CrossRef]
- Otuki, M.F.; Ferreira, J.; Lima, F.V.; Meyre-Silva, C.; Malheiros, A.; Muller, L.A.; Calixto, J.B. Antinociceptive properties of mixture of α-amyrin and β-amyrin triterpenes: Evidence for participation of protein kinase C and protein kinase A pathways. J. Pharmacol. Exp. Ther. 2005, 313, 310–318. [Google Scholar] [CrossRef]
- Soldi, C.; Pizzolatti, M.G.; Luiz, A.P.; Marcon, R.; Meotti, F.C.; Mioto, L.A.; Santos, A.R. Synthetic derivatives of the α-and β-amyrin triterpenes and their antinociceptive properties. Bioorg. Med. Chem. 2008, 16, 3377–3386. [Google Scholar] [CrossRef]
- Chicca, A.; Marazzi, J.; Gertsch, J. The antinociceptive triterpene β-amyrin inhibits 2-arachidonoylglycerol (2-AG) hydrolysis without directly targeting cannabinoid receptors. Br. J. Pharmacol. 2012, 167, 1596–1608. [Google Scholar] [CrossRef]
- Ferraz-Filha, Z.S.; Araújo, M.C.D.P.M.; Ferrari, F.C.; Dutra, I.P.A.R. Tabebuia roseoalba: In vivo hypouricemic and anti-inflammatory effects of its ethanolic extract and constituents. Planta Med. 2016, 82, 1395–1402. [Google Scholar] [CrossRef]
- Hernandez-Vázquez, L.; Palazón Barandela, J.; Navarro-Ocaña, A. The Pentacyclic Triterpenes α, β-Amyrins: A Review of Sources and Biological Activities; IntechOpen: London, UK, 2012; pp. 487–502. [Google Scholar]
- Lin, K.W.; Huang, A.M.; Tu, H.Y.; Lee, L.Y.; Wu, C.C.; Hour, T.C.; Lin, C.N. Xanthine oxidase inhibitory triterpenoid and phloroglucinol from Guttiferaceous plants inhibit growth and induced apoptosis in human NTUB1 cells through a ROS-dependent mechanism. J. Agric. Food Chem. 2011, 59, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Park, H.J.; Gao, Q.; Lee, H.E.; Park, S.J.; Hong, E.; Ryu, J.H. Positive effects of β-amyrin on pentobarbital-induced sleep-in mice via GABAergic neurotransmitter system. Behav. Brain Res. 2015, 291, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Aragao, G.F.; Carneiro, L.M.V.; Juniora, A.P.F.; Bandeira, P.N.; Lemos, T.L.G.; Viana, G.S.d.B. Evidence for excitatory and inhibitory amino acids participation in the neuropharmacological activity of alpha-and beta-amyrin acetate. Open Pharm. Sci. 2009, 3, 9–16. [Google Scholar]
- Oliveira, F.A.; Costa, C.L.; Chaves, M.H.; Almeida, F.R.; Cavalcante, Í.J.; Lima, A.F.; Rao, V.S. Attenuation of capsaicin-induced acute and visceral nociceptive pain by α-and β-amyrin, a triterpene mixture isolated from Protium heptaphyllum resin in mice. J. Life Sci. 2005, 77, 2942–2952. [Google Scholar] [CrossRef]
- Subarnas, A.N.A.S.; Tadano, T.; Oshima, Y.; Kisara, K.; Ohizumi, Y. Pharmacological properties of β-amyrin palmitate, a novel centrally acting compound, isolated from Lobelia inflata leaves. J. Pharm. Pharmacol. 1993, 45, 545–550. [Google Scholar] [CrossRef]
- Park, H.J.; Kwon, H.; Lee, J.H.; Cho, E.; Lee, Y.C.; Moon, M.; Jung, J.W. β-Amyrin ameliorates Alzheimer’s disease-like aberrant synaptic plasticity in the mouse hippocampus. Biomol. Ther. 2020, 28, 74–82. [Google Scholar] [CrossRef]
- Frota Aragão, G.; Oliveira Nogueira, A.; Félix Xavier Júnior, F.A.; Azul Monteiro Evangelista, J.S.; Nogueira Bandeira, P.; Fernandes, C.; Sampaio Assreuy, A.M. Acute toxicity study of the isomeric mixture of alpha and beta amyrin from Protium heptaphyllum (Aubl.) Marchand. Acta Sci. Biol. Sci. 2023, 45, e66144. [Google Scholar] [CrossRef]
- Wei, C.C.; Chang, C.H.; Liao, V.H.C. Anti-Parkinsonian effects of β-amyrin are regulated via LGG-1 involved autophagy pathway in Caenorhabditis elegans. Phytomedicine 2017, 36, 118–125. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, R.; Chang, M.; Jin, Q.; Zhang, H.; Wang, X. Health benefits of 4, 4-dimethyl phytosterols: An exploration beyond 4-desmethyl phytosterols. Food Funct. 2020, 11, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Darley-Usmar, V.; Zhang, J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol. 2014, 2, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Sigmond, T.; Barna, J.; Tóth, M.L.; Takács-Vellai, K.; Pásti, G.; Kovács, A.L.; Vellai, T. Autophagy in Caenorhabditis elegans. Methods Enzymol. 2008, 451, 521–540. [Google Scholar] [PubMed]
- Braak, H.; Del Tredici, K. Invited Article: Nervous system pathology in sporadic Parkinson disease. J. Neurol. 2008, 70, 1916–1925. [Google Scholar] [CrossRef]
- Huang, X.; Chen, H.; Miller, W.C.; Mailman, R.B.; Woodard, J.L.; Chen, P.C.; Poole, C. Lower low-density lipoprotein cholesterol levels are associated with Parkinson’s disease. Mov. Disord. 2007, 22, 377–381. [Google Scholar] [CrossRef]
- Kamaraj, M.; Olikkavi, K.; Vennila, L.; Bose, S.S.; Raj, S.M. In silico docking and anti-cancer activity of the isolated compounds (Alpha and Beta Amyrin) from methanolic bark extract of Shorea robusta. Int. J. Pure Med. Res. 2019, 4, 11–15. [Google Scholar]
- Lima, E.M.; Nascimento, A.M.; Lenz, D.; Scherer, R.; Meyrelles, S.S.; Boëchat, G.A.; Endringer, D.C. Triterpenes from the Protium heptaphyllum resin-chemical composition and cytotoxicity. Rev. Bras. Farmacogn. 2014, 24, 399–407. [Google Scholar] [CrossRef]
- Wen, S.; Gu, D.; Zeng, H. Antitumor effects of beta-amyrin in Hep-G2 liver carcinoma cells are mediated via apoptosis induction, cell cycle disruption and activation of JNK and P38 signalling pathways. J. BUON 2018, 23, 965–970. [Google Scholar]
- Zahid, S.; Malik, A.; Waqar, S.; Zahid, F.; Tariq, N.; Khawaja, A.I.; Ali, Q. Countenance and implication of Β-sitosterol, Β-amyrin and epiafzelechin in nickel exposed Rat: In-silico and in-vivo approach. Sci. Rep. 2023, 13, 21351. [Google Scholar] [CrossRef] [PubMed]
- Victor, M.M.; David, J.M.; dos Santos, M.A.; Barreiros, A.L.; Barreiros, M.L.; Andrade, F.S.; Pessoa, C. Synthesis and evaluation of cytotoxic effects of amino-ester derivatives of natural α, β-amyrin mixture. Bioorg. Med. Chem. 2017, 28, 2155–2162. [Google Scholar] [CrossRef]
- Barros, F.W.; Bandeira, P.N.; Lima, D.J.; Meira, A.S.; de Farias, S.S.; Albuquerque, M.R.J.; do Ó Pessoa, C. Amyrin esters induce cell death by apoptosis in HL-60 leukemia cells. Bioorg. Med. Chem. 2011, 19, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Anburaj, J.; Tamilselvi, E.; Swapna, S.; Amuthavalli, K. β-Amyrin Modulates P38 MAPK and Jnk Pathway to Inhibit Cell Proliferation and Induce ROS-mediated Apoptosis in HeLa Cells. Indian J. Pharm. Sci. 2020, 82, 420–428. [Google Scholar] [CrossRef]
- Park, S.; Hwang, K.; Na, J.R.; Lee, K.; Jeong, E.S.; Kim, S. Triterpenoids from the leaves of Dendropanax morbifera Léveille and its cytotoxic activity toward breast MCF-7 and lung A549 cancer cells. J. Food Sci. Preserv. 2018, 25, 471–481. [Google Scholar] [CrossRef]
- Keawsa-Ard, S.; Liawruangrath, B.; Kongtaweelert, S. Bioactive compounds from Mesua ferrea stems. Chiang Mai J. Sci. 2015, 42, 185–955. [Google Scholar]
- Han, G.; Lee, D.G. Antibacterial mode of action of β-Amyrin promotes apoptosis-like death in Escherichia coli by producing reactive oxygen species. J. Microbiol. Biotechnol. 2022, 32, 1547. [Google Scholar] [CrossRef]
- Asif, M.; Al-Mansoub, M.A.; Khan, M.S.S.; Yehya, A.H.S.; Ezzat, M.O.; Oon, C.E.; Majid, A.M.S.A. Molecular mechanisms responsible for programmed cell death-inducing attributes of terpenes from Mesua ferrea stem bark towards human colorectal carcinoma HCT 116 cells. J. Appl. Biomed. 2017, 15, 71–80. [Google Scholar] [CrossRef]
- Neto, S.F.; Prada, A.L.; Achod, L.D.R.; Torquato, H.F.V.; Lima, C.S.; Paredes-Gamero, E.J.; Amado, J.R.R. α-amyrin-loaded nanocapsules produce selective cytotoxic activity in leukemic cells. Biomed. Pharmacother. 2021, 139, 111656. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Bandeira, P.N.; Lemos, T.L.; Dos Santos, H.S.; Scherf, J.R.; Rocha, J.E.; Teixeira, A.M. In silico and in vitro evaluation of efflux pumps inhibition of α, β-amyrin. J. Biomol. Struct. Dyn. 2022, 40, 12785–12799. [Google Scholar] [CrossRef]
- Bata, M.M.; Adeshina, G.O.; Onaolapo, J.A.; Musa, A.M.; Mshelia, E.H.; Salihu, M.S.; Dauda, G. Antibacterial Activity of A and Β Amyrin Isolated from Morinda lucida Against Some Multidrug Resistant Enterobacteriaceae. J. Biol. Today’s World 2023, 14, 1–9. [Google Scholar]
- Choi, J.W.; Cho, E.J.; Lee, D.G.; Choi, K.; Ku, J.; Park, K.W.; Lee, S. Antibacterial activity of triterpenoids from Clerodendron trichotomum. J. Appl. Biol. Chem. 2012, 55, 169–172. [Google Scholar] [CrossRef][Green Version]
- Chen, D.F.; Zhang, S.X.; Wang, H.K.; Zhang, S.Y.; Sun, Q.Z.; Cosentino, L.M.; Lee, K.H. Novel anti-HIV lancilactone C and related triterpenes from Kadsura lancilimba. J. Nat. Prod. 1999, 62, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Triterpene esters from Uncaria rhynchophylla hooks as potent HIV-1 protease inhibitors and their molecular docking study. Sci. Rep. 2024, 14, 31576. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ma, C.M.; Chen, D.Y.; Hattori, M. Anti-HIV-1 protease triterpenoids from Stauntonia obovatifoliola Hayata subsp. intermedia. Phytochemistry 2008, 69, 1875–1879. [Google Scholar] [CrossRef]
- Kongkum, N.; Tuchinda, P.; Pohmakotr, M.; Reutrakul, V.; Piyachaturawat, P.; Jariyawat, S.; Napaswad, C. Cytotoxic, antitopoisomerase IIα, and anti-HIV-1 activities of triterpenoids isolated from leaves and twigs of Gardenia carinata. J. Nat. Prod. 2013, 76, 530–537. [Google Scholar] [CrossRef]
- Callies, O.; Bedoya, L.M.; Beltrán, M.; Muñoz, A.; Calderón, P.O.; Osorio, A.A.; Bazzocchi, I.L. Isolation, structural modification, and HIV inhibition of pentacyclic lupane-type triterpenoids from Cassine xylocarpa and Maytenus cuzcoina. J. Nat. Prod. 2015, 78, 1045–1055. [Google Scholar] [CrossRef]
- Qian, K.; Kuo, R.Y.; Chen, C.H.; Huang, L.; Morris-Natschke, S.L.; Lee, K.H. Anti-AIDS agents 81. Design, synthesis, and structure—Activity relationship study of betulinic acid and moronic acid derivatives as potent HIV maturation inhibitors. J. Med. Chem. 2010, 53, 3133–3141. [Google Scholar] [CrossRef]
- Kesselheim, A.S.; Sinha, M.S.; Avorn, J.; Sarpatwari, A. Pharmaceutical policy in the United States in 2019: An overview of the landscape and avenues for improvement. Stanf. Law Policy Rev. 2019, 30, 421. [Google Scholar]
- Tichy, E.M.; Hoffman, J.M.; Suda, K.J.; Rim, M.H.; Tadrous, M.; Cuellar, S.; Schumock, G.T. National trends in prescription drug expenditures and projections for 2022. Am. J. Health Pharm. 2022, 79, 1158–1172. [Google Scholar] [CrossRef]
- Klein, E.Y.; Impalli, I.; Poleon, S.; Denoel, P.; Cipriano, M.; Van Boeckel, T.P.; Nandi, A. Global trends in antibiotic consumption during 2016–2023 and future projections through 2030. Proc. Natl. Acad. Sci. USA 2024, 121, e2411919121. [Google Scholar] [CrossRef]
- Lee, H.; Park, D.; Kim, D.S. Determinants of growth in prescription drug spending using 2010–2019 health insurance claims data. Front. Pharmacol. 2021, 12, 681492. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.D.; Rehm, C.D.; Haas, J.S.; Chan, A.T.; Giovannucci, E.L. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015, 314, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Gabbay, R.A. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2023. Diabetes Care 2023, 46, S158–S190. [Google Scholar] [CrossRef] [PubMed]
- Makam, P.; Matsa, R. “Big Three” infectious diseases: Tuberculosis, malaria and HIV/AIDS. Curr. Top. Med. Chem. 2021, 21, 2779–2799. [Google Scholar] [CrossRef]
- Nandi, A.; Pecetta, S.; Bloom, D.E. Global antibiotic use during the COVID-19 pandemic: Analysis of pharmaceutical sales data from 71 countries, 2020–2022. EClinicalMedicine 2023, 57, 101848. [Google Scholar] [CrossRef]
- Haider, R.H.R. Pharmaceutical Market: An Overview. IJIS 2023, 2, 2087–2104. [Google Scholar] [CrossRef]
- Alshehri, S.; Alshammari, R.; Alyamani, M.; Dabbagh, R.; Almalki, B.; Aldosari, O.; Shakeel, F. Current and future prospective of pharmaceutical manufacturing in Saudi Arabia. Saudi Pharm. J. 2023, 31, 605–616. [Google Scholar] [CrossRef]
- Fisher, W.; Okediji, R.L.; Sampath, P.G. Fostering production of pharmaceutical products in developing countries. Mich. J. Int. Law 2022, 43, 1. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Marques, C.M.; Moniz, S.; de Sousa, J.P.; Barbosa-Povoa, A.P.; Reklaitis, G. Decision-support challenges in the chemical-pharmaceutical industry: Findings and future research directions. Comput. Chem. Eng. 2020, 134, 106672. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef] [PubMed]
- ALSAEDI, H.K.; Alwan, N.A.; Al-Masoudi, E.A. Physiological and biochemical effect of α-Amyrin: A review. J. Med. Life Sci. 2024, 6, 443–452. [Google Scholar] [CrossRef]
- Oboh, M.; Govender, L.; Siwela, M.; Mkhwanazi, B.N. Anti-diabetic potential of plant-based pentacyclic triterpene derivatives: Progress made to improve efficacy and bioavailability. Molecules 2021, 26, 7243. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [CrossRef]
- Placha, D.; Jampilek, J. Chronic inflammatory diseases, anti-inflammatory agents and their delivery nanosystems. Pharmaceutics 2021, 13, 64. [Google Scholar] [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, e2306152. [Google Scholar] [CrossRef]
- Robb, C.T.; Regan, K.H.; Dorward, D.A.; Rossi, A.G. Key mechanisms governing resolution of lung inflammation. Semin. Immunopathol. 2016, 38, 425–448. [Google Scholar] [CrossRef]
- Suhana, M.I.; Farha, A.; Hassan, B.M. Inflammation of the Gums. Malays. Fam. Physician. 2020, 15, 71. [Google Scholar]
- Turner, J.D.; Naylor, A.J.; Buckley, C.; Filer, A.; Tak, P.P. Fibroblasts and osteoblasts in inflammation and bone damage. Adv. Exp. Med. Biol. 2018, 1060, 37–54. [Google Scholar]
- Lee, C.H.; Giuliani, F. The role of inflammation in depression and fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Rezus, E.; Cardoneanu, A.; Burlui, A.; Luca, A.; Codreanu, C.; Tamba, B.I.; Rezuș, C. The link between inflammaging and degenerative joint diseases. Int. J. Mol. Sci. 2019, 20, 614. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The role of inflammation in cardiovascular disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Munn, L.L. Cancer and inflammation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1370. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Júnior, W.F.; Bezerra de Menezes, D.L.; de Oliveira, L.C.; Koester, L.S.; Oliveira de Almeida, P.D.; Lima, E.S.; Neves de Lima, Á.A. Inclusion complexes of β and HPβ-cyclodextrin with α, β amyrin and in vitro anti-inflammatory activity. Biomolecules 2019, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Ditmer, M.; Gabryelska, A.; Turkiewicz, S.; Białasiewicz, P.; Małecka-Wojciesko, E.; Sochal, M. Sleep problems in chronic inflammatory diseases: Prevalence, treatment, and new perspectives: A narrative review. J. Clin. Med. 2021, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, Y.; Galalain, A.; Yunusa, U. A modern overview on diabetes mellitus: A chronic endocrine disorder. Eur. J. Biol. 2020, 5, 1–14. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Pang, M.G. Role of insulin in health and disease: An update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef]
- Mohajan, D.; Mohajan, H.K. Hyperglycaemia among Diabetes Patients: A Preventive Approach. Innov. Sci. Technol. 2023, 2, 27–33. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Abensur, H.; Betonico, C.C.; Machado, A.D.; Parente, E.B.; Queiroz, M.; Vencio, S. Interactions between kidney disease and diabetes: Dangerous liaisons. Diabetol. Metab. Syndr. 2016, 8, 50. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic kidney disease: Challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Umpierrez, G.E. Hyperglycemic crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med. Clin. N. Am. 2020, 101, 587–606. [Google Scholar]
- Bharucha, A.E.; Kudva, Y.C.; Prichard, D.O. Diabetic gastroparesis. Endocr. Rev. 2019, 40, 1318–1352. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef]
- Edmonds, M.; Kesavan, R.; Bal, A. Evaluation and Examination of the Diabetic Foot. In Functional Limb Salvage; Springer: Cham, Switzerland, 2023; pp. 107–131. [Google Scholar]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers: A review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Libianto, R.; Batu, D.; MacIsaac, R.J.; Cooper, M.E.; Ekinci, E.I. Pathophysiological links between diabetes and blood pressure. Can. J. Cardiol. 2018, 34, 585–594. [Google Scholar] [CrossRef]
- Andamari, I.; Thio, H.B.; Soebono, H. Potential skin problems of diabetes mellitus patients: A review. J. Med. Sci. 2022, 54, 3. [Google Scholar] [CrossRef]
- Bruschi, L.K.M.; da Rocha, D.A.; Gesteira Filho, E.L.; Barboza, N.D.M.P.; Frisanco, P.A.B.; Callegaro, R.M.; Arbex, A.K. Diabetes mellitus and diabetic peripheral neuropathy. Open J. Endocr. Metab. Dis. 2017, 7, 12–21. [Google Scholar] [CrossRef]
- Kropp, M.; Golubnitschaja, O.; Mazurakova, A.; Koklesova, L.; Sargheini, N.; Vo, T.T.K.S.; Thumann, G. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications—Risks and mitigation. EPMA J. 2023, 14, 21–42. [Google Scholar] [CrossRef]
- Standl, E.; Khunti, K.; Hansen, T.B.; Schnell, O. The global epidemics of diabetes in the 21st century: Current situation and perspectives. Eur. J. Prev. Cardiol. 2019, 26 (Suppl. S2), 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kakadiya, J. Causes, symptoms, pathophysiology and diagnosis of atherosclerosis–a review. PharmacologyOnline 2009, 3, 420–442. [Google Scholar]
- Badimon, L.; Padró, T.; Vilahur, G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013, 339, 161–166. [Google Scholar] [CrossRef]
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: A review. JAMA 2021, 325, 1088–1098. [Google Scholar] [CrossRef]
- Garg, P.K.; O’Neal, W.T.; Mok, Y.; Heiss, G.; Coresh, J.; Matsushita, K. Life’s simple 7 and peripheral artery disease risk: The atherosclerosis risk in community study. Am. J. Prev. Med. 2018, 55, 642–649. [Google Scholar] [CrossRef]
- Valdivielso, J.M.; Rodríguez-Puyol, D.; Pascual, J.; Barrios, C.; Bermúdez-López, M.; Sánchez-Niño, M.D.; Ortiz, A. Atherosclerosis in chronic kidney disease: More, less, or just different? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1938–1966. [Google Scholar] [CrossRef]
- Cortelli, P.; Giannini, G.; Favoni, V.; Cevoli, S.; Pierangeli, G. Nociception and autonomic nervous system. Neurol. Sci. 2013, 34, 41–46. [Google Scholar] [CrossRef]
- Poulsen, I.; Balle, M.; Givard, K.L. Nociception coma scale–revised: Nurses’ experience in clinical practice. Pain. Manag. Nurs. 2019, 20, 592–598. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Guo, F.; Wu, Y.; Li, Y. Antinociceptive and anti-inflammatory activities of a standardized extract of bis-iridoids from Pterocephalus hookeri. J. Ethnopharmacol. 2018, 216, 233–238. [Google Scholar] [CrossRef]
- Lin, C.C.J.; Chen, W.N.; Chen, C.J.; Lin, Y.W.; Zimmer, A.; Chen, C.C. An antinociceptive role for substance P in acid-induced chronic muscle pain. Proc. Natl. Acad. Sci. USA 2012, 109, E76–E83. [Google Scholar] [CrossRef]
- Araujo, I.W.F.; Chaves, H.V.; Pachêco, J.M.; Val, D.R.; Vieira, L.V.; Santos, R.; Benevides, N.M.B. Role of central opioid on the antinociceptive effect of sulfated polysaccharide from the red seaweed Solieria filiformis in induced temporomandibular joint pain. Int. Immunopharmacol. 2017, 44, 160–167. [Google Scholar] [CrossRef]
- Diwan, A.D.; Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 2023, 6, e1231. [Google Scholar] [CrossRef]
- Yamamotova, A. Endogenous antinociceptive system and potential ways to influence it. Physiol. Res. 2019, 68, S195–S205. [Google Scholar] [CrossRef]
- Zhang, W.; Suo, M.; Yu, G.; Zhang, M. Antinociceptive and anti-inflammatory effects of cryptotanshinone through PI3K/Akt signaling pathway in a rat model of neuropathic pain. Chem. Biol. Interact. 2019, 305, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.R.; Hussain, M.K.; Zakaria, Z.A.; Somchit, M.N.; Moin, S.; Mohamad, A.S.; Israf, D.A. Evaluation of the antinociceptive activity of Ficus deltoidea aqueous extract. Fitoterapia 2008, 79, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Fokunang, C.; Fokunang, E.T.; Frederick, K.; Ngameni, B.; Ngadjui, B. Overview of non-steroidal anti-inflammatory drugs (nsaids) in resource limited countries. MOJ Toxicol. 2018, 4, 5–13. [Google Scholar]
- Santenna, C.; Kumar, S.; Balakrishnan, S.; Jhaj, R.; Ahmed, S.N. A comparative experimental study of analgesic activity of a novel non-steroidal anti-inflammatory molecule–zaltoprofen, and a standard drug–piroxicam, using murine models. J. Exp. Pharmacol. 2019, 11, 85–91. [Google Scholar] [CrossRef]
- Freo, U.; Ruocco, C.; Valerio, A.; Scagnol, I.; Nisoli, E. Paracetamol: A review of guideline recommendations. J. Clin. Med. 2021, 10, 3420. [Google Scholar] [CrossRef]
- Kaur, M. Mechanism of Action, Kinetics and a Bioactive Metabolites AM404 of Paracetamol. J. Clin. Med. Res. 2020, 1, 1–9. [Google Scholar]
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An old disease in new perspective–A review. J. Adv. Res. 2017, 8, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, F.; Dalbeth, N.; Bardin, T. A review of uric acid, crystal deposition disease, and gout. Adv. Ther. 2015, 32, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Tandon, P.; Wadhwa, K.; Melkani, I.; Singh, A.P.; Singh, A.P. The complex pathophysiology of urolithiasis (kidney stones) and the effect of combinational drugs. J. Drug Deliv. Ther. 2022, 12, 194–204. [Google Scholar] [CrossRef]
- Pattamapaspong, N.; Vuthiwong, W.; Kanthawang, T.; Louthrenoo, W. Value of ultrasonography in the diagnosis of gout in patients presenting with acute arthritis. Skeletal. Radiol. 2017, 46, 759–767. [Google Scholar] [CrossRef]
- Oh, Y.J.; Moon, K.W. Presence of tophi is associated with a rapid decline in the renal function in patients with gout. Sci. Rep. 2021, 11, 5684. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Vivekanandan, S. Urate crystal deposition, prevention and various diagnosis techniques of GOUT arthritis disease: A comprehensive review. Health Inf. Sci. Syst. 2018, 6, 19. [Google Scholar] [CrossRef]
- Anaizi, N. The impact of uric acid on human health: Beyond gout and kidney stones. Adv. Biomed. Res. 2023, 45, 158–169. [Google Scholar] [CrossRef]
- Borghi, C.; Agabiti-Rosei, E.; Johnson, R.J.; Kielstein, J.T.; Lurbe, E.; Mancia, G.; Tsioufis, K.P. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur. J. Intern. Med. 2020, 80, 1–11. [Google Scholar] [CrossRef]
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Eur. J. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef]
- Cipolletta, E.; Tata, L.J.; Nakafero, G.; Avery, A.J.; Mamas, M.A.; Abhishek, A. Association between gout flare and subsequent cardiovascular events among patients with gout. JAMA 2022, 328, 440–450. [Google Scholar] [CrossRef]
- Singh, J.A. Any sleep is a dream far away: A nominal group study assessing how gout affects sleep. Rheumatology 2018, 57, 1925–1932. [Google Scholar] [CrossRef]
- Pascual, E.; Addadi, L.; Andrés, M.; Sivera, F. Mechanisms of crystal formation in gout—A structural approach. Nat. Rev. Rheumatol. 2015, 11, 725–730. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. Uric acid level, gout and bone mineral density: A Mendelian randomization study. Eur. J. Clin. Investig. 2019, 49, e13156. [Google Scholar] [CrossRef] [PubMed]
- Akeju, O.; Brown, E.N. Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr. Opin. Neurobiol. 2017, 44, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Devi, C.B.P.; Samreen, S.; Kumari, N.K.; Sharma, J.V.C. A review on insomnia: The sleep disorder. Pharma Innov. J. 2018, 7, 227–230. [Google Scholar]
- Morin, C.M.; Drake, C.L.; Harvey, A.G.; Krystal, A.D.; Manber, R.; Riemann, D.; Spiegelhalder, K. Insomnia disorder. Nat. Rev. Dis. Primers 2015, 1, 15026. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Akbar, U.; McQueen, R.B.; Bemski, J.; Carter, J.; Goy, E.R.; Kutner, J.; Kluger, B. Prognostic predictors relevant to end-of-life palliative care in Parkinson’s disease and related disorders: A systematic review. J. Neurol. Neurosurg. Psychiatry 2021, 92, 629–636. [Google Scholar] [CrossRef]
- Hinson, V.K.; Bergmann, K.J.; Revuelta, G.J.; Vaughan, C.L. A primer on Parkinson’s disease. J. Mov. Disord. 2014, 25, 812–833. [Google Scholar]
- Mattiuzzi, C.; Lippi, G. Current cancer epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Glob. Cancer Stat. 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar]
- Ghufran, M.S.; Soni, P.; Duddukuri, G.R. The global concern for cancer emergence and its prevention: A systematic unveiling of the present scenario. In Bioprospecting of Tropical Medicinal Plants; Springer: Cham, Switzerland, 2023; pp. 1429–1455. [Google Scholar]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 6, 7493–7519. [Google Scholar] [CrossRef] [PubMed]
- Mather, A.E.; Gilmour, M.W.; Reid, S.W.; French, N.P. Foodborne bacterial pathogens: Genome-based approaches for enduring and emerging threats in a complex and changing world. Nat. Rev. Microbiol. 2024, 22, 543–555. [Google Scholar] [CrossRef]
- Soni, J.; Sinha, S.; Pandey, R. Understanding bacterial pathogenicity: A closer look at the journey of harmful microbes. Front. Microbiol. 2024, 15, 1370818. [Google Scholar] [CrossRef]
- Vouga, M.; Greub, G. Emerging bacterial pathogens: The past and beyond. Clin. Microbiol. Infect. 2016, 22, 12–21. [Google Scholar] [CrossRef]
- Poulain, B.; Popoff, M.R. Why are botulinum neurotoxin-producing bacteria so diverse and botulinum neurotoxins so toxic? Toxins 2019, 11, 34. [Google Scholar] [CrossRef]
- Wang, H.; Wei, C.X.; Min, L.; Zhu, L.Y. Good or bad: Gut bacteria in human health and diseases. Biotechnol. Biotechnol. Equip. 2018, 32, 1075–1080. [Google Scholar] [CrossRef]
- Rana, D.S.; Sharma, V.; Sheershwal, A. Understanding host-pathogen interactions in urinary tract infections and advancements in diagnostic methods. Urol. Sci. 2025, 36, 61–75. [Google Scholar] [CrossRef]
- Wang, J.; Sang, L.; Chen, Y.; Sun, S.; Chen, D.; Xie, X. Characterisation of Staphylococcus aureus strain causing severe respiratory disease in rabbits. World Rabbit. Sci. 2019, 27, 41–48. [Google Scholar] [CrossRef]
- Froes, F.; Roche, N.; Blasi, F. Pneumococcal vaccination and chronic respiratory diseases. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, ume 12, 3457–3468. [Google Scholar] [CrossRef]
- Otshudiema, J.O.; Acosta, A.M.; Cassiday, P.K.; Hadler, S.C.; Hariri, S.; Tiwari, T.S. Respiratory illness caused by Corynebacterium diphtheriae and C. ulcerans, and use of diphtheria antitoxin in the United States, 1996–2018. Clin. Infect. Dis. 2021, 73, e2799–e2806. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Yatera, K.; Kawanami, T.; Yamasaki, K.; Naito, K.; Akata, K.; Mukae, H. The clinical features of respiratory infections caused by the Streptococcus anginosus group. BMC Pulm. Med. 2015, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Arellano, K.; Park, H.; Todorov, S.D.; Kim, B.; Kang, H.; Holzapfel, W.H. Assessment of the safety and anti-inflammatory effects of three Bacillus strains in the respiratory tract. Environ. Microbiol. 2021, 23, 3077–3098. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Imran, A.; Malik, A.; Chaudhary, A.A.; Rub, A.; Jan, A.T.; Rolfo, C. Bacterial imbalance and gut pathologies: Association and contribution of E. coli in inflammatory bowel disease. Crit. Rev. Clin. Lab. Sci. 2019, 56, 1–17. [Google Scholar] [CrossRef]
- Zha, L.; Garrett, S.; Sun, J. Salmonella infection in chronic inflammation and gastrointestinal cancer. Diseases 2019, 7, 28. [Google Scholar] [CrossRef]
- Matanza, X.M.; Clements, A. Pathogenicity and virulence of Shigella sonnei: A highly drug-resistant pathogen of increasing prevalence. Virulence 2023, 14, 2280838. [Google Scholar] [CrossRef]
- Agyei, F.K.; Scharf, B.; Duodu, S. Vibrio cholerae bacteremia: An enigma in cholera-endemic African countries. Trop. Med. Infect. 2024, 9, 103. [Google Scholar] [CrossRef]
- Fotopoulou, E.T.; Jenkins, C.; Painset, A.; Amar, C. Listeria monocytogenes: The silent assassin. J. Med. Microbiol. 2024, 73, 001800. [Google Scholar] [CrossRef]
- Jerse, A.E.; Wu, H.; Packiam, M.; Vonck, R.A.; Begum, A.A.; Garvin, L.E. Estradiol-treated female mice as surrogate hosts for Neisseria gonorrhoeae genital tract infections. Front. Microbiol. 2011, 2, 107. [Google Scholar] [CrossRef] [PubMed]
- Theel, E.S.; Katz, S.S.; Pillay, A. Molecular and direct detection tests for Treponema pallidum subspecies pallidum: A review of the literature, 1964–2017. Clin. Infect. Dis. 2020, 71, S4–S12. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Spinola, S.M. Infections caused by Haemophilus ducreyi: One organism, two stories. Clin. Microbiol. Rev. 2024, 37, e00135-24. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, E.; Harding-Esch, E.; Mabey, D.; Holland, M.J. When bacteria and viruses collide: A tale of Chlamydia trachomatis and sexually transmitted viruses. Viruses 2023, 15, 1954. [Google Scholar] [CrossRef]
- Jensen, J.S.; Cusini, M.; Gomberg, M.; Moi, H.; Wilson, J.; Unemo, M. 2021 European guideline on the management of Mycoplasma genitalium infections. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 641–650. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; He, Y.; Lv, Z.; Liang, Z.; Chen, J.; Liu, X. Exploring the role of Staphylococcus aureus in inflammatory diseases. Toxins 2022, 14, 464. [Google Scholar] [CrossRef]
- Martín-Romero, M.; Clavero-Martínez, D.; Castillo-Navarro, A.M.; García-Vázquez, E. Neisseria meningitidis bacteraemia and SARS-CoV-2 infection: A coinfection that reminds previous epidemic outbreaks. Rev. Esp. Quimioter. 2022, 35, 293. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, Q.; Zhang, T.; Zhen, S.; Wang, J.; Jiang, E.; Feng, S. Pseudomonas aeruginosa bloodstream infection in patients with hematological diseases: Clinical outcomes and prediction model of multidrug-resistant infections. J. Infect. 2023, 86, 66–117. [Google Scholar] [CrossRef]
- Vinayaka, A.C.; Golabi, M.; Than, T.L.Q.; Wolff, A.; Bang, D.D. Point-of-care diagnosis of invasive non-typhoidal Salmonella enterica in bloodstream infections using immunomagnetic capture and loop-mediated isothermal amplification. N. Biotechnol. 2022, 66, 1–7. [Google Scholar] [CrossRef]
- Kravtsov, A.L.; Bugorkova, S.A.; Klyueva, S.N.; Shmelkova, T.P.; Kozhevnikov, V.A. Human blood granulocyte degranulation and lysis intensity during interaction with Yersinia pestis in the ex vivo model of bacteriemia. J. Microbiol. epidemiology Immunobiol. 2025, 102, 80–90. [Google Scholar] [CrossRef]
- Méric, G.; Mageiros, L.; Pensar, J.; Laabei, M.; Yahara, K.; Pascoe, B.; Sheppard, S.K. Disease-associated genotypes of the commensal skin bacterium Staphylococcus epidermidis. Nat. Commun. 2018, 9, 5034. [Google Scholar] [CrossRef]
- German Advisory Committee Blood (Arbeitskreis Blut), Subgroup ‘Assessment of Pathogens Transmissible by Blood’. Human immunodeficiency virus (HIV). Transfus Med. Hemother. 2016, 43, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Kapila, A.; Chaudhary, S.; Sharma, R.B.; Vashist, H.; Sisodia, S.S.; Gupta, A. A review on: Hiv aids. IJPBR. 2016, 4, 69–73. [Google Scholar]
- Duggal, S.; Chugh, T.D.; Duggal, A.K. HIV and malnutrition: Effects on immune system. J. Immunol. Res. 2012, 2012, 784740. [Google Scholar] [CrossRef] [PubMed]
- Antony, B. Opportunistic Infections in HIV/AIDS: An Overview. Holist. Approach. Infect. Dis. 2017, 217–230. [Google Scholar]
- Prabhu, S.R.; van Wagoner, N. Human immunodeficiency virus infection and acquired immunodeficiency syndrome (HIV/AIDS): An overview. Sexually Transm. Oral Dis. 2023, 51–71. [Google Scholar]
- Saini, A.K.; Gupta, A.A.; Keservani, R.K.; Kachave, R.N.; Dharmamoorthy, G.; Kesharwani, R.K.; Patil, S.J. HIV/AIDS neurological disorders. In A Review on Diverse Neurological Disorders; Academic Press: Cambridge, MA, USA, 2024; pp. 291–298. [Google Scholar]
- Hsue, P.Y.; Waters, D.D. HIV infection and coronary heart disease: Mechanisms and management. Nat. Rev. Cardiol. 2019, 16, 745–759. [Google Scholar] [CrossRef]
- Kaspar, M.B.; Sterling, R.K. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol. 2017, 4. [Google Scholar] [CrossRef]
- Alfano, G.; Cappelli, G.; Fontana, F.; Di Lullo, L.; Di Iorio, B.; Bellasi, A.; Guaraldi, G. Kidney disease in HIV infection. J. Clin. Med. 2019, 8, 1254. [Google Scholar] [CrossRef]
- Gordin, F.M.; Roediger, M.P.; Girard, P.M.; Lundgren, J.D.; Miro, J.M.; Palfreeman, A.; Slater, L.N. Pneumonia in HIV-infected persons: Increased risk with cigarette smoking and treatment interruption. Am. J. Respir. Crit. Care Med. 2008, 178, 630–636. [Google Scholar] [CrossRef]
- Elfstrand, L.; Florén, C.H. Management of chronic diarrhea in HIV-infected patients: Current treatment options, challenges and future directions. HIV AIDS 2010, 219–224. [Google Scholar] [CrossRef]
- Bailin, S.S.; Gabriel, C.L.; Wanjalla, C.N.; Koethe, J.R. Obesity and weight gain in persons with HIV. Curr. HIV/AIDS Rep. 2020, 17, 138–150. [Google Scholar] [CrossRef]
- King, A.B. Elemental Diet in HIV Infection: Dietary Management of the Patient with Diarrhea or Malabsorption. Uses Elem. Diet Clin. Sit. 2018, 219–241. [Google Scholar]
- Kumar, M.; Murmu, N.; Kujur, A.; Singh, S.; Kumar, D.; Sagar, V.; Singh, S.B. A Study on the Quality of Life and Economic Burden Among People Living With HIV/AIDS and Attending a Tertiary Care Hospital in Jharkhand, India. Cureus 2025, 17. [Google Scholar] [CrossRef]
- Kumar, T. Molecular docking studies possible treatment of diabetes using vasicine against islet amyloid polypeptide. Int. J. Res. Appl. Sci. Eng. Technol. 2021, 9, 4202–4209. [Google Scholar] [CrossRef]
- Ching, J.; Lin, H.S.; Tan, C.H.; Koh, H.L. Quantification of α-and β-amyrin in rat plasma by gas chromatography–mass spectrometry: Application to preclinical pharmacokinetic study. J. Mass Spectrom. 2011, 46, 457–464. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Silva Junior, W.F.; Veiga Junior, V.F.; Lima, Á.A.; Lima, E.S. Physicochemical characterization and biological activities of the triterpenic mixture α, β-amyrenone. Molecules 2017, 22, 298. [Google Scholar] [CrossRef]
- Angsusing, J.; Singh, S.; Samee, W.; Tadtong, S.; Stokes, L.; O’Connell, M.; Chittasupho, C. Anti-inflammatory activities of Yataprasen Thai traditional formulary and its active compounds, beta-amyrin and stigmasterol, in RAW264. 7 and THP-1 cells. Pharmaceuticals 2024, 17, 1018. [Google Scholar] [CrossRef]
- Krishnan, K.; Mathew, L.E.; Vijayalakshmi, N.R.; Helen, A. Anti-inflammatory potential of β-amyrin, a triterpenoid isolated from Costus igneus. Inflammopharmacology 2014, 22, 373–385. [Google Scholar] [CrossRef]
- Melo, C.M.; Morais, T.C.; Tomé, A.R.; Brito, G.A.C.; Chaves, M.H.; Rao, V.S.; Santos, F.A. Anti-inflammatory effect of α, β-amyrin, a triterpene from Protium heptaphyllum, on cerulein-induced acute pancreatitis in mice. J. Inflamm. Res. 2011, 60, 673–681. [Google Scholar] [CrossRef]
- Hryhorowicz, S.; Kaczmarek-Ryś, M.; Zielińska, A.; Scott, R.J.; Słomski, R.; Pławski, A. Endocannabinoid system as a promising therapeutic target in inflammatory bowel disease a systematic review. Front. Immunol. 2021, 12, 790803. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, C.; Lu, G.; Mu, Z.; Cui, W.; Gao, H.; Wang, Y. Anti-diabetic effect of mulberry leaf polysaccharide by inhibiting pancreatic islet cell apoptosis and ameliorating insulin secretory capacity in diabetic rats. Int. Immunopharmacol. 2014, 22, 248–257. [Google Scholar] [CrossRef]
- Perera, H.K.I.; Premadasa, W.K.V.K.; Poongunran, J. α-glucosidase and glycation inhibitory effects of Costus speciosus leaves. BMC Complement. Altern. Med. 2015, 16, 2. [Google Scholar] [CrossRef]
- Thouvenot, K.; Turpin, T.; Taïlé, J.; Clément, K.; Meilhac, O.; Gonthier, M.P. Links between insulin resistance and periodontal bacteria: Insights on molecular players and therapeutic potential of polyphenols. Biomolecules. 2022, 12, 378. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; El Zalabani, S.M.; Sabry, M.M. Role of Dietary Supplements in Cardiovascular Diseases. J. Cardioprot. Nat. Prod. Prom. Hop. 2018, 193–246. [Google Scholar]
- Sunil, C.; Irudayaraj, S.S.; Duraipandiyan, V.; Al-Dhabi, N.A.; Agastian, P.; Ignacimuthu, S. Antioxidant and free radical scavenging effects of β-amyrin isolated from Symplocos cochinchinensis Moore. leaves. Ind. Crops Prod. 2014, 61, 510–516. [Google Scholar] [CrossRef]
- Philpott, H.T.; McDougall, J.J. Combatting joint pain and inflammation by dual inhibition of monoacylglycerol lipase and cyclooxygenase-2 in a rat model of osteoarthritis. Arthritis Res. Ther. 2020, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- De-Almeida, S.C.X.; da-Silva, Â.C.; Sousa, N.R.T.; Amorim, I.H.F.; Leite, B.G.; Neves, K.R.T.; de-Barros Viana, G.S. Antinociceptive and anti-inflammatory activities of a triterpene-rich fraction from Himatanthus drasticus. Braz. J. Med. Biol. Res. 2019, 52, e7798. [Google Scholar] [CrossRef]
- Vikrama Chakravarthi, P.; Murugesan, S.; Arivuchelvan, A.; Sukumar, K.; Arulmozhi, A.; Jagadeeswaran, A. Therapeutic antigout and antioxidant activity of Piper betle L. in gout-induced broilers. Br. Poult. Sci. 2022, 63, 324–331. [Google Scholar] [CrossRef]
- Fischer, S.P.M.; Brusco, I.; Camponogara, C.; Piana, M.; Faccin, H.; Gobo, L.A.; Oliveira, S.M. Arctium minus crude extract presents antinociceptive effect in a mice acute gout attack model. Inflammopharmacology 2018, 26, 505–519. [Google Scholar] [CrossRef]
- Banik, B.; Das, S.; Das, M.K. Medicinal Plants with Potent Anti-inflammatory and Anti-arthritic Properties Found in Eastern Parts of the Himalaya: An Ethnomedicinal Review. Pharmacogn. Rev. 2020, 14, 121–137. [Google Scholar] [CrossRef]
- Baburaj, R.; Sandur, V.R.; Das, K. Investigation of the Pro-active Role of Alpha Amyrin Nanoemulsions in Quashing Neurodegeneration, Excitotoxicity, and Neuronal Inflammation-A Combined in vivo and in silico Approach. Indian J. Pharm. Educ. Res. 2024, 58, 240–253. [Google Scholar] [CrossRef]
- Liu, J.; Meng, T.; Wang, C.; Cheng, W.; Zhang, Q.; Cheng, G. Natural products for the treatment of depression: Insights into signal pathways influencing the hypothalamic–pituitary–adrenal axis. Medicine. 2023, 102, e35862. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Barres, B.A. The relationship between neuronal survival and regeneration. Annu. Rev. Neurosci. 2000, 23, 579–612. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Suliman, R.S.; Aljammaz, N.A.; Kahtani, K.M.; Aljatli, D.A.; Albadrani, G.M. Natural products as novel neuroprotective agents; computational predictions of the molecular targets, ADME properties, and safety profile. Plants 2022, 11, 549. [Google Scholar] [CrossRef]
- Ranjbar, M.M.; Assadolahi, V.; Yazdani, M.; Nikaein, D.; Rashidieh, B. Virtual Dual inhibition of COX-2/5-LOX enzymes based on binding properties of alpha-amyrins, the anti-inflammatory compound as a promising anti-cancer drug. EXCLI J. 2016, 15, 238. [Google Scholar]
- Drif, A.I.; Avula, B.; Khan, I.A.; Efferth, T. COX2-Inhibitory and Cytotoxic Activities of Phytoconstituents of Matricaria chamomilla L. J. Appl. Sci. 2023, 13, 8935. [Google Scholar] [CrossRef]
- Beg, M.A.; Shivangi; Afzal, O.; Akhtar, M.S.; Altamimi, A.S.A.; Hussain, A.; Imam, M.A.; Ahmad, M.N.; Chopra, S.; Athar, F. Potential efficacy of β-amyrin targeting mycobacterial universal stress protein by in vitro and in silico approach. Molecules 2022, 27, 4581. [Google Scholar] [CrossRef]
- Kwun, M.S.; Lee, H.J.; Lee, D.G. β-amyrin-induced apoptosis in Candida albicans triggered by calcium. J. Microbiol. Biotechnol. 2021, 125, 630–636. [Google Scholar] [CrossRef]
- Mi, G.; Shi, D.; Wang, M.; Webster, T.J. Reducing bacterial infections and biofilm formation using nanoparticles and nanostructured antibacterial surfaces. Adv. Healthc. Mater. 2018, 7, 1800103. [Google Scholar] [CrossRef]
- Mamidala, E.; Munipally, P. Inhibitory potential of α-Amyrin from Calotropis procera against HIV-1 reverse transcriptase: Insights from in silico and in vitro assays. Pharmaceuticals 2025, 16, 258–288. [Google Scholar]
- Hasan, K.; Ferdianti, F.N.; Paryati, S.P. Anti-HIV Transcriptase Herbs: A Review. Acta Med. Health Sci. 2023, 2, 96–108. [Google Scholar] [CrossRef]
- Rüdiger, A.L.; Siani, A.C.; Junior, V.V. The chemistry and pharmacology of the South America genus Protium Burm. f. (Burseraceae). Pharmacogn. Rev. 2007, 1, 93–104. [Google Scholar]
- Okoye, N.N.; Ajaghaku, D.L.; Okeke, H.N.; Ilodigwe, E.E.; Nworu, C.S.; Okoye, F.B.C. Beta-amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity. Pharm. Biol. 2014, 52, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Sirat, H.M.; Susanti, D.; Ahmad, F.; Takayama, H.; Kitajima, M. Amides, triterpene, and flavonoids from the leaves of Melastoma malabathricum L. J. Nat. Med. 2010, 64, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Ara, L.; Hajimehdipoor, H.; Read, R.W.; Arshadi, S.; Nikan, M. Chemical constituents of Swertia longifolia Boiss. with α-amylase inhibitory activity. Res. Pharm. Sci. 2016, 11, 23–32. [Google Scholar] [PubMed]
- Quan, N.V.; Xuan, T.D.; Tran, H.D.; Thuy, N.T.D.; Trang, L.T.; Huong, C.T.T.; Tuyen, P.T. Antioxidant, α-amylase and α-glucosidase inhibitory activities and potential constituents of Canarium tramdenum bark. Molecules 2019, 24, 605. [Google Scholar] [CrossRef]

| Disease | Cell Line (Receptors) | Duration | Doses of α- and β-Amyrins | Assay | References |
|---|---|---|---|---|---|
| Periodontal | TNF-α | 24 h | 5–10 mg/kg | Vivo | [24] |
|
Persistent Inflammatory and neuropathic hyperalgesia | CB1, CB2 | 12 h | 30 mg/kg | Vivo | [25] |
| Colitis | COX-2, VEGF, NF-κB | 72 h | 3 mg/kg | Vivo | [26] |
| Colitis | ICAM-1, VCAM-1, PCAM-1, β2-integrin, CD68, P-selectin | 0–7 days | 1, 3, and 10 mg/kg | Vivo | [27] |
| Acute pancreatitis | (TNF-α), (IL-6) | 24 h | 10, 30, and 100 mg/kg | Vivo | [28] |
| Disease | Cell Line (Receptors) | Duration | Doses of α- and β-Amyrins | Assay | References |
|---|---|---|---|---|---|
|
Diabetes, Cardiovascular | Beta cell | 12 h | 10, 30, and 100 mg/kg | Vivo | [29] |
| Diabetes | Beta cell | 24 h | 50 µg/kg | Vivo | [30] |
| Diabetes | - | - | 10 µg/mL | Vitro | [31] |
| Diabetes | 3T3-L1 | 24 h | 1,10, and 100 µg/mL | Vivo | [32] |
| Diabetes | HK-2 | 24 h | 100 µg/kg | Vivo | [33] |
| - | - | 19.50 µg/mL | Vitro | [34] |
| Disease | Cell Line (Receptors) | Duration | Doses of α- and β-Amyrins | Assay | References |
|---|---|---|---|---|---|
| Atherosclerosis | HepG2 | - | 200 μmol/L | Vitro | [35] |
| Type II diabetes, and atherosclerosis | IL-6, TNF-α | - | 0.01 μM | Vitro | [36] |
| Nonalcoholic fatty liver | Lipid levels | 15 weeks | 10, 20, 50 mg/kg | Vivo | [37] |
|
Vascular disorders | SVEC4-10 | - | 0.6 và 0.3 µM | Vitro | [38] |
| Vascular | HUVECs | 24–72 h | 0.025–10 μM) | Vitro | [39] |
| Obesity | PHE, ACh, SNP | 15 days | 20 mg/kg | Vivo | [40] |
| Disease | Cell Line (Receptors) | Duration | Doses of α- and β-Amyrins | Assay | References |
|---|---|---|---|---|---|
| Antinociceptive | Capsaicin, naloxone | 10–20 min | 10, 30, and 100 mg/kg | Vivo | [41] |
| Antinociceptive | Protein kinase A, protein kinase C | - | 0.1–100 mg/kg | Vivo | [42] |
| Visceral pain | KBr pellets, Bruker AC | - | 45–90% | Vitro | [43] |
|
Novel analgesic | CHO-K1 cell, Cannabinoid CB1 and CB2 receptors | - | >10 µM | Vitro | [44] |
| Disease | Enzyme (Receptors) | Duration | Doses of α- and β-Amyrins | Assay | References |
|---|---|---|---|---|---|
| Gout | XO | - | 258.22 µg/mL | Vitro | [23] |
| Gout | XO, Urate crystals | - | - | Vivo | [45,46] |
| Gout | NTUB1 | 24 h | - | Vitro | [46] |
| Disease | Cell Line (Receptors) | Duration | Doses of α- and β-Amyrins | Assay | References |
|---|---|---|---|---|---|
| Insomnia | GABAergic | 12 h | 1, 3, or 10 mg/kg | Vivo | [48] |
| Convulsant, Sedative, Anxiolytic | Glutamate, Aspartate, Taurine | 12 h | 2.5; 5; 10; 25 µg/mL | Vitro | [49] |
| Analgesia | TRPV1, Opioid | 12 h | 3–100 mg/kg | Vivo | [50] |
| Sedative, Depressant | TRPV1, Ruthenium red | 15 h | 5, 10, 20 mg/kg | Vivo | [51] |
| Alzheimer | pPI3K, PI3K, pAkt, Akt | 24 h | 4 µg/mL | Vitro | [52] |
| Protective central and peripheral nervous systems | Triglycerides | - | 2000 mg/kg | Vivo | [52] |
| Disease | Cell Line (Receptors) | Duration | Doses of α- and β-Amyrins | Assay | References |
|---|---|---|---|---|---|
| Parkinson | 6-OHDA | 72 h | 5, 10, 15, 30 µM | Vitro | [54] |
| Parkinson | LGG-1 | 12 h | 5–30 µM | Vitro | [55] |
| Parkinson | LGG-1 | - | - | Vitro | [57] |
| Parkinson | LGG-1 | - | - | Vitro | [58] |
| Parkinson | LDL-C | - | - | Vitro | [59] |
| Cancer Deases | Incidence | Mortality | ||
|---|---|---|---|---|
| Rank | New Cases | Rank | Deaths | |
| Lung | 1 | 2,480,301 | 1 | 18,171,722 |
| Female breast | 2 | 2,308,897 | 4 | 665,684 |
| Colorectum | 3 | 1,926,118 | 2 | 903,859 |
| Prostate | 4 | 1,466,680 | 8 | 396,792 |
| Stomach | 5 | 968,350 | 5 | 659,853 |
| Liver | 6 | 865,269 | 3 | 757,948 |
| Thyroid | 7 | 821,173 | 24 | 47,485 |
| Cervix uteri | 8 | 661,021 | 9 | 348,189 |
| Bladder | 9 | 613,791 | 13 | 220,349 |
| Non-Hodgkin | 10 | 553,010 | 11 | 250,475 |
| Esophagus | 11 | 510,716 | 7 | 445,129 |
| Pancreas | 12 | 510,566 | 6 | 467,005 |
| Leukemia | 13 | 486,777 | 10 | 305,033 |
| Kidney | 14 | 434,419 | 16 | 155,702 |
| Corpus uteri | 15 | 420,242 | 19 | 97,704 |
| Lip, oral cavity | 16 | 389,485 | 15 | 188,230 |
| Skin | 17 | 331,647 | 22 | 58,645 |
| Ovary | 18 | 324,398 | 14 | 206,839 |
| Brain | 19 | 321,476 | 12 | 248,305 |
| Larynx | 20 | 188,960 | 18 | 103,216 |
| Disease | Cell Line (Receptors) | Duration | Doses of α- and β-Amyrins | Assay | References |
|---|---|---|---|---|---|
| Liver cancer | Hepatocellular | - | −9.36 and −8.90 kcal/mol | Docking | [60] |
| Breast cancer | MCF-7, ATCC-HTB22 | 72 h | 2.35–2.48 µg/ml | Vitro | [61] |
| Liver cancer | Hep-G2 | - | 25 µM | Vitro | [62] |
| Colon cancer | VEGF, MMP-9, IL-10 | 30 days | 100 mg/kg | Vivo | [63] |
|
Prostate Carcinoma | PC3, HL60 | 72 h | 13.9–25.4% | Vitro | [64] |
| Leukemia cancer | HL-60, MDAMB-435, SF-295, HCT-8 | - | 1.8–3 μM | Vitro | [65] |
| Cervical cancer | HeLa | - | 10–200 μM | Vitro | [66] |
| Breast cancer | MCF-7 | - | 28.45 μM | Vitro | [67] |
| Skin cancer | KB-oral | - | 18.01 μM | Vitro | [68] |
| Lung cancer | NCI-H187 | 18.42 μM | Vitro | [69] | |
| Colon cancer | HCT116 | - | - | Vitro | [70] |
| Leukemia cancer | Kasumi-1 | 1 year | - | Nano | [71] |
| Related Diseases | Type of Bacteria | Reference |
|---|---|---|
| Respiratory | Staphylococcus | [175] |
| Pneumococcus | [176] | |
| Diphtheria | [177] | |
| Streptococcus | [178] | |
| Bacillus | [179] | |
| Gastrointestinal | E. coli | [180] |
| Salmonella | [181] | |
| Shigella | [182] | |
| Vibrio cholerae | [183] | |
| Listeria monocytogenes | [184] | |
| Genital | Neisseria gonorrhoeae | [185] |
| Treponema pallidum | [186] | |
| Haemophilus ducreyi | [187] | |
| Chlamydia trachomatis | [188] | |
| Mycoplasma genitalium | [189] | |
| Blood | Staphylococcus aureus | [190] |
| Neisseria meningitidis | [191] | |
| Pseudomonas aeruginosa | [192] | |
| Salmonella typhi | [193] | |
| Yersinia pestis | [194] | |
| Staphylococcus epidermidis | [195] |
| Bacterial | Receptors | Doses of α- and β-Amyrins | Assay | References |
|---|---|---|---|---|
| Escherichia coli, Staphylococcus aureus | NorA, MepA | - | Docking | [72] |
| Klebsiella, Pragia, Serratia, Enterobacter, Providencia, E. coli. | Inhibition zones | 0.093 µg/ml | Vitro | [73] |
| E. coli, S. aureus, H. pylori | Inhibition zones | 3.4 mg/mL | Vitro | [74] |
| Disease | Research Subject (Receptors) | Doses of α- and β-Amyrins | Assay | References |
|---|---|---|---|---|
| HIV | NMR spectral- | 1.4 μM | Vitro | [75] |
| HIV | SAR of HIV-1 PR inhibitors | 0.34 μM | Vitro | [76] |
| HIV | HR-EI/FAB-MS and 1D and 2D NMR | - | Vitro | [77] |
| HIV | A549 | 0.6–4.8 μM | Vitro | [78] |
| HIV | 1D and 2D NMR | 4.08, 4.18, 1.70 μM | Vivo | [79] |
| HIV | C-3 pharmacophore | 0.0006 μM | Vitro | [80] |
| Pharmaceutical Potentials | Mechanisms |
|---|---|
| Anti-inflammatory |
|
| Antidiabetic |
|
| Antiatherosclerotic |
|
| Antinociceptive |
|
| Antigout |
|
| Positive Effects On Nerves | |
| Anti-Parkinsonian |
|
| Anticancer |
|
| Antibacterial |
|
| Anti-HIV |
|
| Level Studies | In Vivo | In Vitro | In Clinical | Not Cytotoxicity | |
|---|---|---|---|---|---|
| Activities | |||||
| Anti-inflammatory | ✔ | ✔ | - | ✔ | |
| Antidiabetic | ✔ | ✔ | - | ✔ | |
| Antiatherosclerotic | ✔ | ✔ | - | ✔ | |
| Analgesic | ✔ | ✔ | - | ✔ | |
| Antigout | ✔ | ✔ | - | ✔ | |
| Neuroprotective | ✔ | ✔ | - | ✔ | |
| Anti-Parkinsonian | ✔ | ✔ | - | ✔ | |
| Anticancer | ✔ | ✔ | - | ✔ | |
| Antibacterial | ✔ | ✔ | - | ✔ | |
| Anti-HIV activities | ✔ | ✔ | - | ✔ | |
| Source of Extraction | Extraction Efficiency (g/kg Dry Weight) | References |
|---|---|---|
| Protium kleinii | 2.40 | [238] |
| Symplocos cochinchinensis | 1.70 | [221] |
| Swertia longifolia | 2.0 | [239] |
| Melastoma malabathricum | 0.60 | [240] |
| Swertia longifolia | 1.00 | [241] |
| Canarium tramdenum | 1.52 | [242] |
| Celastrus hindsii | 10.75 | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viet, T.D.; Anh, L.H.; Xuan, T.D.; Dong, N.D. The Pharmaceutical Potential of α- and β-Amyrins. Nutraceuticals 2025, 5, 21. https://doi.org/10.3390/nutraceuticals5030021
Viet TD, Anh LH, Xuan TD, Dong ND. The Pharmaceutical Potential of α- and β-Amyrins. Nutraceuticals. 2025; 5(3):21. https://doi.org/10.3390/nutraceuticals5030021
Chicago/Turabian StyleViet, Tran Duc, La Hoang Anh, Tran Dang Xuan, and Ngo Duy Dong. 2025. "The Pharmaceutical Potential of α- and β-Amyrins" Nutraceuticals 5, no. 3: 21. https://doi.org/10.3390/nutraceuticals5030021
APA StyleViet, T. D., Anh, L. H., Xuan, T. D., & Dong, N. D. (2025). The Pharmaceutical Potential of α- and β-Amyrins. Nutraceuticals, 5(3), 21. https://doi.org/10.3390/nutraceuticals5030021









