Abstract
Short-chain fatty acids (SCFAs) are metabolites derived from the fermentation of dietary fibre by gut bacteria. SCFAs function as essential regulators of host-microbiome interactions by participating in numerous physiological and pathological processes within the gastrointestinal (GI) tract. In recent years, the depletion of SCFAs has been increasingly linked to the pathogenesis of GI diseases. In this review, we summarize the current understanding of the therapeutic mechanisms of SCFAs in GI diseases, including inflammatory bowel disease, irritable bowel syndrome, metabolic dysfunction-associated steatotic liver disease, and acute pancreatitis. We next highlight potential therapeutic approaches that increase the endogenous production of SCFAs, including prebiotics, probiotics, and fecal microbiota transplantation. We conclude that, although SCFAs are promising therapeutic agents, further research is necessary due to variability in treatment efficacy, inconsistent clinical outcomes, and a limited understanding of SCFAs’ mechanisms of action.
1. Introduction
Gastrointestinal (GI) diseases are conditions that affect the GI tract and the accessory organs involved in digestion. They disrupt normal digestive function and lead to symptoms such as pain, bloating, diarrhea, and constipation. In 2019, the global prevalence and incidence of GI diseases were 7.32 billion and 2.86 billion, respectively, remaining essentially unchanged between 1990 and 2019 [1]. Gut microbiota-derived metabolites, including short-chain fatty acids (SCFAs), have been shown to play a crucial role in the development of GI diseases. Acetate (C2), propionate (C3), and butyrate (C4) are the most prevalent colonic SCFAs produced from the intestinal microbial fermentation of dietary fibre. They act as signal molecules by activating G-protein-coupled receptors (GPCRs) GPR41, GPR43, and GPR109A, and inhibiting histone deacetylase (HDAC) activity [2,3,4]. The activation of GPCRs, in turn, regulates diverse cellular functions, including inflammatory response, metabolism, and immunity.
2. Therapeutic Roles of SCFAs in GI Diseases
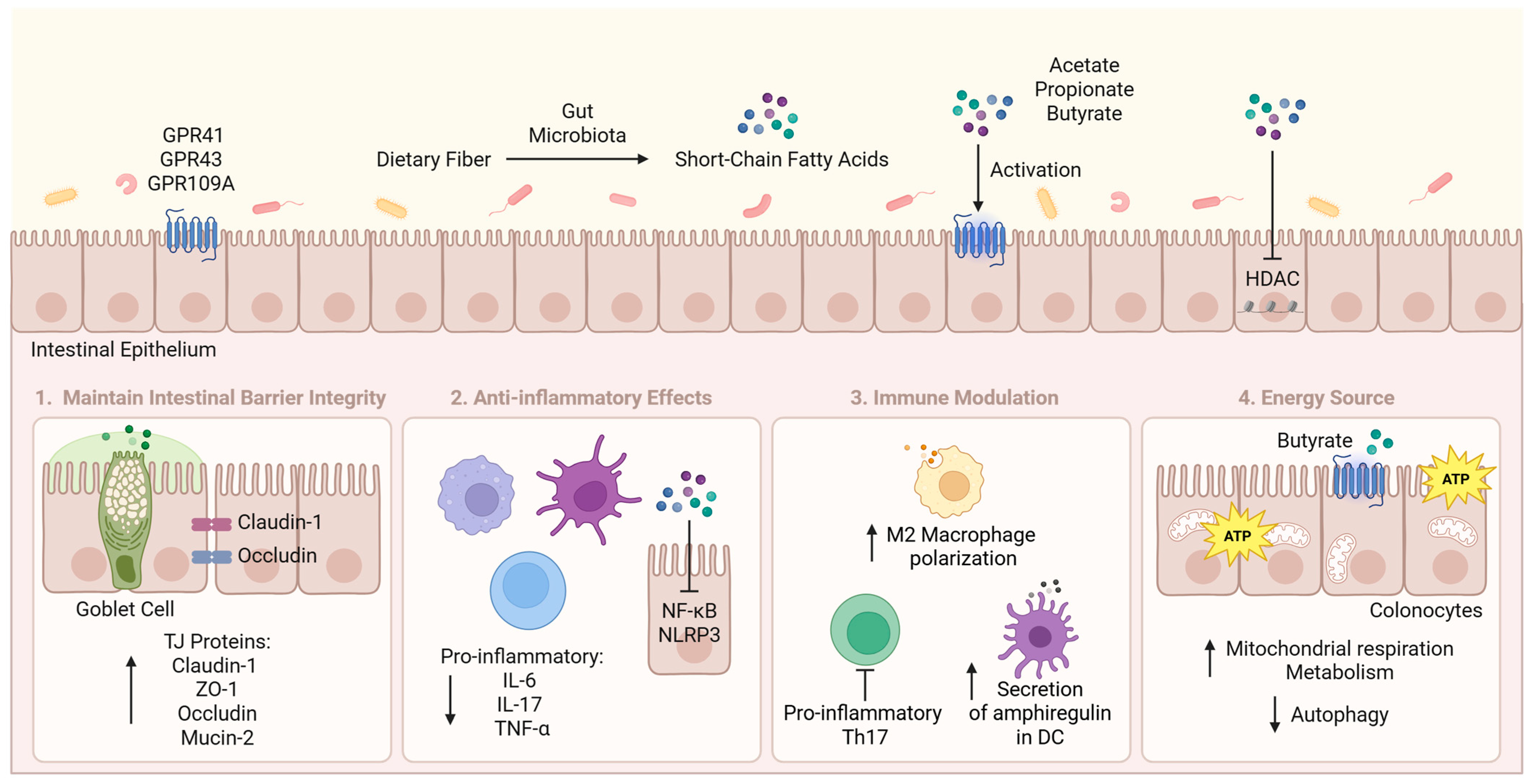
SCFAs exert their therapeutic effects primarily by maintaining intestinal barrier integrity through the promotion of tight junction (TJ) proteins and mucus production, suppressing inflammation by inhibiting pro-inflammatory cytokines and downregulating key inflammatory signalling pathways, modulating the immune system, and enhancing energy production in colonocytes (Figure 1). SCFA levels are significantly reduced in GI diseases due to dysbiosis, resulting in compromised intestinal integrity, increased inflammation, and disrupted microbial balance, ultimately contributing to disease progression [5,6,7].
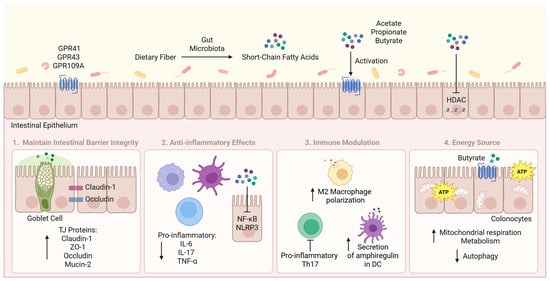
Figure 1.
Main therapeutic mechanisms of SCFAs in GI Diseases. SCFAs are produced from the intestinal microbial fermentation of dietary fibre. They mainly act through maintaining intestinal barrier integrity, reducing inflammation, modulating immune responses, and providing energy for colonocytes. ATP, adenosine triphosphate; DC, dendritic cell; GPR, G protein-coupled receptor; HDAC, histone deacetylase; IL, interleukin; NF-κB, nuclear factor kappa B; NLRP3, NOD-like receptor protein 3; Th17, T helper 17; TJ, tight junction; TNF-α, tumour necrosis factor-alpha; ZO-1, zonula occludens-1. This figure was created with BioRender (https://biorender.com/, accessed on 11 July 2025).
3. Inflammatory Bowel Disease
Inflammatory bowel disease (IBD), which includes Crohn’s disease (CD) and ulcerative colitis (UC), is a group of chronic inflammatory conditions that affect the GI tract. A growing body of evidence supports the therapeutic effects of SCFAs in IBD. A recent multi-omics study of the intestinal microbiota ecosystem in IBD patients revealed a general decrease in SCFAs, which corroborates the observed depletion of butyrate-producing species Faecalibacterium prausnitzii and Roseburia hominis [5]. UC patients with a lower abundance of SCFA-producing species experienced more severe colonic inflammation and were less responsive to treatments [8]. In vitro studies have shown that administering high acetate concentrations to patient-derived epithelial monolayers induced anti-inflammatory effects and enhanced epithelial resistance [9]. Administration of sodium butyrate or propionate in mouse models significantly improved inflammation and intestinal barrier integrity [4,10].
There are several pathways by which SCFAs alleviate IBD. Firstly, they contribute to maintaining intestinal barrier function. Butyrate upregulates TJ proteins claudin-1, zonula occludens-1 (ZO-1), and occludin, which form a barrier between epithelia cells to control molecular movement and maintain cellular polarity, and mitigates lipopolysaccharide (LPS)-induced barrier impairment [11,12]. Propionate stimulates the differentiation of goblet cells and enhances the expression of genes related to mucus production [13]. Secondly, SCFAs exert potent anti-inflammatory properties by promoting the production of anti-inflammatory cytokines, suppressing pro-inflammatory cytokines, and inhibiting signalling pathways that trigger inflammatory responses. Butyrate mitigated colitis in mice by attenuating the expression of pro-inflammatory cytokines, including interleukin-6 (IL-6), IL-17, tumour necrosis factor-alpha (TNF-α), and IL-1β [14]. Administration of sodium butyrate improved inflammation in mice by activating GPR109A while suppressing protein kinase B and nuclear factor kappa B (NF-κB) p65 signalling pathways [4]. Cyclic GMP-AMP synthase (cGAS) and stimulator of interferon genes (STING) form the cGAS-STING pathway that activates NOD-like receptor protein 3 (NLRP3) inflammasome. Butyrate reduced intestinal inflammation in in vitro and in vivo CD models by inhibiting cGAS-STING-NLRP3 axis-driven pyroptosis in intestinal epithelial cells [15]. However, the anti-inflammatory actions of SCFAs are not universal and may be dependent on the immunological and metabolic context. For instance, Vancamelbeke et al. found that butyrate was ineffective and could exacerbate inflammation-induced barrier dysfunction in UC patient-derived cell lines, possibly due to butyrate hypersensitivity [16]. Moreover, butyrate and propionate activated NLRP3 inflammasome in human macrophages under inflammatory conditions by inhibiting the transcription of FLICE-like inhibitory protein and IL-10 through HDAC inhibition [17]. Nevertheless, these findings highlight the context-dependent roles of SCFAs in modulating disease progression. Thirdly, SCFAs are involved in modulating immune cells. Butyrate alleviated colitis in mice by inhibiting pro-inflammatory T helper 17 (Th17) cells via the activation of Sirtuin 1 and mammalian target of rapamycin [14]. Additionally, butyrate promoted M2 macrophage polarization, which attenuated intestinal inflammation in in vitro and in vivo colitis models [18]. The expression of amphiregulin in dendritic cells, a crucial regulator of cell proliferation and repair, relies on butyrate and its interactions with GPR43 and B lymphocyte-induced maturation protein 1 [19]. Butyrate suppressed the capacity of neutrophils to produce pro-inflammatory mediators and form neutrophil extracellular traps in mice [3]. Lastly, SCFAs, particularly butyrate, regulate intestinal metabolism by enhancing energy production. Adding butyrate to germ-free colonocytes restored oxidative phosphorylation and suppressed autophagy [20]. Butyrate metabolism enhanced mitochondrial respiration in normal human colon mucosal epithelial cells, supporting transporter-mediated butyrate utilization and thereby maintaining barrier integrity [21]. These synergistic actions collectively alleviate diarrhea, rectal bleeding, and abdominal pain symptoms.
Given their critical role in intestinal homeostasis and disease modulation, current research primarily focuses on prebiotics, probiotics, and fecal microbiota transplantation (FMT), which utilize the body’s natural capacity to enhance SCFA production. Oral administration of sodium butyrate as a postbiotic therapy is not preferred due to its rancid odour and limited efficacy, owing to rapid proximal small bowel absorption that results in low colonic bioavailability [22].
Prebiotics are non-digestible food compounds that are selectively utilized by host microorganisms to confer health benefits [23]. They act as substrates for the production of SCFAs through microbial fermentation. Common types of prebiotics include fructooligosaccharides (FOS), galactooligosaccharides (GOS), and polysaccharides. Some studies propose that SCFAs mediate the therapeutic effects of prebiotics, while others find no significant impact. For instance, Astragalus polysaccharides increased the abundance of SCFA-producing bacteria in UC mice, thereby suppressing NF-κB activation and improving the balance between Th17 and regulatory T (Treg) cells [24]. In contrast, a randomized controlled trial (RCT) involving patients with mild to moderate UC found that the efficacy of 1-ketose was not associated with significant changes in SCFA levels [25].
Probiotics are live microorganisms that confer health benefits to the host when administered in adequate amounts [23]. Preclinical data support probiotics as a promising therapy for IBD in dextran sodium sulphate (DSS)-induced colitis mouse model through multiple SCFA-related mechanisms that act in concert. Commonly studied single-strain probiotics include various species of Bifidobacterium and Lactobacillus. Firstly, prebiotics play a crucial role in maintaining intestinal barrier function. For instance, administration of Lactobacillus plantarum (L. plantarum) HNU082 in DSS mice protected the intestinal barrier by enhancing the production of SCFAs, ZO-1, ZO-2, occludin, goblet cells, and mucin-2 [26]. Escherichia coli Nissle 1917 (EcN) with genes encoding Elafin improved butyrate and valerate production and restored ZO-1’s activity in mice [27]. Acetic acid-producing Bifidobacterium bifidum (B. bifidum) H3-R2 and propionic acid-producing Propionibacterium freudenreichii B1 enhanced the production of ZO-1, occludin, and claudin-1 in mice through the rho-associated coiled-coil containing protein kinase 1 and Wnt/β-catenin signalling pathways, with the latter exerting more substantial protective effects [28].
Secondly, probiotics play a role in immune modulation. Oral administration of B. breve strains H4-2 and H9-3 in mice produced exopolysaccharides, which mitigated inflammation by promoting SCFA production and inhibiting the NF-κB signalling pathway [29]. Upregulation of SCFAs by B. breve Bif11, B. longum Bif10, and B. longum Bif16 helped to modulate immune responses in mice by limiting the translocation of LPS into the bloodstream, with B. longum Bif10 and B. breve Bif11 exerting stronger protective effects [30]. L. plantarum L15 led to a marked reduction in the translocation of NF-κB p65 to the nucleus through increasing colonic acetate and butyrate levels in mice [31]. Administration of low-dose L. rhamnosus CY12 in mice produced acetic acid, which inhibited the translocation of NF-κB p65 and the signalling pathway comprising toll-like receptor 4, myeloid differentiation primary response 88, and NF-κB [32]. Acetate-producing Bacillus paralicheniformis HMPM220325 suppressed the NLRP3 inflammasome signalling pathway and relieved UC in mice [33].
Lastly, probiotics help regulate the microbiota composition by promoting the growth of beneficial bacteria while suppressing the growth of harmful ones. L. plantarum ZJ316 enriched butyrate-producing genera in mice, including Faecalibacterium, Agathobacter, and Roseburia [34]. L. plantarum DMDL 9010 elevated the relative abundance of Bacteroidetes and Firmicutes and mitigated symptoms of depression-like behaviour in mice [35]. Similarly, L. rhamnosus strains SHA113 and KBL2290 alleviated colitis in mice by increasing the abundance of SCFA-producing genera [36,37]. EcN-Sj16 encoding a schistosome immunoregulatory protein (Sj16) increased butyrate levels through a selective enrichment of Ruminococcaceae, leading to elevated retinoic acid production, which relieved colitis in mice by promoting Treg cell activity and suppressing Th17 [38].
Most single-strain probiotics are still in the early stages of preclinical testing, with few having progressed to clinical trials. Three RCTs demonstrated that EcN’s effect on maintaining the remission of UC was comparable to that of mesalazine [39,40,41]. However, the clinical efficacy of EcN may not be associated with the production of SCFAs [42]. Nevertheless, it is increasingly being explored as a next-generation probiotic that could be engineered to enhance SCFA production. For example, EcNL4, a genetically engineered EcN strain to produce ketone body (R)-3-hydroxybutyrate, increased SCFA levels by 3.1 times in mice compared to the wild-type [43]. Moreover, many studies have not investigated changes in SCFA levels. For instance, B. longum 536 (BB536) and L. rhamnosus GG (LGG) have demonstrated clinical efficacy in inducing and maintaining remission in UC patients, respectively [44,45]. Despite previous indications of their ability to produce SCFAs, limited preclinical and clinical evidence measures changes in SCFA levels and correlates them with disease improvement [46,47]. Therefore, additional evidence is required to conclude whether SCFAs directly contribute to the prophylaxis of IBD.
Multi-strain probiotics exhibit synergistic effects that are superior to those of single-strain probiotics and are more commonly explored in clinical studies. VSL#3, a diverse formulation comprising eight bacterial strains, is one of the most effective probiotics. It exhibited anti-inflammatory effects in Muc2 mucin-deficient mice by stimulating acetate production, which facilitated the suppression of chemokines and reactive oxygen species while upregulating growth factors [48]. Patients with mild to moderate UC achieved better remission with VSL#3 and low-dose balsalazide than with balsalazide alone or mesalazine [49]. Three double-blind RCTs demonstrated that the daily administration of 3.6 × 1012 CFU of VSL#3 was safe and effective in inducing remission in adult patients with mild to moderate UC [50,51,52]. A body weight-dependent dose of VSL#3 was reported to be safe and maintained remission in children with UC [53]. Administration of VLS#3 in CD patients resulted in reduced mucosal inflammation and a lower post-surgery endoscopic recurrence rate [54].
Bifidobacteria-fermented milk (BFM) contains Yakult strains of B. breve, B. bifidum, and L. acidophilus. It significantly increased SCFA levels and butyrate utilization in the colonic mucosa of UC patients in an RCT [55]. Two RCTs demonstrated that administering BFM at 100 mL/day was superior to standard treatment and effective in maintaining remission of UC [55,56]. An RCT of B. breve strain Yakult, combined with the prebiotic GOS, markedly improved the clinical status of patients with mild to moderate UC [57]. A double-blind RCT on patients with quiescent UC did not corroborate the conclusion and found BFM ineffective in reducing relapse time and incidence compared to placebo [58]. Mixed results could be attributed to the difference in disease states and dosage frequency.
SymproveTM (Symprove Ltd., Farnham, UK), a four-strain probiotic, exhibited anti-inflammatory and wound-healing properties in in vitro UC and co-culture models by modulating the microbiota composition and promoting lactate and butyrate synthesis [59]. A single-centre RCT of SymproveTM showed no adverse effects or significant differences in IBD-related quality of life (QoL) scores between the treatment and placebo groups [60]. However, treatment significantly lowered fecal calprotectin levels and reduced inflammation in UC patients, while no statistical differences were observed in CD patients. An RCT of Bifidobacterium triple viable combined with mesalazine enhanced the therapeutic efficacy for UC by achieving a response rate of 91.11% [61]. Treatment enriched SCFA-producing genera, including Bifidobacteria, but direct changes in SCFA levels were not measured.
Other multi-strain probiotics have also demonstrated the capacity to stimulate SCFA production, which conferred protective effects in preclinical studies. GUT-108, an 11-strain probiotic, reversed colitis in mouse models [62]. Mechanisms include the upregulation of acetate, butyrate, and propionate, which promote mucosal healing and immunomodulation. A combination of seven SCFA-producing strains, 7-mix, promoted M2 macrophage polarization via inactivation of a signalling pathway involving Janus kinase (JAK), signal transducer and activator of transcription (STAT), and forkhead box O3 (FOXO3) [63]. This subsequently suppressed the inflammatory response and improved mucosal healing in in vitro and in vivo models. A four-strain probiotic’s ability to enhance CD-like ileitis in SAMP mice was associated with a significant increase in SCFAs and their capacity to act as GPCRs to maintain gut homeostasis [64].
FMT involves the transfer of microbial community, including SCFA-producing microbes, from a healthy donor to a recipient to restore the microbial imbalance. It has been effective in helping UC patients achieve remission by enriching SCFA-producing strains and SCFA biosynthesis [65]. FMT combined with probiotics containing Clostridium butyricum enriched fecal butyric acid content, and significantly prolonged remission in UC patients [66]. Lima et al. identified a core set of human donor-derived transferable strains where Odoribacter splanchnicus played a key role in alleviating colonic inflammation in mice, with its protective effects mediated by lymphocytes, IL-10, and SCFAs [67].
SCFAs demonstrated their potential in treating IBD by maintaining intestinal barrier integrity, suppressing inflammation, modulating the immune system, and supporting colonocyte function (Table 1). Oral supplementation of SCFAs is ineffective due to limited colonic bioavailability, whereas prebiotics, probiotics, and FMT enhance endogenous SCFA production, leading to sustained and localized effects. The probiotic VSL#3 has shown the most consistent clinical evidence for maintaining remission in UC patients. The clinical efficacy of SCFA therapies is more significant in UC patients than in CD patients, due to their distinct pathophysiological characteristics. Inflammation in UC is typically restricted to the colon and therefore responds more effectively to treatments such as probiotics. UC patients who show the highest response rates consist of those with mild to moderate disease activity, relapsing UC, and those experiencing dysbiosis with low baseline SCFA production. SCFA therapies are less effective for CD due to its transmural inflammation, which makes immune-targeted treatments, such as aminosalicylates, corticosteroids, and immunomodulators, the primary option.

Table 1.
A comparative summary of SCFA therapeutics in GI diseases.
4. Irritable Bowel Syndrome
Irritable Bowel Syndrome (IBS) is a chronic GI disorder that affects the colon. It is classified into four subtypes based on bowel habit abnormalities: diarrhea-predominant IBS (IBS-D), constipation-predominant IBS (IBS-C), IBS with mixed bowel habits (IBS-M), and unclassified IBS (IBS-U). Studies reported that IBS patients have differentially abundant SCFA-producing bacteria and altered levels of SCFAs. The reduction in butyrate-producing bacteria is a hallmark of IBS-M and IBS-D [6]. In contrast, IBS patients exhibited a significantly higher abundance of Veillonella and Lactobacillus, along with acetate and propionate, which were positively correlated with the severity of their GI symptoms [68]. Fecal propionate levels were also documented as a potential biomarker for diagnosing the IBS-D subtype [69].
There are several mechanisms by which SCFAs, particularly butyrate, alleviate IBS. One key factor in the pathophysiology of IBS is visceral hypersensitivity, which refers to the abnormally heightened sensitivity of the internal organs that leads to discomfort [119]. Butyrate attenuated visceral hypersensitivity in mice by lowering colonic expression of IL-1 receptor-associated kinase 1 [70]. Butyrate also alleviated visceral allodynia and colonic hyperpermeability in rats, likely through adenosine monophosphate-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor γ, involving nitric oxide, opioid, and central dopamine D2 pathways [71]. Additionally, butyrate is involved in gut–brain axis communication by acting through the central nervous system to enhance intestinal integrity and visceral nociception via cannabinoid signalling in rats [72]. Due to the complex nature of IBS, SCFA therapies encompass a range of interventions designed to enhance the endogenous production of SCFA through diet, prebiotics, probiotics, and FMT rather than direct supplementation.
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) normalizes abnormal fecal acetate and propionate levels and is the most effective treatment for IBS-D [69]. A prospective RCT involving 101 IBS-D patients found that both a short-term strict and a long-term modified low FODMAP diet (LFD) significantly improved QoL, alleviated symptoms such as abdominal pain frequency and bloating, and significantly decreased IBS medication use compared to traditional dietary advice [73]. However, restricted fibre intake may negatively impact gut health by altering the gut microbiota composition and limiting the production of SCFAs. Three RCTs showed a significant reduction in Bifidobacteria following an LFD [74]. Fibre-fix, a prebiotic supplement comprising different fibres, increased colonic fermentation and production of SCFAs. In a double-blind RCT, consumption of Fibre-fix alongside an LFD promoted butyrate production, which increased neurotransmitter synthesis and metabolism [75]. This led to improved mental health without inducing IBS symptoms. In an RCT, an LFD combined with prebiotic β-GOS relieved IBS symptoms but could not prevent the decline in fecal Bifidobacteria and butyrate, reaffirming the adverse effects of long-term LFD use [76]. Prebiotic partially hydrolyzed guar gum (PHGG) increased the relative abundance of Bifidobacteria and Faecalibacteria and induced the production of acetic and butyric acids in vitro [77]. Administration of PHGG at 6 g/day significantly relieved bloating in IBS patients, but was ineffective against abdominal pain, stool frequency, or QoL [78]. Intake of inulin at 10 g/day, together with choline and silymarin, improved abdominal pain and bloating in IBS-C patients [79]. Stool frequency and consistency were also improved, though the changes were not statistically significant. However, high doses of prebiotics may worsen symptoms due to excessive colonic gas production. For example, 20 g/day of FOS did not outperform the control in providing symptom relief. It led to a transient worsening of abdominal flatulence at the start of the treatment [120].
A probiotic cocktail, Bifico, improved visceral hypersensitivity in IBS mice, with Bifidobacteria playing a key role by increasing propionate, butyrate, and valerate production while reducing IL-6 and TNF-α [80]. A crossover RCT investigated the effect of a combination of BB536 and L. rhamnosus HN001 with vitamin B6 (LBB) in IBS-C and IBS-D patients [81]. LBB treatment significantly reduced abdominal pain, bloating, and disease severity. These effects may be attributed to the restoration of the gut microbiota and a significant increase in propionate, butyrate, and pentanoate levels, which improved colonic permeability. Stool consistency was significantly normalized in IBS-D patients, whereas only slight improvements were observed in IBS-C patients. A pilot RCT on L. plantarum CCFM8610 demonstrated a significant reduction in bloating and restoration of dysbiosis in IBS-D patients, likely through increasing butyrate-producing bacteria [82].
Numerous RCTs have investigated FMT as a therapeutic intervention for IBS, demonstrating varying degrees of efficacy. El-Salhy et al. compared the effectiveness of gastroscopic delivery of 30 g or 60 g FMT to placebo in a double-blind RCT involving 164 patients [83]. Treatment improved abdominal symptoms, fatigue, and QoL in IBS patients, with the response to treatment positively correlated with the dosage received. A continuation of the study found that the significant increase in fecal butyric acid levels in both treatment groups was negatively correlated to IBS symptoms, suggesting a potential involvement of SCFAs in the pathophysiology of IBS [84]. A 3-year follow-up study of 125 patients reported high response rates and long-lasting effects with few self-limiting adverse events [85]. Nasojejunal delivery of FMT in patients with refractory IBS led to a transient reduction in abdominal bloating. Although the effects diminished over a year, a second FMT was able to restore symptom relief in patients who had previously responded [86]. An RCT involving a single FMT via colonoscopy following bowel cleansing could relieve symptoms, but did not outperform autologous FMT [87]. Another RCT of single colonoscopic delivery induced a lasting change in the microbiota of IBS patients, but only provided temporary symptom relief [88]. Patients who received FMT via oral capsules also experienced increased fecal microbial diversity, but this did not translate into clinical improvement [89]. In contrast, the placebo group showed greater symptom alleviation than the treatment group. Similarly, bacterial engraftment in IBS-D patients following oral FMT did not result in superior symptom relief compared to placebo [90]. While the studies by El-Salhy et al. showed promising results, the overall results were inconsistent. The route of FMT delivery also affects its relative efficacy. A meta-analysis of eight RCTs, including the study by El-Salhy et al., found endoscopic delivery effective but not oral capsules [91].
Butyrate stands out as a potential therapeutic option for IBS as it reduces visceral hypersensitivity while enhancing intestinal barrier integrity. In contrast, propionate may serve as a biomarker for IBS-D. An LFD provided effective control of IBS-D symptoms, yet long-term strict compliance may reduce the diversity of the gut microbiota. Prebiotics like PHGG and inulin provided IBS-C patients with better relief from bloating symptoms, but high doses of prebiotics can render them ineffective and may exacerbate symptoms. Probiotics showed effectiveness mainly in IBS-D patients, whereas FMT yielded inconsistent results, depending on factors such as dosage, frequency, and route of administration. Many studies did not distinguish between the IBS subtypes as they are not always easily differentiated. This may lead to a heterogeneous patient population with varied responses, making it difficult to determine whether a treatment is effective for one subtype over another due to potential dilution of the average effects.
5. Metabolic Dysfunction-Associated Steatotic Liver Disease
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD), is characterized by abnormal hepatic lipid accumulation [121]. It encompasses a spectrum of diseases from simple steatosis to metabolic-associated steatohepatitis and cirrhosis. Although the terminology has changed, previous studies referenced in this review used NAFLD as the definition and diagnostic criteria. Recent multi-omics analyses have shown that patients with NAFLD exhibit distinct changes in gut microbiota and fecal SCFAs compared to healthy controls (HC), including a significant reduction in fecal acetic and butyric acids [7]. Moreover, SCFA levels could be used as a diagnostic tool to predict disease severity. Butyric acid could differentiate mild NAFLD from HC, while acetic acid could distinguish moderate NAFLD and severe NAFLD from HC.
SCFAs play a crucial role in managing MASLD primarily through reducing hepatic steatosis, enhancing insulin sensitivity, and inhibiting inflammation. Sodium butyrate inhibited steatosis in mice mainly via activating a key metabolic pathway involving liver kinase B1, AMPK, and insulin-induced gene [92]. Similarly, sodium acetate reduced steatosis in mice by activating AMPK [93]. Acetate is associated with reduced adipocyte lipolysis in healthy volunteers, which lowers the flux of free fatty acids to the liver, while propionate suppressed lipogenesis in vitro [94,95]. Sodium butyrate attenuated type 2 diabetes-induced NAFLD in mice by modulating intestinal tight junctions and restoring intestinal Takeda G-protein-coupled expression, increasing serum glucagon-like peptide-1 levels and insulin sensitivity [96]. Administration of sodium acetate, propionate, and butyrate significantly lowered serum levels of alanine aminotransferase (ALT) and aspartate transaminase (AST) in mice, suggesting an attenuation of hepatic injury and inflammation [93]. Sodium acetate demonstrated superior anti-inflammatory effects, which were primarily mediated by the inhibition of macrophage pro-inflammatory activation. In addition, SCFAs have also shown mild antifibrotic effects in preclinical models; however, their clinical efficacy in attenuating liver fibrosis remains limited. Butyrate protected mice from diet-induced NAFLD and fibrosis by attenuating collagen synthesis in hepatic stellate cells, whereas acetate and propionate did not exert similar effects [97]. Commonly studied SCFA therapies of MASLD/NAFLD include prebiotics, probiotics, and FMT.
Inulin is one of the most commonly explored prebiotics. Butyrate produced from prebiotic fermentation of inulin mediated the expression of Paneth cell α-defensins, potentially through histone deacetylation and STAT3 activation, leading to improved diet-induced barrier dysfunction in mice [98]. Acetate, another fermentation product of inulin, modulated hepatic lipid metabolism and insulin sensitivity through free fatty acid receptor 2 in hepatocytes, thereby attenuating NAFLD progression in mice [99]. Other prebiotics include FOS, which improved steatohepatitis, visceral fat accumulation, and chronic inflammation by increasing fecal and serum SCFA levels [100]. Sinapine, a prebiotic polyphenol derived from rapeseed, led to an enrichment of probiotic bacteria and SCFA-mediated expression of GPR43, which suppressed inflammation in mice [101]. Resistant starch from green bananas improved SCFA production and obesity-related steatosis in mice [102].
B. pseudolongum significantly attenuated NAFLD-hepatocellular carcinoma in mice by producing acetate, which binds to GPR43 on hepatocytes, inhibiting the IL-6/JAK1/STAT3 signalling pathway and suppressing tumour formation [103]. LGG combined with oat beta-glucan improved NAFLD by increasing liver acetate content, which reduced de novo lipogenesis in mice [104]. Astragalus polysaccharides-enriched Desulfovibrio vulgaris alleviated hepatic steatosis in mice, potentially through acetic acid production [105]. Acetic acid reduced high-fat diet (HFD)-induced weight gain and suppressed the expression of fatty acid synthase, an enzyme that can act as the rate-limiting step in hepatic fatty acid synthesis. A meta-analysis of 35 RCTs involving 2212 NAFLD patients revealed that a combination of Lactobacillus, Bifidobacterium, and Streptococcus may be the most effective in improving liver enzymes (ALT, AST, and gamma glutamyl transpeptidase), lipid profiles (low-density lipoprotein and total cholesterol), and inflammation factors (TNF-α) [106]. However, most of the studies did not assess changes in SCFA levels. One study that tested changes in fecal SCFA concentration following the administration of a synbiotic containing FOS and B. animalis subsp. lactis BB-12 found no association between changes in SCFA levels and liver fat percentage [107]. While the synbiotic was effective in promoting the growth of Bifidobacterium and Faecalibacterium, it did not lead to improvements in liver fat content or fibrosis markers. These findings highlight the need for further studies to evaluate the relationship between SCFA modulation by probiotics and therapeutic outcomes in MASLD/NAFLD.
FMT attenuated estrogen deficiency-induced NAFLD in mice by restoring butyrate concentration, thereby maintaining gut barrier integrity and preventing the translocation of LPS [108]. FMT of a nine-strain bacterial consortium comprising butyrate and propionate producers prevented the progression of NAFLD in rats [109]. The association between the increase in cecal butyrate and propionate levels and disease improvement is to be determined.
On the contrary, some studies indicated deleterious effects of SCFAs. Fecal acetate and propionate concentrations were elevated in NAFLD patients, along with a higher abundance of SCFA-producing genera, which were correlated with immunological markers of disease progression [110]. Likewise, Ruminococcaceae and Veillonellaceae were identified as key microbial contributors associated with fibrosis severity in non-obese NAFLD patients [111]. Moreover, fecal propionate levels were elevated in those with significant fibrosis. HFD led to gut dysbiosis in mice, characterized by an increased serum acetate level, which was normalized by Bacillus species through the reduction in acetate and GPR43 [112]. Nevertheless, these findings suggest that SCFAs play a role in MASLD/NAFLD regulation and could serve as potential diagnostic markers.
Overall, SCFAs alleviate MASLD/NAFLD by attenuating hepatic steatosis, increasing glucose sensitivity, and inhibiting inflammation. Current SCFA therapies, including prebiotics, probiotics, and FMT, have shown therapeutic promise in MASLD/NAFLD management, though their clinical application requires further validation. The greatest therapeutic benefits could be achieved in patients with early-stage disease, characterized by simple steatosis without fibrosis. Although SCFAs exhibit mild antifibrotic properties, their principal value lies in reducing fibrogenesis rather than reversing established scarring in advanced fibrosis or cirrhosis stages.
6. Acute Pancreatitis
Acute pancreatitis (AP), or the inflammation of the pancreas, is characterized by an increase in the production of serum pancreatic enzymes that promote the necrosis of acinar cells. AP exacerbation is strongly associated with increased barrier permeability, SFCA depletion, and alteration of the gut microbiota [122]. Administration of butyrate in animal models could attenuate the disease by reducing systemic inflammation and promoting intestinal barrier integrity [113,114]. Possible mechanisms include upregulating TJ proteins and FOXP3, suppressing pro-inflammatory mediators, and inhibiting the NLRP3 inflammasome. Administration of butyrate in mice notably enhanced intestinal barrier function compared to ineffective antibiotic treatment [115,116]. Acetate production via Parabacteroides administration could also mitigate AP in mice by reducing neutrophil infiltration [117]. Not all findings, however, support the therapeutic role of SCFAs in AP treatment. Ammer-Herrmenau et al. found that all sixteen differentially abundant microbe species over-represented in severe AP patients compared to those with non-severe AP were common producers of SCFAs [118]. Despite that, the finding highlights the role of SCFAs in AP severity and their significance as therapeutic targets in disease management.
7. Conclusions and Future Perspectives
Collectively, there is increasing evidence on the association between gut microbiota and GI diseases. The local modulatory effects of SCFAs within the gut are demonstrated in IBD and IBS. At the same time, MASLD extends its influence to the gut–liver axis, and AP reveals a broader systemic impact that links gut dysbiosis and SCFA depletion to pancreatic inflammation. These results underscore the pivotal roles of SCFAs in GI diseases and establish them as viable targets for therapy. However, there is a need for additional research to fill existing knowledge gaps before these treatments can achieve full effectiveness. Treatment efficacy may differ among patients due to individual variability in gut microbiota, highlighting the importance of precision therapy. For instance, microbiome profiling enables clinicians to identify individual-specific microbiota, guiding the selection of appropriate probiotics. Moreover, current approaches lack standardized protocols and clinical trials to assess specific interventions among diverse populations over extended periods. Additionally, the mechanisms of action of SCFAs are not well understood. Many studies have not measured SCFA changes or associated them with improvements in disease. As SCFAs are often used as adjunct therapies, further research is needed to investigate their interactions with conventional treatments to ensure safety and efficacy.
Author Contributions
Writing—original draft preparation, M.T.Z.; writing—review and editing, J.W.J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, Y.; Huang, Y.; Chase, R.C.; Li, T.; Ramai, D.; Li, S.; Huang, X.; Antwi, S.O.; Keaveny, A.P.; Pang, M. Global Burden of Digestive Diseases: A Systematic Analysis of the Global Burden of Diseases Study, 1990 to 2019. Gastroenterology 2023, 165, 773–783.e15. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-Chain Fatty Acids Activate GPR41 and GPR43 on Intestinal Epithelial Cells to Promote Inflammatory Responses in Mice. Gastroenterology 2013, 145, 396–406.e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. eBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci. Rep. 2015, 5, 12693. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, J.; Yang, L.; Hu, Q.; Li, L.; Yang, Y.; Hu, J.; Pan, D.; Zhao, Q. Altered gut microbial profile accompanied by abnormal short chain fatty acid metabolism exacerbates nonalcoholic fatty liver disease progression. Sci. Rep. 2024, 14, 22385. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.J.; Plichta, D.; Hogstrom, L.; Borren, N.Z.; Lau, H.; Gregory, S.M.; Tan, W.; Khalili, H.; Clish, C.; Vlamakis, H.; et al. Multi-omics reveal microbial determinants impacting responses to biologic therapies in inflammatory bowel disease. Cell Host Microbe 2021, 29, 1294–1304.e1294. [Google Scholar] [CrossRef] [PubMed]
- Deleu, S.; Arnauts, K.; Deprez, L.; Machiels, K.; Ferrante, M.; Huys, G.R.B.; Thevelein, J.M.; Raes, J.; Vermeire, S. High Acetate Concentration Protects Intestinal Barrier and Exerts Anti-Inflammatory Effects in Organoid-Derived Epithelial Monolayer Cultures from Patients with Ulcerative Colitis. Int. J. Mol. Sci. 2023, 24, 768. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.C.; Wang, Y.; Wang, Z.B.; Liu, W.Y.; Sun, S.; Li, L.; Su, D.F.; Zhang, L.C. Propionate Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Front. Pharmacol. 2016, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, Z.; Wang, Q.; Wu, C.; Sun, Y.; Wang, Z.; Xu, X.; Xue, W.; Cao, Z.; Zhang, M.; et al. Bacteroides methylmalonyl-CoA mutase produces propionate that promotes intestinal goblet cell differentiation and homeostasis. Cell Host Microbe 2024, 32, 63–78.e67. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, W.; Li, F.; Xiang, L.; Lv, P.; Chen, Y. Treatment with butyrate alleviates dextran sulfate sodium and Clostridium difficile-induced colitis by preventing activity of Th17 cells via regulation of SIRT1/mTOR in mice. J. Nutr. Biochem. 2023, 111, 109155. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, Z.; Huang, Z.; Lv, X.; Jiang, D.; Huang, Z.; Han, B.; Lin, G.; Liu, G.; Li, S.; et al. Butyrate attenuates intestinal inflammation in Crohn’s disease by suppressing pyroptosis of intestinal epithelial cells via the cGSA-STING-NLRP3 axis. Int. Immunopharmacol. 2024, 143, 113305. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Laeremans, T.; Vanhove, W.; Arnauts, K.; Ramalho, A.S.; Farré, R.; Cleynen, I.; Ferrante, M.; Vermeire, S. Butyrate Does Not Protect Against Inflammation-induced Loss of Epithelial Barrier Function and Cytokine Production in Primary Cell Monolayers From Patients with Ulcerative Colitis. J. Crohn’s Colitis 2019, 13, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dernst, A.; Martin, B.; Lorenzi, L.; Cadefau-Fabregat, M.; Phulphagar, K.; Wagener, A.; Budden, C.; Stair, N.; Wagner, T.; et al. Butyrate and propionate are microbial danger signals that activate the NLRP3 inflammasome in human macrophages upon TLR stimulation. Cell Rep. 2024, 43, 114736. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Shu, D.; Zheng, M.; Wang, J.; Luo, C.; Wang, Y.; Guo, F.; Zou, X.; Lv, X.; Li, Y.; et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 2016, 6, 24838. [Google Scholar] [CrossRef] [PubMed]
- Xiu, W.; Chen, Q.; Wang, Z.; Wang, J.; Zhou, Z. Microbiota-derived short chain fatty acid promotion of Amphiregulin expression by dendritic cells is regulated by GPR43 and Blimp-1. Biochem. Biophys. Res. Commun. 2020, 533, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Conder, E.; Shay, H.C.; Vekaria, H.; Erinkitola, I.; Bhogoju, S.; Goretsky, T.; Sullivan, P.; Barrett, T.; Kapur, N. Butyrate-induced mitochondrial function improves barrier function in inflammatory bowel disease (IBD). Gastroenterology 2023, 164, S91. [Google Scholar] [CrossRef]
- Pietrzak, A.; Banasiuk, M.; Szczepanik, M.; Borys-Iwanicka, A.; Pytrus, T.; Walkowiak, J.; Banaszkiewicz, A. Sodium Butyrate Effectiveness in Children and Adolescents with Newly Diagnosed Inflammatory Bowel Diseases-Randomized Placebo-Controlled Multicenter Trial. Nutrients 2022, 14, 3283. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, W.; Qin, H.; Chen, Z.; Zhou, Y.; Zhou, Z.; Wang, J.; Wang, K. Astragalus polysaccharides alleviate DSS-induced ulcerative colitis in mice by restoring SCFA production and regulating Th17/Treg cell homeostasis in a microbiota-dependent manner. Carbohydr. Polym. 2025, 349, 122829. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, S.; Nakamura, M.; Honda, T.; Yamamura, T.; Maeda, K.; Sawada, T.; Ishikawa, E.; Yamamoto, K.; Furune, S.; Ishikawa, T.; et al. Efficacy of 1-kestose supplementation in patients with mild to moderate ulcerative colitis: A randomised, double-blind, placebo-controlled pilot study. Aliment. Pharmacol. Ther. 2023, 57, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jha, R.; Li, A.; Liu, H.; Zhang, Z.; Zhang, C.; Zhai, Q.; Zhang, J. Probiotics (Lactobacillus plantarum HNU082) Supplementation Relieves Ulcerative Colitis by Affecting Intestinal Barrier Functions, Immunity-Related Gene Expression, Gut Microbiota, and Metabolic Pathways in Mice. Microbiol. Spectr. 2022, 10, e01651-22. [Google Scholar] [CrossRef] [PubMed]
- Teng, G.; Liu, Z.-Z.; Liu, Y.; Wu, T.; Dai, Y.; Wang, H.; Wang, W. Probiotic Escherichia coli Nissle 1917 Expressing Elafin Protects Against Inflammation and Restores the Gut Microbiota. Front. Microbiol. 2022, 13, 819336. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shang, J.; Liu, L.; Tang, Z.; Meng, X. Strains producing different short-chain fatty acids alleviate DSS-induced ulcerative colitis by regulating intestinal microecology. Food Funct. 2022, 13, 12156–12169. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.M.; Guo, H.X.; Cai, J.W.; Meng, X.C. Bifidobacterium breve Alleviates DSS-Induced Colitis in Mice by Maintaining the Mucosal and Epithelial Barriers and Modulating Gut Microbes. Nutrients 2022, 14, 3671. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhatia, R.; Khare, P.; Sharma, S.; Rajarammohan, S.; Bishnoi, M.; Bhadada, S.K.; Sharma, S.S.; Kaur, J.; Kondepudi, K.K. Anti-inflammatory Bifidobacterium strains prevent dextran sodium sulfate induced colitis and associated gut microbial dysbiosis in mice. Sci. Rep. 2020, 10, 18597. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L.; Tang, H.; Zhang, K.; Chen, Q.; Liu, C.; Guo, Y.; Li, M.; Guo, Z.; Li, B. In vivo evidence of the prevents DSS-induced colitis of Lactiplantibacillus plantarum L15. Front. Microbiol. 2022, 13, 1028919. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ahmad, A.A.; Yang, C.; Liang, Z.; Shen, W.; Liu, J.; Yan, Z.; Han, J.; Yang, Y.; Dong, P.; et al. Orally Administered Lactobacillus rhamnosus CY12 Alleviates DSS-Induced Colitis in Mice by Restoring the Intestinal Barrier and Inhibiting the TLR4-MyD88-NF-κB Pathway via Intestinal Microbiota Modulation. J. Agric. Food Chem. 2024, 72, 9102–9116. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Yang, X.; Pan, P.; Zhang, G.; Sheng, K.; Wang, J.; Liang, X.; Wang, Y. Bacillus paralicheniformis, an acetate-producing probiotic, alleviates ulcerative colitis via protecting the intestinal barrier and regulating the NLRP3 inflammasome. Microbiol. Res. 2024, 287, 127856. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Xia, C.; Liu, N.; Chen, Z.; Zhou, Q.; Li, P. Lactobacillus plantarum ZJ316 alleviates ulcerative colitis by inhibiting inflammation and regulating short-chain fatty acid levels and the gut microbiota in a mouse model. Food Funct. 2023, 14, 3982–3993. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Wu, Y.P.; Jia, X.Z.; Lin, J.; Xiao, L.F.; Liu, D.M.; Liang, M.H. Lactiplantibacillus plantarum DMDL 9010 alleviates dextran sodium sulfate (DSS)-induced colitis and behavioral disorders by facilitating microbiota-gut-brain axis balance. Food Funct. 2022, 13, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Jin, H.; Liao, N.; Li, J.; Jiang, C.; Shao, D.; Shi, J. Lactobacillus rhamnosus from human breast milk ameliorates ulcerative colitis in mice via gut microbiota modulation. Food Funct. 2021, 12, 5171–5186. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Min, S.G.; Kwon, H.; Park, S.; Jo, M.J.; Ko, G. Lactobacillus rhamnosus KBL2290 Ameliorates Gut Inflammation in a Mouse Model of Dextran Sulfate Sodium-Induced Colitis. J. Microbiol. 2023, 61, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liao, Y.; Yang, R.; Zhu, Z.; Zhang, L.; Wu, Z.; Sun, X. An engineered probiotic secreting Sj16 ameliorates colitis via Ruminococcaceae/butyrate/retinoic acid axis. Bioeng. Transl. Med. 2021, 6, e10219. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Schütz, E.; Fric, P.; Fixa, B.; Judmaier, G.; Stolte, M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 1997, 11, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Rembacken, B.J.; Snelling, A.M.; Hawkey, P.M.; Chalmers, D.M.; Axon, A.T.R. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: A randomised trial. Lancet 1999, 354, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Frič, P.; Pokrotnieks, J.; Lukáš, M.; Fixa, B.; Kaščák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Nzakizwanayo, J.; Dedi, C.; Standen, G.; Macfarlane, W.M.; Patel, B.A.; Jones, B.V. Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Sci. Rep. 2015, 5, 17324. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, X.-Y.; Zhang, D.; Zhang, Y.-D.; Li, Z.-H.; Liu, X.; Wu, F.; Chen, G.-Q. Construction of a sustainable 3-hydroxybutyrate-producing probiotic Escherichia coli for treatment of colitis. Cell. Mol. Immunol. 2021, 18, 2344–2357. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, H.; Nakase, H.; Inoue, S.; Kawanami, C.; Itani, T.; Ohana, M.; Kusaka, T.; Uose, S.; Hisatsune, H.; Tojo, M.; et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig. Endosc. 2016, 28, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zocco, M.A.; Dal Verme, L.Z.; Cremonini, F.; Piscaglia, A.C.; Nista, E.C.; Candelli, M.; Novi, M.; Rigante, D.; Cazzato, I.A.; Ojetti, V.; et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 2006, 23, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, H.; Odamaki, T.; Fukuda, S.; Kato, T.; Xiao, J.-Z.; Abe, F.; Kikuchi, J.; Ohno, H. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci. Rep. 2015, 5, 13548. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Ma, K.; Li, J.; Ren, Z.; Zhang, J.; Shan, A. Lactobacillus rhamnosus GG ameliorates DON-induced intestinal damage depending on the enrichment of beneficial bacteria in weaned piglets. J. Anim. Sci. Biotechnol. 2022, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kissoon-Singh, V.; Coria, A.L.; Moreau, F.; Chadee, K. Probiotic mixture VSL#3 reduces colonic inflammation and improves intestinal barrier function in Muc2 mucin-deficient mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 312, G34–G45. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Giorgetti, G.; Forti, G.; Modeo, M.; Gigliobianco, A. Low-dose balsalazide plus high-potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2004, 10, PI126–PI131. [Google Scholar]
- Sood, A.; Midha, V.; Makharia, G.K.; Ahuja, V.; Singal, D.; Goswami, P.; Tandon, R.K. The Probiotic Preparation, VSL#3 Induces Remission in Patients with Mild-to-Moderately Active Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1202–1209.e1. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Papa, A.; Giglio, A.; Elisei, W.; Giorgetti, G.M.; Forti, G.; Morini, S.; Hassan, C.; Pistoia, M.A.; et al. Treatment of Relapsing Mild-to-Moderate Ulcerative Colitis with the Probiotic VSL#3 as Adjunctive to a Standard Pharmaceutical Treatment: A Double-Blind, Randomized, Placebo-Controlled Study. Off. J. Am. Coll. Gastroenterol. ACG 2010, 105, 2218–2227. [Google Scholar]
- Ng, S.C.; Plamondon, S.; Kamm, M.A.; Hart, A.L.; Al-Hassi, H.O.; Guenther, T.; Stagg, A.J.; Knight, S.C. Immunosuppressive effects via human intestinal dendritic cells of probiotic bacteria and steroids in the treatment of acute ulcerative colitis. Inflamm. Bowel Dis. 2010, 16, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Pascarella, F.; Giannetti, E.; Quaglietta, L.; Baldassano, R.N.; Staiano, A. Effect of a Probiotic Preparation (VSL#3) on Induction and Maintenance of Remission in Children with Ulcerative Colitis. Off. J. Am. Coll. Gastroenterol. ACG 2009, 104, 437–443. [Google Scholar]
- Fedorak, R.N.; Feagan, B.G.; Hotte, N.; Leddin, D.; Dieleman, L.A.; Petrunia, D.M.; Enns, R.; Bitton, A.; Chiba, N.; Paré, P.; et al. The Probiotic VSL#3 Has Anti-inflammatory Effects and Could Reduce Endoscopic Recurrence After Surgery for Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Akedo, I.; Umesaki, Y.; Tanaka, R.; Imaoka, A.; Otani, T. Randomized Controlled Trial of the Effect of Bifidobacteria-Fermented Milk on Ulcerative Colitis. J. Am. Coll. Nutr. 2003, 22, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Mizuno, S.; Umesaki, Y.; Ishii, Y.; Sugitani, M.; Imaoka, A.; Otsuka, M.; Hasunuma, O.; Kurihara, R.; Iwasaki, A.; et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment. Pharmacol. Ther. 2004, 20, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Matsumoto, S.; Ohashi, Y.; Imaoka, A.; Setoyama, H.; Umesaki, Y.; Tanaka, R.; Otani, T. Beneficial Effects of Probiotic Bifidobacterium and Galacto-Oligosaccharide in Patients with Ulcerative Colitis: A Randomized Controlled Study. Digestion 2011, 84, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Uemura, Y.; Kanai, T.; Kunisaki, R.; Suzuki, Y.; Yokoyama, K.; Yoshimura, N.; Hibi, T. Efficacy of Bifidobacterium breve Fermented Milk in Maintaining Remission of Ulcerative Colitis. Dig. Dis. Sci. 2018, 63, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Ghyselinck, J.; Verstrepen, L.; Moens, F.; Van den Abbeele, P.; Said, J.; Smith, B.; Bjarnason, I.; Basit, A.W.; Gaisford, S. A 4-strain probiotic supplement influences gut microbiota composition and gut wall function in patients with ulcerative colitis. Int. J. Pharm. 2020, 587, 119648. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, I.; Sission, G.; Hayee, B.H. A randomised, double-blind, placebo-controlled trial of a multi-strain probiotic in patients with asymptomatic ulcerative colitis and Crohn’s disease. Inflammopharmacology 2019, 27, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.E.; Yang, S.M.; Zhou, X.J.; Zhang, Y. Effects of mesalazine combined with bifid triple viable on intestinal flora, immunoglobulin and levels of cal, MMP-9, and MPO in feces of patients with ulcerative colitis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 935–942. [Google Scholar] [CrossRef] [PubMed]
- van der Lelie, D.; Oka, A.; Taghavi, S.; Umeno, J.; Fan, T.-J.; Merrell, K.E.; Watson, S.D.; Ouellette, L.; Liu, B.; Awoniyi, M.; et al. Rationally designed bacterial consortia to treat chronic immune-mediated colitis and restore intestinal homeostasis. Nat. Commun. 2021, 12, 3105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhou, Y.; Xu, J.; Zhang, Y.; Wang, H.; Zhao, C.; Huang, H.; Yang, J.; Huang, C.; Li, Y.; et al. Short-chain fatty acid-producing bacterial strains attenuate experimental ulcerative colitis by promoting M2 macrophage polarization via JAK/STAT3/FOXO3 axis inactivation. J. Transl. Med. 2024, 22, 369. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, L.; Osme, A.; Ghannoum, M.; Cominelli, F. A Novel Probiotic Combination Ameliorates Crohn’s Disease—Like Ileitis by Increasing Short-Chain Fatty Acid Production and Modulating Essential Adaptive Immune Pathways. Inflamm. Bowel Dis. 2023, 29, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; et al. Specific Bacteria and Metabolites Associated with Response to Fecal Microbiota Transplantation in Patients with Ulcerative Colitis. Gastroenterology 2019, 156, 1440–1454.e2. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-M.; Huang, H.-L.; Xu, J.; He, J.; Zhao, C.; Peng, Y.; Zhao, H.-L.; Huang, W.-Q.; Cao, C.-Y.; Zhou, Y.-J.; et al. Cross-Talk Between Butyric Acid and Gut Microbiota in Ulcerative Colitis Following Fecal Microbiota Transplantation. Front. Microbiol. 2021, 12, 658292. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.F.; Gogokhia, L.; Viladomiu, M.; Chou, L.; Putzel, G.; Jin, W.-B.; Pires, S.; Guo, C.-J.; Gerardin, Y.; Crawford, C.V.; et al. Transferable Immunoglobulin A–Coated Odoribacter splanchnicus in Responders to Fecal Microbiota Transplantation for Ulcerative Colitis Limits Colonic Inflammation. Gastroenterology 2022, 162, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Umesaki, Y.; Imaoka, A.; Handa, T.; Kanazawa, M.; Fukudo, S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 2010, 22, 512–519, e114–e115. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Jiang, Z.; Ma, J.; Yang, D. Changes in Fecal Short-Chain Fatty Acids in IBS Patients and Effects of Different Interventions: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1727. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tan, Y.; Zhu, J.; Wu, X.; Huang, Z.; Chen, H.; Yang, M.; Chen, D. Effect of sodium butyrate regulating IRAK1 (interleukin-1 receptor-associated kinase 1) on visceral hypersensitivity in irritable bowel syndrome and its mechanism. Bioengineered 2021, 12, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Nozu, T.; Miyagishi, S.; Nozu, R.; Takakusaki, K.; Okumura, T. Butyrate inhibits visceral allodynia and colonic hyperpermeability in rat models of irritable bowel syndrome. Sci. Rep. 2019, 9, 19603. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Nozu, T.; Ishioh, M.; Igarashi, S.; Kumei, S.; Ohhira, M. Centrally administered butyrate improves gut barrier function, visceral sensation and septic lethality in rats. J. Pharmacol. Sci. 2021, 146, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Goyal, O.; Batta, S.; Nohria, S.; Kishore, H.; Goyal, P.; Sehgal, R.; Sood, A. Low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol diet in patients with diarrhea-predominant irritable bowel syndrome: A prospective, randomized trial. J. Gastroenterol. Hepatol. 2021, 36, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Schumann, D.; Klose, P.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Nutrition 2018, 45, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Murphy, M.; Genoni, A.; Marlow, E.; Dunican, I.C.; Lo, J.; Andrew, L.; Devine, A.; Christophersen, C.T. Does Fibre-fix provided to people with irritable bowel syndrome who are consuming a low FODMAP diet improve their gut health, gut microbiome, sleep and mental health? A double-blinded, randomised controlled trial. BMJ Open Gastroenterol. 2020, 7, e000448. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Rossi, M.; Kanno, T.; Parkes, G.C.; Anderson, S.; Mason, A.J.; Irving, P.M.; Lomer, M.C.; Whelan, K. β-Galactooligosaccharide in Conjunction with Low FODMAP Diet Improves Irritable Bowel Syndrome Symptoms but Reduces Fecal Bifidobacteria. Off. J. Am. Coll. Gastroenterol. ACG 2020, 115, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.-E.; Fu, H.; Yang, X.-X.; Yu, Z.-C.; Teng, W.-L.; Zhang, Y.; Ye, X.-W.; Quan, H.H.; Lu, L.-Z.; Liu, W. Bacterial, short-chain fatty acid and gas profiles of partially hydrolyzed guar gum in vitro fermentation by human fecal microbiota. Food Chem. 2024, 430, 137006. [Google Scholar] [CrossRef] [PubMed]
- Niv, E.; Halak, A.; Tiommny, E.; Yanai, H.; Strul, H.; Naftali, T.; Vaisman, N. Randomized clinical study: Partially hydrolyzed guar gum (PHGG) versus placebo in the treatment of patients with irritable bowel syndrome. Nutr. Metab. 2016, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Bărboi, O.B.; Chirilă, I.; Ciortescu, I.; Anton, C.; Drug, V.L. Inulin, Choline and Silymarin in the Treatment of Irritable Bowel Syndrome with Constipation-Randomized Case-Control Study. J. Clin. Med. 2022, 11, 2248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, F.; Mao, L.; Feng, T.; Wang, K.; Xu, M.; Lv, B.; Wang, X. Bifico relieves irritable bowel syndrome by regulating gut microbiota dysbiosis and inflammatory cytokines. Eur. J. Nutr. 2023, 62, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Bonfrate, L.; Di Palo, D.M.; Celano, G.; Albert, A.; Vitellio, P.; De Angelis, M.; Gobbetti, M.; Portincasa, P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur. J. Clin. Investig. 2020, 50, e13201. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, X.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Xue, Z.; Zhai, Q.; et al. Lactobacillus plantarum CCFM8610 Alleviates Irritable Bowel Syndrome and Prevents Gut Microbiota Dysbiosis: A Randomized, Double-Blind, Placebo-Controlled, Pilot Clinical Trial. Engineering 2021, 7, 376–385. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020, 69, 859. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Valeur, J.; Hausken, T.; Gunnar Hatlebakk, J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2021, 33, e13983. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Winkel, R.; Casen, C.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. Efficacy of Fecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome at 3 Years After Transplantation. Gastroenterology 2022, 163, 982–994.e914. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, T.; Joossens, M.; Vázquez-Castellanos, J.F.; Christiaens, E.; Heyerick, L.; Boelens, J.; Verhasselt, B.; van Vlierberghe, H.; De Vos, M.; Raes, J.; et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients with Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-term Results From a Placebo-Controlled Randomized Trial. Gastroenterology 2021, 160, 145–157.e8. [Google Scholar] [CrossRef] [PubMed]
- Holster, S.; Lindqvist, C.M.; Repsilber, D.; Salonen, A.; de Vos, W.M.; König, J.; Brummer, R.J. The Effect of Allogenic Versus Autologous Fecal Microbiota Transfer on Symptoms, Visceral Perception and Fecal and Mucosal Microbiota in Irritable Bowel Syndrome: A Randomized Controlled Study. Clin. Transl. Gastroenterol. 2019, 10, e00034. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, P.; Jalanka, J.; Hartikainen, A.; Mattila, E.; Hillilä, M.; Punkkinen, J.; Koskenpato, J.; Anttila, V.-J.; Tillonen, J.; Satokari, R.; et al. Randomised clinical trial: Faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2020, 51, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Halkjær, S.I.; Christensen, A.H.; Lo, B.Z.S.; Browne, P.D.; Günther, S.; Hansen, L.H.; Petersen, A.M. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-blind placebo-controlled study. Gut 2018, 67, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Alm, E.J.; Kelley, J.M.; Cheng, V.; Smith, M.; Kassam, Z.; Nee, J.; Iturrino, J.; Lembo, A. Effect of antibiotic pretreatment on bacterial engraftment after Fecal Microbiota Transplant (FMT) in IBS-D. Gut Microbes 2022, 14, 2020067. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xie, X.; Zhao, S.; Ma, X.; Wang, Z.; Zhang, Y. Fecal microbiota transplantation for irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. Front. Immunol. 2023, 14, 1136343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-H.; Wang, Z.-X.; Zhou, D.; Han, Y.; Ma, F.; Hu, Z.; Xin, F.-Z.; Liu, X.-L.; Ren, T.-Y.; Zhang, F.; et al. Sodium Butyrate Supplementation Inhibits Hepatic Steatosis by Stimulating Liver Kinase B1 and Insulin-Induced Gene. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Qu, F.; Chen, L.; Liu, C.; Zhang, M.; Ren, F.; Guo, H.; Zhang, H.; Ge, S.; Wu, C.; et al. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J. Endocrinol. 2020, 245, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.R.; Gerson, C.D.; DeCarli, L.M.; Lieber, C.S. Role of acetate in the reduction of plasma free fatty acids produced by ethanol in man. J. Lipid Res. 1968, 9, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Nishina, P.M.; Freedland, R.A. Effects of Propionate on Lipid Biosynthesis in Isolated Rat Hepatocytes. J. Nutr. 1990, 120, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yang, H.; Heng, C.; Wang, H.; Chen, S.; Hu, Y.; Jiang, Z.; Yu, Q.; Wang, Z.; Qian, S.; et al. Amelioration of non-alcoholic fatty liver disease by sodium butyrate is linked to the modulation of intestinal tight junctions in db/db mice. Food Funct. 2020, 11, 10675–10689. [Google Scholar] [CrossRef] [PubMed]
- Gart, E.; van Duyvenvoorde, W.; Toet, K.; Caspers, M.P.M.; Verschuren, L.; Nielsen, M.J.; Leeming, D.J.; Souto Lima, E.; Menke, A.; Hanemaaijer, R.; et al. Butyrate Protects against Diet-Induced NASH and Liver Fibrosis and Suppresses Specific Non-Canonical TGF-β Signaling Pathways in Human Hepatic Stellate Cells. Biomedicines 2021, 9, 1954. [Google Scholar] [CrossRef] [PubMed]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Günther, C.; Bischoff, S.C. Prebiotic Inulin and Sodium Butyrate Attenuate Obesity-Induced Intestinal Barrier Dysfunction by Induction of Antimicrobial Peptides. Front. Immunol. 2021, 12, 678360. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Onuki, M.; Hattori, K.; Ito, M.; Yamada, T.; Kamikado, K.; Kim, Y.G.; Nakamoto, N.; Kimura, I.; Clarke, J.M.; et al. Commensal microbe-derived acetate suppresses NAFLD/NASH development via hepatic FFAR2 signalling in mice. Microbiome 2021, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Takai, A.; Kikuchi, K.; Ichimura, M.; Tsuneyama, K.; Moritoki, Y.; Matsumoto, K.; Tsunashima, H.; Onda, T.; Kuniyoshi, N.; Nariyama, T.; et al. Fructo-oligosaccharides ameliorate steatohepatitis, visceral adiposity, and associated chronic inflammation via increased production of short-chain fatty acids in a mouse model of non-alcoholic steatohepatitis. BMC Gastroenterol. 2020, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Su, Q.; Liu, Y. Sinapine reduces non-alcoholic fatty liver disease in mice by modulating the composition of the gut microbiota. Food Funct. 2019, 10, 3637–3649. [Google Scholar] [CrossRef] [PubMed]
- Rosado, C.P.; Rosa, V.H.C.; Martins, B.C.; Soares, A.C.; Santos, I.B.; Monteiro, E.B.; Moura-Nunes, N.; da Costa, C.A.; Mulder, A.d.R.P.; Daleprane, J.B. Resistant starch from green banana (Musa sp.) attenuates non-alcoholic fat liver accumulation and increases short-chain fatty acids production in high-fat diet-induced obesity in mice. Int. J. Biol. Macromol. 2020, 145, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, X.; Liu, W.; Wei, H.; Liang, W.; Zhou, Y.; Ding, Y.; Ji, F.; Ho-Kwan Cheung, A.; Wong, N.; et al. Bifidobacterium pseudolongum-generated acetate suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma. J. Hepatol. 2023, 79, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Yau, Y.F.; El-Nezami, H.; Galano, J.-M.; Kundi, Z.M.; Durand, T.; Lee, J.C.-Y. Lactobacillus rhamnosus GG and Oat Beta-Glucan Regulated Fatty Acid Profiles along the Gut-Liver-Brain Axis of Mice Fed with High Fat Diet and Demonstrated Antioxidant and Anti-Inflammatory Potentials. Mol. Nutr. Food Res. 2020, 64, 2000566. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Sheng, L.; Zhong, J.; Tao, X.; Zhu, W.; Ma, J.; Yan, J.; Zhao, A.; Zheng, X.; Wu, G.; et al. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes 2021, 13, 1930874. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, L.; Wu, J.; Hu, J.; Wan, M.; Bie, J.; Li, J.; Pan, D.; Sun, G.; Yang, C. Optimal probiotic combinations for treating nonalcoholic fatty liver disease: A systematic review and network meta-analysis. Clin. Nutr. 2024, 43, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Afolabi, P.R.; Miles, E.A.; Smith, D.E.; Almehmadi, A.; Alshathry, A.; Childs, C.E.; Del Fabbro, S.; Bilson, J.; Moyses, H.E.; et al. Synbiotics Alter Fecal Microbiomes, But Not Liver Fat or Fibrosis, in a Randomized Trial of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, Q.; Li, T.; Shao, K.; Zhu, X.; Cong, Y.; Zhao, X. Gut microbiota and butyrate contribute to nonalcoholic fatty liver disease in premenopause due to estrogen deficiency. PLoS ONE 2022, 17, e0262855. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, I.; Barberá, A.; Raurell, I.; Estrella, F.; de Leeuw, M.; Bolca, S.; Gottardi, D.; Horscroft, N.; Possemiers, S.; Salcedo, M.T.; et al. A Nine-Strain Bacterial Consortium Improves Portal Hypertension and Insulin Signaling and Delays NAFLD Progression In Vivo. Biomedicines 2022, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; You, H.; Bajaj, J.; Joo, S.; Yu, J.; Park, S.; Kang, H.; Park, J.; Kim, J.; Dong Hyeon, L.; et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kwon, J.; Kim, M.S.; Park, H.; Ji, Y.; Holzapfel, W.; Hyun, C.K. Protective effects of Bacillus probiotics against high-fat diet-induced metabolic disorders in mice. PLoS ONE 2018, 13, e0210120. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Fang, X.; Wang, F.; Li, H.; Niu, W.; Liang, W.; Wu, C.; Li, J.; Tu, X.; Pan, L.L.; et al. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br. J. Pharmacol. 2019, 176, 4446–4461. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Jing, S.; Jiakui, S.; Lei, Z.; Ying, L.; Han, L.; Xinwei, M.; Weiqin, L. Butyrate Ameliorates Intestinal Epithelial Barrier Injury Via Enhancing Foxp3+ Regulatory T-Cell Function in Severe Acute Pancreatitis Model. Turk. J. Gastroenterol. 2022, 33, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Guo, J.; Shen, J.; Jiang, S.; Han, S.; Li, L. Butyrate ameliorated the intestinal barrier dysfunction and attenuated acute pancreatitis in mice fed with ketogenic diet. Life Sci. 2023, 334, 122188. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Guo, J.; Shen, J.; Jiang, S.; Han, S.; Li, L. Ketogenic Diet Exacerbates L-Arginine-Induced Acute Pancreatitis and Reveals the Therapeutic Potential of Butyrate. Nutrients 2023, 15, 4427. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Tang, L.; Liu, S.; Hu, S.; Wu, L.; Liu, Y.; Yang, M.; Huang, S.; Tang, X.; Tang, T.; et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome 2021, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Ammer-Herrmenau, C.; Antweiler, K.L.; Asendorf, T.; Beyer, G.; Buchholz, S.M.; Cameron, S.; Capurso, G.; Damm, M.; Dang, L.; Frost, F.; et al. Gut microbiota predicts severity and reveals novel metabolic signatures in acute pancreatitis. Gut 2024, 73, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Bahramsoltani, R.; Abdollahi, M.; Rahimi, R. The Role of Visceral Hypersensitivity in Irritable Bowel Syndrome: Pharmacological Targets and Novel Treatments. J. Neurogastroenterol. Motil. 2016, 22, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Olesen, M.; Gudmand-Høyer, E. Efficacy, safety, and tolerability of fructooligosaccharides in the treatment of irritable bowel syndrome. Am. J. Clin. Nutr. 2001, 72, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mei, Q.; Fu, Y.; Zeng, Y. Alteration of gut microbiota in acute pancreatitis and associated therapeutic strategies. Biomed. Pharmacother. 2021, 141, 111850. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).