Mixed Sweeteners Supplemented with Chromium Picolinate (CrPic) Improved Some Diabetes-Related Markers and Complications in a Type 2 Diabetic Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Animals/Grouping
2.3. Experimental Design and Type 2 Diabetes Induction
2.4. Blood Collection and Serum and Tissue Preparation
2.5. Estimation of Serum Insulin
2.6. Effect of MSSC on Hexokinase and α-Glucosidase Activities
2.7. The Effect of MSSC on the Serum Lipid Profile
2.8. The Effect of MSSC on the Liver and Kidney Function Indices
2.9. The Effect of MSSC on the Pancreatic Antioxidant Status
2.10. Histopathological Analysis of Liver and Pancreas
2.11. Statistical Analysis
3. Results
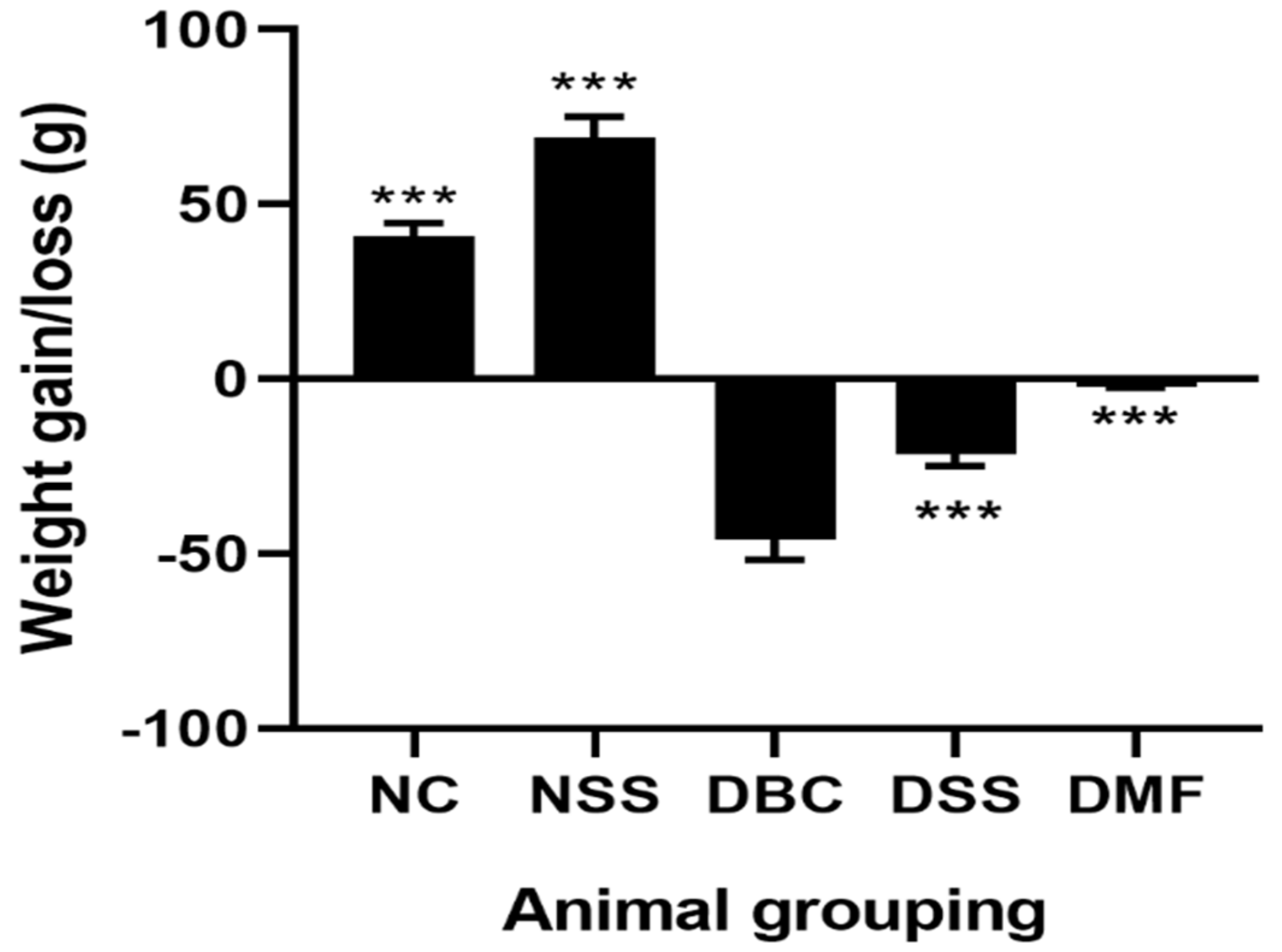
3.1. The Effect of MSSC on Body Weight
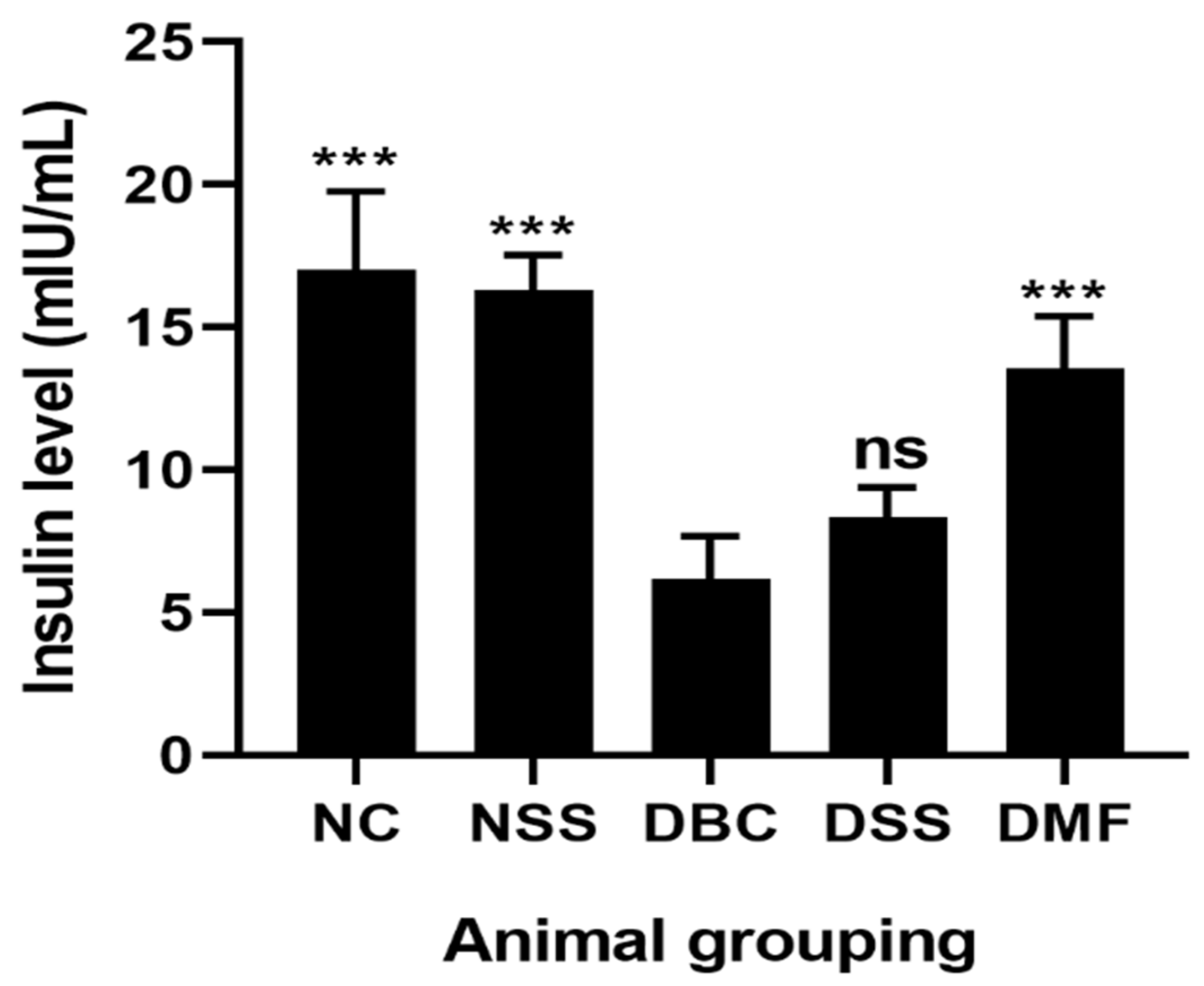
3.2. Effect of MSSC on Fasting Blood Glucose (FBGL) and Insulin Level
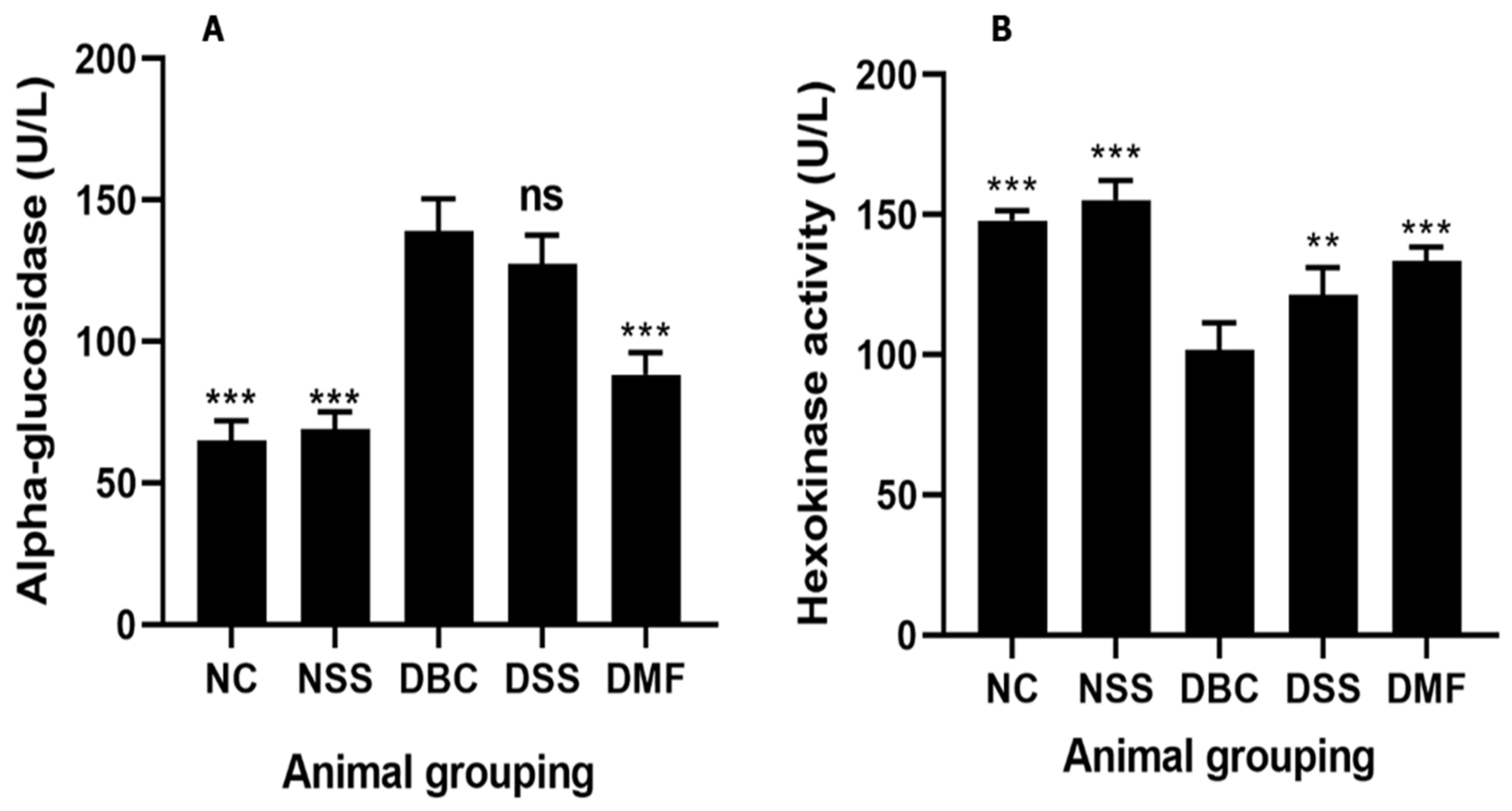
3.3. Effect of MSSC on Hepatic Alpha-Glucosidase and Hexokinase Activity
3.4. Effect of MSSC on Serum Lipid Profile
3.5. Effect of MSSC on In Vivo Antioxidant Enzyme Activity
3.6. The Effect of MSSC on the Liver Functional Indices
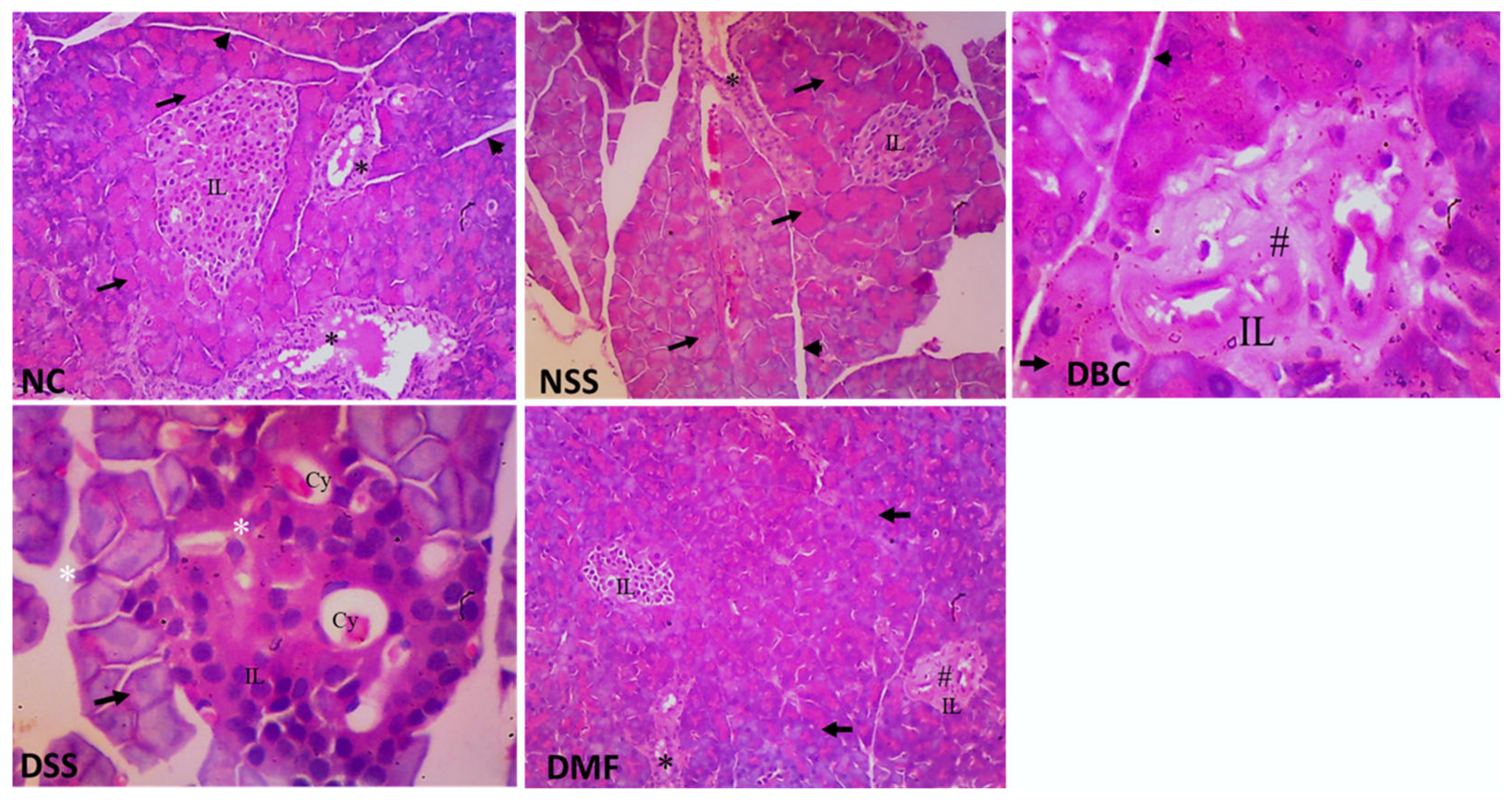
3.7. Histopathological Studies of Pancreas
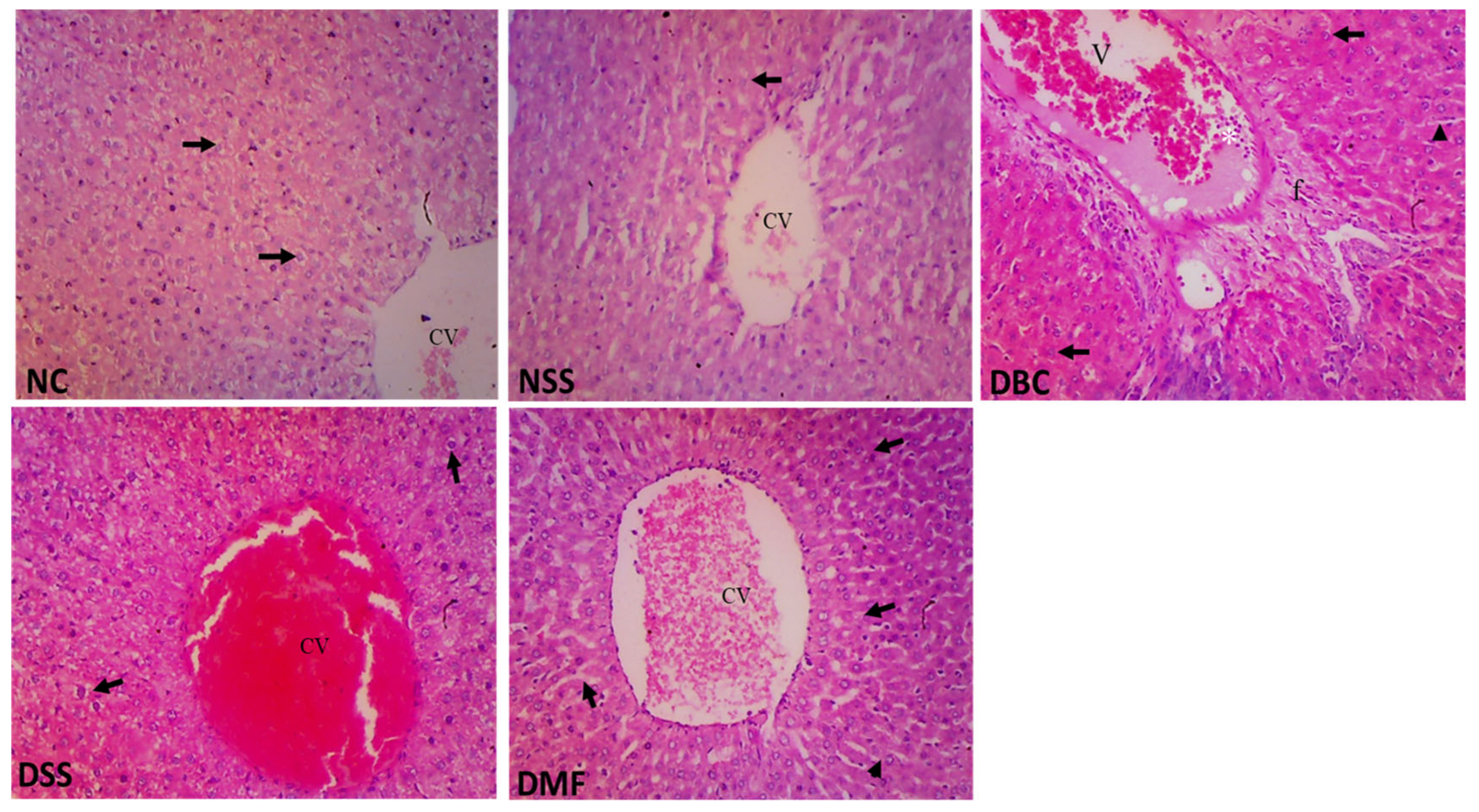
3.8. Histopathological Studies of Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Newary, S.A.; Afifi, S.M.; Aly, M.S.; Ahmed, R.; El Gendy, A.E.G.; Abd-ElGawad, A.M.; Farag, M.A.; Elgamal, A.M.; Elshamy, A.I. Chemical profile of Launaea nudicaulis ethanolic extract and its antidiabetic effect in streptozotocin-induced rats. Molecules 2021, 26, 1000. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Obesity and Overweight; World Health Organisation: Geneva, Switzerland, 2021. [Google Scholar]
- Pavanello, S.; Moretto, A.; La Vecchia, C.; Alicandro, G. Non-sugar sweeteners and cancer: Toxicological and epidemiological evidence. Regul. Toxicol. Pharmacol. 2023, 139, 105369. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Zhao, X.; Liu, B. Current Advances and Future Aspects of Sweetener Synergy: Properties, Evaluation Methods and Molecular Mechanisms. Appl. Sci. 2022, 12, 5096. [Google Scholar] [CrossRef]
- Bordier, V.; Teysseire, F.; Schlotterbeck, G.; Senner, F.; Beglinger, C.; Meyer-Gerspach, A.C.; Wölnerhanssen, B.K. Effect of a chronic intake of the natural sweeteners Xylitol and Erythritol on glucose absorption in humans with obesity. Nutrients 2021, 13, 3950. [Google Scholar] [CrossRef]
- Baird, I.M.; Shephard, N.W.; Merritt, R.J.; Hildick-Smith, G. Repeated dose study of sucralose tolerance in human subjects. Food Chem. Toxicol. 2000, 38, S123–S129. [Google Scholar] [CrossRef]
- Barakat, H.; Al-Roug, K.; Algonaiman, R.; Althwab, S.A.; Alfheeaid, H.A.; Alhomaid, R.M.; Almujaydil, M.S.; Bushnaq, T.; Ebeid, T.A. Biological assessment of stevioside and sucralose as sucrose substitutes for diabetics on STZ-induced diabetes in rats. Molecules 2023, 28, 940. [Google Scholar] [CrossRef]
- Mbambo, N.P.; Dlamini, S.N.; Chukwuma, C.I.; Islam, M. Comparative effects of commonly used commercially available non-nutritive sweeteners on diabetes-related parameters in non-diabetic rats. J. Food Biochem. 2020, 44, e13453. [Google Scholar] [CrossRef]
- Lohner, S.; Kuellenberg de Gaudry, D.; Toews, I.; Ferenci, T.; Meerpohl, J.J. Non-nutritive sweeteners for diabetes mellitus. Cochrane Database Syst. Rev. 2020, 5, CD012885. [Google Scholar] [CrossRef]
- Anderson, R.A. Recent Advances in the clinical and bio-chemical manifestation of chromium deficiency in Human and Animal Nutrition. J. Trace Elem. Med. Biol. 1998, 11, 241–250. [Google Scholar] [CrossRef]
- Qi, S.S.; Zheng, H.X.; Jiang, H.; Yuan, L.P.; Dong, L.C. Protective effects of chromium picolinate against diabetic-induced renal dysfunction and renal fibrosis in streptozotocin-induced diabetic rats. Biomolecules 2020, 10, 398. [Google Scholar] [CrossRef]
- Harrington, E.O.; Vang, A.; Braza, J.; Shil, A.; Chichger, H. Activation of the sweet taste receptor, T1R3, by the artificial sweetener sucralose regulates the pulmonary endothelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L165–L176. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, E.A.; Takahashi, H.; Karakas, E. Structural basis for activation and gating of IP3 receptors. Nat. Commun. 2022, 13, 1408. [Google Scholar] [CrossRef] [PubMed]
- Margolskee, R.F.; Dyer, J.; Kokrashvili, Z.; Salmon, K.; Ilegems, E.; Daly, K.; Maillet, E.L.; Ninomiya, Y.; Mosinger, B.; Shirazi-Beechey, S.P. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl. Acad. Sci. USA 2007, 104, 15075–15080. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Ribeiro, D.; Freitas, M.; Fernandes, E. Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3137–3207. [Google Scholar] [CrossRef]
- Wilson, R.D.; Islam, M.S. Fructose-fed streptozotocin-injected rat: An alternative model for type 2 diabetes. Pharmacol. Rep. 2012, 64, 129–139. [Google Scholar] [CrossRef]
- Kalaivani, K.; Sankaranarayanan, C. Modulatory effect of isopulegol on hepatic key enzymes of glucose metabolism in high-fat diet/streptozotocin-induced diabetic rats. Arch. Physiol. Biochem. 2021, 127, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Jayaprasad, B.; Sharavanan, P.S.; Sivaraj, R. Antidiabetic effect of Chloroxylon swietenia bark extracts on streptozotocin induced diabetic rats. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 61–69. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. J. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Nichans, W.G.; Samuelson, B. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968, 6, 126–130. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Islam, M.S. Sorbitol increases muscle glucose uptake ex vivo and inhibits intestinal glucose absorption ex vivo and in normal and type 2 diabetic rats. Appl. Physiol. Nutr. Metab. 2017, 42, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Meenatchi, M.; Vellapandian, C. Exploring the long-term effect of artificial sweeteners on metabolic health. Cureus 2024, 16, e70043. [Google Scholar] [CrossRef]
- Vajdi, M.; Khajeh, M.; Safaei, E.; Moeinolsadat, S.; Mousavi, S.; Seyedhosseini-Ghaheh, H.; Abbasalizad-Farhangi, M.; Askari, G. Effects of chromium supplementation on body composition in patients with type 2 diabetes: A dose-response systematic review and meta-analysis of randomized controlled trials. J. Trace Elem. Med. Biol. 2024, 81, 127338. [Google Scholar] [CrossRef] [PubMed]
- Stamataki, N.S.; Scott, C.; Elliott, R.; McKie, S.; Bosscher, D.; McLaughlin, J.T. Stevia Beverage Consumption prior to Lunch Reduces Appetite and Total Energy Intake without Affecting Glycemia or Attentional Bias to Food Cues: A Double-Blind Randomized Controlled Trial in Healthy Adults. J. Nutr. 2020, 150, 1126–1134. [Google Scholar] [CrossRef]
- Orellana-Paucar, A.M. Steviol glycosides from stevia rebaudiana: An updated overview of their sweetening activity, pharmacological properties, and safety aspects. Molecules 2023, 28, 1258. [Google Scholar] [CrossRef]
- Assaei, A.; Mokarram, P.; Dastghaib, S.; Darbandi, S.; Darbandi, M.; Zal, F.; Akmali, M.; Ranjbar Omrani, G.H. Hypoglycemic Effect of Aquatic Extract of Stevia in Pancreas of Diabetic Rats: PPARγ-dependent Regulation or Antioxidant Potential. Avicenna J. Med. Biotechnol. 2016, 8, 65–74. [Google Scholar] [PubMed]
- Cefalu, W.T. Concept, strategies, and feasibility of noninvasive insulin delivery. Diabetes Care 2004, 27, 239–246. [Google Scholar] [CrossRef]
- Hua, Y.; Clark, S.; Ren, J.; Sreejayan, N. Molecular mechanisms of chromium in alleviating insulin resistance. J. Nutr. Biochem. 2012, 23, 313–319. [Google Scholar] [CrossRef]
- Ekakitie, L.; Ajiboye, B.O.; Oyinloye, B.E.; Owero-Ozeze, O.S.; Onikanni, S.A.; Ojo, O.A. Attenuation of diabetic nephropathy in alloxan-induced diabetic rats by Solanum macrocarpon Linn aqueous leaves extract. Comp. Clin. Pathol. 2021, 30, 173–179. [Google Scholar] [CrossRef]
- Boubekri, N.; Amrani, A.; Zama, D.; Dendougi, H.; Benayache, F.; Benayache, S. In vitro antioxidant and in vivo antidiabetic potential of n-butanol extract of Chrysanthemum fuscatum in streptozotocin induced diabetic rats. Int. J. Pharm. Sci. Rev. Res. 2016, 41, 214–221. [Google Scholar]
- Kumar, R.; Kumar, N.; Rajput, S.K.; Mallan, S.; Kumar, A.; Rawat, B.S.; Rangra, N.K.; Singh, S. Antidiabetic Evaluation of Kigelia pinnata Root Bark Extract in Streptozotocin-Induced Type-2 Diabetes Model of Rats. Assay. Drug Dev. Technol. 2024, 22, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Talevi, A. Beneficial Effects of Stevia rebaudiana Bertoni and Steviol-Related Compounds on Health, in Sweeteners: Pharmacology, Biotechnology, and Applications; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–22. [Google Scholar]
- Sharma, S.; Walia, S.; Singh, B.; Kumar, R. Comprehensive review on agro technologies of low-calorie natural sweetener stevia (Stevia rebaudiana Bertoni): A boon to diabetic patients. J. Sci. Food Agric. 2016, 96, 1867–1879. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, F.; Ye, S.; Zhu, J.; Ma, Y.; Shan, M.; Li, S.; Chen, Y.; Li, J. Correlation between fruit consumption and 10-year all-cause mortality in patients with dyslipidemia. Front. Nutr. 2024, 11, 1471737. [Google Scholar] [CrossRef]
- Romo-Romo, A.; Aguilar-Salinas, C.A.; Brito-Córdova, G.X.; Gómez Díaz, R.A.; Vilchis Valentín, D.; Almeda-Valdes, P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: Systematic review of observational prospective studies and clinical trials. PLoS ONE 2016, 11, e0161264. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Salleh, N.; Henry, J.; Forde, C.G. Effects of aspartame-, monk fruit-, stevia- and sucrose-sweetened beverages on postprandial glucose, insulin and energy intake. Int. J. Obes. 2017, 41, 450–457. [Google Scholar] [CrossRef]
- Mchunu, N.; Chukwuma, C.I.; Ibrahim, M.A.; Oyebode, O.A.; Dlamini, S.N.; Islam, M.S. Commercially available non-nutritive sweeteners modulate the antioxidant status of type 2 diabetic rats. J. Food Biochem. 2019, 43, e12775. [Google Scholar] [CrossRef]
- Kooshki, F.; Tutunchi, H.; Vajdi, M.; Karimi, A.; Niazkar, H.R.; Shoorei, H.; Gargari, B.P. Comprehensive insight into the effect of chromium supplementation on oxidative stress indices in diabetes mellitus: A systematic review. Clin. Exp. Pharmacol. Physiol. 2021, 48, 291–309. [Google Scholar] [CrossRef]
- Mohamed; Nazratun, N. A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132–e141. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Abensur, H.; Betônico, C.C.; Machado, A.D.; Parente, E.B.; Queiroz, M.; Salles, J.E.; Titan, S.; Vencio, S. Interactions between kidney disease and diabetes: Dangerous liaisons. Diabetol. Metab. Syndr. 2016, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Erukainure, O.L.; Hafizur, R.M.; Kabir, N.; Choudhary, M.I.; Atolani, O.; Banerjee, P.; Preissner, R.; Chukwuma, C.I.; Muhammad, A.; Amonsou, E.O.; et al. Suppressive Effects of Clerodendrum volubile P Beauv. [Labiatae] Methanolic Extract and Its Fractions on Type 2 Diabetes and Its Complications. Front. Pharmacol. 2018, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Oyedemi, S.O.; Nwaogu, G.; Chukwuma, C.I.; Adeyemi, O.T.; Matsabisa, M.G.; Swain, S.S.; Aiyegoro, O.A. Quercetin modulates hyperglycemia by improving the pancreatic antioxidant status and enzymes activities linked with glucose metabolism in type 2 diabetes model of rats: In silico studies of molecular interaction of quercetin with hexokinase and catalase. J. Food Biochem. 2020, 44, e13127. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, F.; Rashid, H.U.; Yesmine, S.; Monjur, F.; Chatterjee, T.K. Preliminary analysis of the effect of Stevia (Stevia rebaudiana) in patients with chronic kidney disease (stage I to stage III). Contemp. Clin. Trials Commun. 2018, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- AbdElwahab, A.H.; Yousuf, A.F.; Ramadan. B.K.; Elimam, H. Comparative Effects of Stevia rebaudiana and Aspartame on hepato-renal function of diabetic rats: Biochemical and Histological Approaches. J. App. Pharm. Sci. 2017, 7, 34–42. [Google Scholar]
- Lenzen, S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef]
| Groups | 0 D | 7 Days | 14 Days | 21 Days | 28 Days | 35 Days | 42 Days | %NFBGR |
|---|---|---|---|---|---|---|---|---|
| NC | 98.8 ± 6.0 a | 98.4 ± 3.05 a | 98.2 ± 3.8 a | 99.8 ± 1.5 a | 101.6 ± 3.1 a | 100.2 ± 2.04 a | 98.4 ± 5.4 a,b | - |
| DBC | 277.8 ± 22.7 c | 296.0 ± 18.6 d | 325.3 ± 23.3 d | 311.8 ± 25.2 c | 328.5 ± 36.5 c | 351.0 ± 37.6 c | 364.3 ± 41.7 c | NR |
| NSS | 103.3 ± 9.1 a | 102.2 ± 12.1 a | 105.3 ± 4.0 a | 111.3 ± 5.7 a | 99.5 ± 4.5 a | 90.8 ± 1.71 a | 83.5 ± 3.7 a | 3.63 ± 0.8 a |
| DSS | 362.8 ± 16.1 d | 389.7 ± 14.6 e | 373.3 ± 20.3 e | 363.0 ± 14.8 d | 358.7 ± 11.4 d | 351.0 ± 6.5 c | 335.3 ± 7.0 c | 7.35 ± 1.2 b |
| DMF | 253.2 ± 31.5 b,c | 241.6 ± 21.0 c | 214.6 ± 32.5 c | 193.4 ± 18.8 b | 172.6 ± 22.6 b | 152.8 ± 18.1 b | 138.4 ± 22.1 b | 45.34 ± 6.0 c |
| Grouping | TC | TAG | HDL-C | LDL-C |
|---|---|---|---|---|
| NC | 2.89 ± 0.19 b | 2.08 ± 0.12 b | 1.50 ± 0.08 a | 0.97 ± 0.19 b |
| DBC | 4.27 ± 0.31 c | 3.23 ± 0.19 c | 1.40 ± 0.01 a | 1.81 ± 0.31 c |
| NSS | 2.92 ± 0.16 b | 2.19 ± 0.65 b | 1.48 ± 0.07 a | 0.47 ± 0.04 a |
| DSS | 2.71 ± 0.15 b | 2.33 ± 0.1 b | 1.40 ± 0.02 a | 0.84 ± 0.16 b |
| DMF | 1.85 ± 0.13 a | 1.99 ± 0.04 a | 1.42 ± 0.0 a | 0.63 ± 0.00 a |
| Grouping | GPx (U/L) | SOD (U/mL) | CAT (U/mL) | MDA (µM) | GSH (mM) |
|---|---|---|---|---|---|
| NC | 18.64 ± 2.34 d | 1.74 ± 0.43 b | 34.44 ± 2.96 c | 2.58 ± 0.73 a | 3.03 ± 0.37 c |
| DBC | 8.42 ± 1.28 a | 0.82 ± 0.07 a | 17.62 ± 2.66 a | 7.47 ± 1.17 a | 1.31 ± 0.05 a |
| NSS | 18.26 ± 1.95 d | 1.92 ± 0.08 b,c | 30.68 ± 1.57 c | 2.27 ± 0.72 a | 2.86 ± 0.48 c |
| DSS | 11.38 ± 2.40 b | 1.18 ± 0.07 b | 25.49 ± 1.61 b | 4.19 ± 0.78 b | 2.03 ± 0.15 b |
| DMF | 15.64 ± 1.93 c | 1.46 ± 0.24 b | 28.56 ± 2.63 b | 3.68 ± 1.07 b | 2.87 ± 0.08 c |
| Parameters. | NC | DBC | NSS | DSS | DMF |
|---|---|---|---|---|---|
| Albumin (g/dL) | 5.12 ± 0.6 b | 3.89 ± 0.4 a | 5.47 ± 0.3 b | 3.8 ± 0.2 a | 5.37 ± 0.6 b |
| Protein (g/dL) | 6.05 ± 0.2 b | 4.75 ± 0.3 a | 6.45 ± 0.2 b | 5.1 ± 0.2 a | 6.18 ± 0.3 b |
| Bilirubin (µmol/L) | 26.25 ± 3.2 c | 11.57 ± 1.7 a | 25.96 ± 1.4 c | 15.6 ± 1.3 b | 26.05 ± 1.7 c |
| Urea (mmol/L) | 1.19 ± 0.1 a | 2.63 ± 0.3 b | 1.42 ± 0.1 a | 2.41 ± 0.2 b | 1.29 ± 0.2 a |
| Creatinine (µmol/L) | 2.86 ± 0.2 a | 4.22 ± 0.3 b | 2.92 ± 0.2 a | 3.03 ± 0.2 a | 2.78 ± 0.2 a |
| ALT (U/L) | 120.23 ± 2.8 a | 132.82 ± 4.5 b | 117.66 ± 2.8 a | 130.76 ± 5.4 b | 130.81 ± 4.7 b |
| ALP (U/L) | 284.53 ± 11.9 a | 1493.08 ± 226.6 e | 438.59 ±31.8 b | 912.69 ±63.3 d | 585.5 ± 55.1 c |
| AST (U/L) | 113.42 ± 9.4 a | 124.96 ± 6.4 c | 127.71 ± 7.5 c | 124.84 ± 6.4 c | 119.0 ± 6.5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inyang, E.; Ijeh, I.I.; Oyedemi, S. Mixed Sweeteners Supplemented with Chromium Picolinate (CrPic) Improved Some Diabetes-Related Markers and Complications in a Type 2 Diabetic Rat Model. Nutraceuticals 2024, 4, 658-672. https://doi.org/10.3390/nutraceuticals4040036
Inyang E, Ijeh II, Oyedemi S. Mixed Sweeteners Supplemented with Chromium Picolinate (CrPic) Improved Some Diabetes-Related Markers and Complications in a Type 2 Diabetic Rat Model. Nutraceuticals. 2024; 4(4):658-672. https://doi.org/10.3390/nutraceuticals4040036
Chicago/Turabian StyleInyang, Ekomobong, Ifeoma Irene Ijeh, and Sunday Oyedemi. 2024. "Mixed Sweeteners Supplemented with Chromium Picolinate (CrPic) Improved Some Diabetes-Related Markers and Complications in a Type 2 Diabetic Rat Model" Nutraceuticals 4, no. 4: 658-672. https://doi.org/10.3390/nutraceuticals4040036
APA StyleInyang, E., Ijeh, I. I., & Oyedemi, S. (2024). Mixed Sweeteners Supplemented with Chromium Picolinate (CrPic) Improved Some Diabetes-Related Markers and Complications in a Type 2 Diabetic Rat Model. Nutraceuticals, 4(4), 658-672. https://doi.org/10.3390/nutraceuticals4040036







